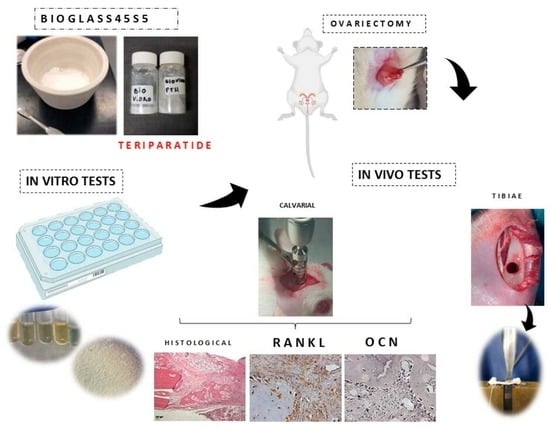
The Local Release of Teriparatide Incorporated in 45S5 Bioglass Promotes a Beneficial Effect on Osteogenic Cells and Bone Repair in Calvarial Defects in Ovariectomized Rats
Abstract
:1. Introduction
2. Materials and Methods
2.1. 45S5 Synthesis
2.2. Sonochemical Technique
2.3. Characterization Biomaterial
2.4. In Vitro Experiment
2.4.1. Cell Adhesion
2.4.2. Cell Viability (MTT)
2.4.3. Protein Content Determination and ALP Assays during hMSC Differentiation
2.4.4. Formation of Mineralization Nodules
2.5. Experimental Design In Vivo Study
2.5.1. Bilateral Ovariectomy
2.5.2. Surgical Procedure at Calvaria
2.5.3. Macroscopic Evaluation of the Uterus and Exfoliative Cytology
2.5.4. Histological and Histomorphometric Analysis
2.5.5. Immunohistochemistry Analysis
2.5.6. Surgical Procedure at Tibiae
2.5.7. Biomechanical Properties
2.6. Statistical Analysis
3. Results
3.1. Sample Characterization
3.2. In Vitro Analysis
3.3. Bone Repair Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An overview and management of osteoporosis. Eur. J. Rheumatol. 2017, 4, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Broken Bones, Broken Lives: A Roadmap to Solve the Fragility Fracture Crisis in Europe. Available online: https://www.osteoporosis.foundation/educational-hub/files/broken-bones-broken-lives-roadmap-solve-fragility-fracture-crisis-europe (accessed on 26 October 2022).
- Li, L.; Wang, Z. Ovarian Aging and Osteoporosis. Adv. Exp. Med. Biol. 2018, 1086, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Kubota, T.; Hasuike, A.; Tsukune, N.; Ozawa, Y.; Yamamoto, T.; Min, S.; Masako, N.; Shuichi, S. Influence of estrogen deficiency on guided bone augmentation: Investigation of rat calvarial model and osteoblast-like MC3T3-E1 cells. Eur. J. Oral Sci. 2018, 126, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Russow, G.; Jahn, D.; Appelt, J.; Märdian, S.; Tsitsilonis, S.; Keller, J. Anabolic Therapies in Osteoporosis and Bone Regeneration. Int. J. Mol. Sci. 2018, 20, 83. [Google Scholar] [CrossRef]
- Van Houdt, C.I.A.; Gabbai-Armelin, P.R.; Perez, P.M.L.; Ulrich, D.J.O.; Jansen, J.A.; Renno, A.C.M.; Beucken, J. Alendronate release from calcium phosphate cement for bone regeneration in osteoporotic conditions. Sci. Rep. 2018, 8, 15398. [Google Scholar] [CrossRef]
- Mori, H.; Manabe, M.; Kurachi, Y.; Nagumo, M. Osteointegration of dental implants in rabbit bone with low mineral density. J. Oral Maxillofac. Surg. 1997, 55, 351–361. [Google Scholar] [CrossRef]
- Yang, C.; Wu, H.; Chen, S.; Kang, G. Three-dimensional bioglass-collagen-phosphatidylserine scaffolds designed with functionally graded structure and mechanical features. Biomed. Tech. 2018, 63, 255–259. [Google Scholar] [CrossRef]
- Banwart, J.C.; Asher, M.A.; Hassanein, R.S. Iliac crest bone graft harvest donor site morbidity: A statistical evaluation. Spine 1995, 20, 1055. [Google Scholar] [CrossRef]
- Mazzonetto, R.; Netto, H.D.; Nascimento, F.F. Enxertos Ósseos em Implantodontia, 1st ed.; Editora Napoleão: São Paulo, Brazil, 2012. [Google Scholar]
- Iaquinta, M.R.; Mazzoni, E.; Manfrini, M.; D’Agostino, A.; Trevisiol, L.; Nocini, R.; Trombelli, L.; Barbanti-Brodano, G.; Martini, F.; Tognon, M. Innovative Biomaterials for Bone Regrowth. Int. J. Mol. Sci. 2019, 20, 618. [Google Scholar] [CrossRef]
- de Vasconcellos, L.M.R.; Santana-Melo, G.F.; Silva, E.; Pereira, V.F.; Araújo, J.C.R.; Silva, A.D.R.; Furtado, A.S.A.; Elias, C.M.V.; Viana, B.C.; Marciano, F.R.; et al. Electrospun Poly(butylene-adipate-co-terephthalate)/Nano-hyDroxyapatite/Graphene Nanoribbon Scaffolds Improved the In Vivo Osteogenesis of the Neoformed Bone. J. Funct. Biomater. 2021, 12, 11. [Google Scholar] [CrossRef]
- Silva, A.D.S.; Rodrigues, B.V.M.; Oliveira, F.C.; Carvalho, J.O.; de Vasconcellos, L.M.R.; de Araújo, J.C.R.; Marciano, F.R.; Lobo, A.O. Characterization and in vitro and in vivo assessment of poly(butylene adipate-co-terephthalate)/nano-hydroxyapatite composites as scaffolds for bone tissue engineering. J. Polym. Res. 2019, 26, 53. [Google Scholar] [CrossRef]
- Duracan, C.; Brown, P.W. Biodegradable hydroxyapatite-polymer composites. Adv. Eng. Mater. 2001, 3, 227–231. [Google Scholar] [CrossRef]
- Johari, B.; Kadivar, M.; Lak, S.; Gholipourmalekabadi, M.; Urbanska, A.M.; Mozafari, M.; Ahmadzadehzarajabad, M.; Azarnezhad, A.; Afshari, S.; Zargan, J.; et al. Osteoblast-seeded bioglass/gelatin nanocomposite: A promising bone substitute in critical-size calvarial defect repair in rat. Int. J. Artif. Organs 2016, 39, 524–533. [Google Scholar] [CrossRef]
- Khoshakhlagh, P.; Rabiee, S.M.; Kiaee, G.; Heidari, P.; Miri, A.K.; Moradi, R.; Moztarzadeh, F.; Ravarian, R. Development and characterization of a bioglass/chitosan composite as an injectable bone substitute. Carbohydr. Polym. 2017, 157, 1261–1271. [Google Scholar] [CrossRef]
- Rizwan, M.; Hamdi, M.; Basirun, W.J. Bioglass® 45S5-based composites for bone tissue engineering and functional applications. J. Biomed. Mater. Res. Part A 2017, 105, 3197–3223. [Google Scholar] [CrossRef]
- Zhang, L.; Ke, X.; Lin, L.; Xiao, J.; Yang, X.; Wang, J.; Yang, G.; Xu, S.; Gou, Z.; Shi, Z. Systematic evaluation of the osteogenic capacity of low-melting bioactive glass-reinforced 45S5 Bioglass porous scaffolds in rabbit femoral defects. Biomed. Mater. 2017, 12, 035010. [Google Scholar] [CrossRef]
- Fernandes, H.R.; Gaddam, A.; Rebelo, A.; Brazete, D.; Stan, G.E.; Ferreira, J.M.F. Bioactive Glasses and Glass-Ceramics for Healthcare Applications in Bone Regeneration and Tissue Engineering. Materials 2018, 11, 2530. [Google Scholar] [CrossRef]
- Lee, S.; Matsugaki, A.; Kasuga, T.; Nakano, T. Development of bifunctional oriented bioactive glass/poly(lactic acid) composite scaffolds to control osteoblast alignment and proliferation. J. Biomed. Mater. Res. Part A 2019, 107, 1031–1041. [Google Scholar] [CrossRef]
- Pazarceviren, A.E.; Evis, Z.; Keskin, D.; Tezcaner, A. Resorbable PCEC/gelatin-bismuth doped bioglass-graphene oxide bilayer membranes for guided bone regeneration. Biomed. Mater. 2019, 14, 035018. [Google Scholar] [CrossRef]
- Hench, L.L.; Paschall, H.A. Direct Chemical Bond of Bioactive Glass-Ceramic Materials to Bone and Muscle. J. Biomed. Mater. Res. 1973, 7, 25–42. [Google Scholar] [CrossRef]
- Cerruti, M.; Greenspan, D.; Powers, K. Effect of pH and ionic strength on the reactivity of Bioglass 45S5. Biomaterials 2005, 26, 1665–1674. [Google Scholar] [CrossRef] [PubMed]
- Slosarczyk, A.; Paszkiewicza, Z.; Paluszkiewicz, C. FTIR and XRD evaluation of carbonated hydroxyapatite powders synthesized by wet methods. J. Mol. Struct. 2005, 744–747, 657–661. [Google Scholar] [CrossRef]
- Anderson, A.; Dallmier, A.; Chudzik, S.; Duran, L.; Guire, P.; Hergenrother, R.; Lodhi, M.; Novak, A.; Ofstead, R.; Wormuth, K.; et al. Technologies for the surface modification of biomaterials. In Biomaterials in Orthopedics; Yaszemski, M.J., Trantolo, D.J., Lewandrowski, K.U., Hasirci, V., Altobelli, D.E., Wise, D.L., Eds.; Marcel Dekker Inc.: New York, NY, USA, 2004; p. 123. [Google Scholar]
- Hench, L.L. The story of Bioglass. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef] [PubMed]
- Zamet, J.; Darbar, U.; Griffiths, G.; Bulman, J.; Brägger, U.; Bürgin, W.; Newman, H. Particulate Bioglass® as a grafting material in the treatment of periodontal intrabony defects. J. Clin. Periodontol. 1997, 24, 410–418. [Google Scholar] [CrossRef]
- Tadjoedin, E.S.; De Lange, G.L.; Lyaruu, D.; Kuiper, L.; Burger, E.H. High concentrations of bioactive glass material (Biogran®) vs. Autogenous bone for sinus floor elevation. Clin. Oral Implants Res. 2002, 13, 428–436. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Miguez-Pacheco, V.; Boccaccini, A.R.; Vitale-Brovarone, C. Bioactive glasses: Special applications outside the skeletal system. J. Non-Cryst. Solids 2016, 432, 15–30. [Google Scholar] [CrossRef]
- Domingues, Z.; Cortés, M.; Gomes, T.; Diniz, H.; Freitas, C.; Gomes, J.; Faria, A.; Sinisterra, R. Bioactive glass as a drug delivery system of tetracycline and tetracycline associated with β-cyclodextrin. Biomaterials 2004, 25, 327–333. [Google Scholar] [CrossRef]
- Lisboa-Filho, P.N.; Gomes-Ferreira, P.H.S.; Batista, F.R.S.; Momesso, G.A.C.; Faverani, L.P.; Okamoto, R. Bone repair with raloxifene and bioglass nanoceramic composite in animal experiment. Connect. Tissue Res. 2018, 59 (Suppl. 1), 97–101. [Google Scholar] [CrossRef]
- Mosqueira, L.; Barrioni, B.R.; Martins, T.; Ocarino, N.M.; Serakides, R.; Pereira, M.M. In vitro effects of the co-release of icariin and strontium from bioactive glass submicron spheres on the reduced osteogenic potential of rat osteoporotic bone marrow mesenchymal stem cells. Biomed. Mater. 2020, 15, 055023. [Google Scholar] [CrossRef]
- Rivadeneira, J.; Di Virgilio, A.L.; Audisio, M.C.; Boccaccini, A.R.; Gorustovich, A.A. Evaluation of the antibacterial effects of vancomycin hydrochloride released from agar-gelatin-bioactive glass composites. Biomed. Mater. 2015, 10, 015011. [Google Scholar] [CrossRef]
- Ning, Z.; Tan, B.; Chen, B.; Lau, D.A.S.; Wong, T.M.; Sun, T.; Peng, S.; Li, Z.; Lu, W.W. Precisely Controlled Delivery of Abaloparatide through Injectable Hydrogel to Promote Bone Regeneration. Macromol. Biosci. 2019, 19, e1900020. [Google Scholar] [CrossRef]
- Wang, C.; Yu, S.; Fretwurst, T.; Larsson, L.; Sugai, J.; Oh, J.; Lehner, K.; Jin, Q.; Giannobile, W. Maresin 1 Promotes Wound Healing and Socket Bone Regeneration for Alveolar Ridge Preservation. J. Dent. Res. 2020, 99, 930–937. [Google Scholar] [CrossRef]
- Ersan, N.; van Ruijven, L.J.; Bronckers, A.L.; Olgaç, V.; Ilgüy, D.; Everts, V. Teriparatide and the treatment of bisphosphonate-related osteonecrosis of the jaw: A rat model. Dentomaxillofac. Radiol. 2014, 43, 20130144. [Google Scholar] [CrossRef]
- Mazziotti, G.; Bilezikian, J.; Canalis, E.; Cocchi, D.; Giustina, A. New understanding and treatments for osteoporosis. Endocrine 2012, 41, 58–69. [Google Scholar] [CrossRef]
- Kubota, T.; Hasuike, A.; Naito, M.; Tsunori, K.; Min, S.; Sato, S. Enhancement of Bone Augmentation in Osteoporotic Conditions by the Intermittent Parathyroid Hormone: An Animal Study in the Calvarium of Ovariectomized Rat. Int. J. Oral Maxillofac. Implants 2018, 33, 1003–1010. [Google Scholar] [CrossRef]
- de Oliveira, D.; de Oliveira Puttini, I.; Silva Gomes-Ferreira, P.H.; Palin, L.P.; Matsumoto, M.A.; Okamoto, R. Effect of intermittent teriparatide (PTH 1-34) on the alveolar healing process in orchiectomized rats. Clin. Oral Investig. 2019, 23, 2313–2322. [Google Scholar] [CrossRef]
- Puttini, I.D.O.; Poli, P.P.; Maiorana, C.; de Vasconcelos, I.R.; Schmidt, L.E.; Colombo, L.T.; Hadad, H.; dos Santos, G.M.; de Carvalho, P.S.P.; Souza, F. Evaluation of Osteoconduction of Biphasic Calcium Phosphate Ceramic in the Calvaria of Rats: Microscopic and Histometric Analysis. J. Funct. Biomater. 2019, 10, 7. [Google Scholar] [CrossRef]
- Jiang, X.; Xu, C.; Shi, H.; Cheng, Q. PTH1-34 improves bone healing by promoting angiogenesis and facilitating MSCs migration and differentiation in a stabilized fracture mouse model. PLoS ONE 2019, 14, e0226163. [Google Scholar] [CrossRef]
- Özer, T.; Başlarlı, Ö.; Aktaş, A.; Barış, E.; Çelik, H.H.; Ocak, M. Locally administrated single-dose teriparatide affects critical-size rabbit calvarial defects: A histological, histomorphometric and micro-CT study. Acta Orthop. Traumatol. Turc. 2019, 53, 478–484. [Google Scholar] [CrossRef]
- Tao, Z.-S.; Zhou, W.-S.; Wu, X.-J.; Wang, L.; Yang, M.; Xie, J.-B.; Xu, Z.-J.; Ding, G.-Z. Single-dose local administration of parathyroid hormone (1-34, PTH) with β-tricalcium phosphate/collagen (β-TCP/COL) enhances bone defect healing in ovariectomized rats. J. Bone Miner. Metab. 2019, 37, 28–35. [Google Scholar] [CrossRef]
- Bakker, A.D.; Zandieh-Doulabi, B.; Klein-Nulend, J. Strontium ranelate affects signaling from mechanically-stimulated osteocytes towards osteoclasts and osteoblasts. Bone 2013, 53, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, J.; Yang, L.; Zhou, Y.; Wang, Y. Dose-dependence of PTH-related peptide-1 on the osteogenic induction of MC3T3-E1 cells in vitro. Medicine 2017, 96, e6637. [Google Scholar] [CrossRef] [PubMed]
- Bang, J.H.; Suslick, K.S. Applications of ultrasound to the synthesis of nanostructured materials. Adv. Mater. 2010, 22, 1039–1059. [Google Scholar] [CrossRef] [PubMed]
- Arruda, L.B.; Orlandi, M.O.; Lisboa-Filho, P.N. Morphological modifications and surface amorphization in ZnO sonochemically treated nanoparticles. Ultrason. Sonochem. 2013, 20, 799–804. [Google Scholar] [CrossRef]
- Gonzalo-Juan, I.; Xie, F.; Becker, M.; Tulyaganov, D.U.; Ionescu, E.; Lauterbach, S.; De Angelis Rigotti, F.; Fischer, A.; Riedel, R. Synthesis of Silver Modified Bioactive Glassy Materials with Antibacterial Properties via Facile and Low-Temperature Route. Materials 2020, 13, 5115. [Google Scholar] [CrossRef]
- Spirandeli, B.R.; Campos, T.M.B.; Ribas, R.G.; Thim, G.P.; Trichês, E.S. Evaluation of colloidal and polymeric routes in sol-gel synthesis of a bioactive glass-ceramic derived from 45S5 bioglass. Ceram. Int. 2020, 46, 20264–20271. [Google Scholar] [CrossRef]
- Zhang, L.; Chan, C. Isolation and enrichment of rat mesenchymalstem cells (MSCs) and separation of single-colony derived MSCs. J. Vis. Exp. 2010, 22, 1852. [Google Scholar] [CrossRef]
- do Prado, R.F.; Esteves, G.C.; Santos, E.; Bueno, D.; Cairo, C.; Vasconcellos, L.G.; Sagnori, R.; Tessarin, F.; Oliveira, F.E.; Oliveira, L.D.; et al. In vitro and in vivo biological performance of porous Ti alloys prepared by powder metallurgy. PLoS ONE 2018, 13, e0196169. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 10993-5. Biological Evaluation of Medical Devices. Part 5: Tests for Cytotoxicity: In Vitro Methods. Available online: https://www.iso.org/standard/36406.html (accessed on 26 October 2022).
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. Br. J. Pharmacol. 2020, 177, 3617–3624. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; de Oliveira, D.; Frigério, P.B.; de Souza Batista, F.R.; Grandfield, K.; Okamoto, R. Teriparatide improves microarchitectural characteristics of peri-implant bone in orchiectomized rats. Osteoporos. Int. 2020, 31, 1807–1815. [Google Scholar] [CrossRef]
- Develos Godoy, D.J.; Banlunara, W.; Jaroenporn, S.; Sangvanich, P.; Thunyakitpisal, P. Collagen and mPCL-TCP scaffolds induced differential bone regeneration in ovary-intact and ovariectomized rats. Biomed. Mater. Eng. 2018, 29, 389–399. [Google Scholar] [CrossRef]
- Luvizuto, E.R.; Queiroz, T.P.; Dias, S.M.D.; Okamoto, T.; Dornelles, R.C.M.; Garcia, I.R.; Okamoto, R. Histomorphometric analysis and immunolocalization of RANKL and OPG during the alveolar healing process in female ovariectomized rats treated with oestrogen or raloxifene. Arch. Oral Biol. 2010, 55, 52–59. [Google Scholar] [CrossRef]
- da Cruz Vegian, M.R.; Costa, B.C.A.; de Fátima Santana-Melo, G.; Godoi, F.H.C.; Kaminagakura, E.; Tango, R.N.; do Prado, R.F.; de Oliveira, L.D.; Federico, C.A.; de Oliveira Marco Avelino, S.; et al. Systemic and local effects of radiotherapy: An experimental study on implants placed in rats. Clin. Oral Investig. 2020, 24, 785–797. [Google Scholar] [CrossRef]
- dos Santos, P.L.; Queiroz, T.P.; Margonar, R.; Gomes de Souza Carvalho, A.C.; Okamoto, R.; de Souza Faloni, A.P.; Garcia Júnior, I.R. Guided implant surgery: What is the influence of this new technique on bone cell viability? J. Oral Maxillofac. Surg. 2013, 71, 505–512. [Google Scholar] [CrossRef]
- dos Santos, P.L.; de Molon, R.S.; Queiroz, T.P.; Okamoto, R.; Faloni, A.P.D.S.; Gulinelli, J.L.; Luvizuto, E.R.; Junior, I.R.G. Evaluation of bone substitutes for treatment of peri-implant bone defects: Biomechanical, histological, and immunohistochemical analyses in the rabbit tibia. J. Periodontal Implant Sci. 2016, 46, 176–196. [Google Scholar] [CrossRef]
- Esteves, J.; Marcantonio, E., Jr.; Faloni, A.; Rocha, F.R.G.; Marcantonio, R.A.; Wilk, K.; Intini, G. Dynamics of bone healing after osteotomy with piezosurgery or conventional drilling—Histomorphometrical, immunohistochemical, and molecular analysis. J. Transl. Med. 2013, 11, 221. [Google Scholar] [CrossRef]
- Queiroz, T.P.; Souza, F.A.; Okamoto, R.; Margonar, R.; Pereira-Filho, V.A.; Garcia, I.R., Jr.; Vieira, E.H. Evaluation of immediate bonecell viability and of drill wear after implant osteotomies: Immunohistochemistry and scanning electron microscopy analysis. J. Oral Maxillofac. Surg. 2008, 66, 1233–1240. [Google Scholar] [CrossRef]
- Raisz, L.G. Pathogenesis of osteoporosis: Concepts, conflicts, and prospects. J. Clin. Investig. 2005, 115, 3318–3325. [Google Scholar] [CrossRef] [Green Version]
- Di Munno, O.; Ferro, F. The effect of biologic agents on bone homeostasis in chronic inflammatory rheumatic diseases. Clin. Exp. Rheumatol. 2019, 7, 502–507. [Google Scholar]
- Zerbini, C.A.F.; Clark, P.; Mendez-Sanchez, L.; Pereira, R.M.R.; Messina, O.D.; Uña, C.R.; Adachi, J.D.; Lems, W.F.; Cooper, C.; Lane, N.E. IOF Chronic Inflammation and Bone Structure (CIBS) Working Group. Biologic therapies and bone loss in rheumatoid arthritis. Osteoporos. Int. 2017, 28, 429–446. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zeng, D.; Weng, W.; Huang, Q.; Zhang, X.; Wen, J.; Wu, J.; Jiang, X. Alendronate delivery on amino modified mesoporous bioactive glass scaffolds to enhance bone regeneration in osteoporosis rats. Artif. Cells Nanomed. Biotechnol. 2018, 46 (Suppl. 2), 171–181. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.; Xia, L.; Li, H.; Jiang, X.; Pan, H.; Xu, Y.; Lu, W.W.; Zhang, Z.; Chang, J. Enhanced osteoporotic bone regeneration by strontium-substituted calcium silicate bioactive ceramics. Biomaterials 2013, 34, 10028–10042. [Google Scholar] [CrossRef]
- Kyllönen, L.; D’Este, M.; Alini, M.; Eglin, D. Local drug delivery for enhancing fracture healing in osteoporotic bone. Acta Biomater. 2015, 11, 412–434. [Google Scholar] [CrossRef]
- Sun, J.; Fan, W.; Wu, D.; Sun, Y. Structure Control of SiO2 Sol-Gels via Addition of PEG. Stud. Surf. Sci. Catal. 1998, 118, 617–624. [Google Scholar] [CrossRef]
- Ajita, J.; Saravanan, S.; Selvamurugan, N. Effect of size of bioactive glass nanoparticles on mesenchymal stem cell proliferation for dental and orthopedic applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 53, 142–149. [Google Scholar] [CrossRef]
- Hoppe, A.; Güldal, N.S.; Boccaccini, A.R. A review of the biological response to ionic dissolution products from bioactive glasses and glass-ceramics. Biomaterials 2011, 32, 2757–2774. [Google Scholar] [CrossRef]
- Gorustovich, A.A.; Roether, J.A.; Boccaccini, A.R. Effect of bioactive glasses on angiogenesis: A review of in vitro and in vivo evidences. Tissue Eng. Part B Rev. 2010, 16, 199–207. [Google Scholar] [CrossRef]
- Xynos, I.D.; Hukkanen, M.V.; Batten, J.J.; Buttery, L.D.; Hench, L.L.; Polak, J.M. Bioglass 45S5 stimulates osteoblast turnover and enhances bone formation in vitro: Implications and applications for bone tissue engineering. Calcif. Tissue Int. 2000, 67, 321–329. [Google Scholar] [CrossRef]
- Andersson, O.H.; Kangasniemi, I. Calcium phosphate formation at the surface of bioactive glass in vivo. J. Non-Cryst. Solids 1990, 119, 290–296. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; Lisboa-Filho, P.N.; da Silva, A.C.; Bim-Júnior, O.; Batista, F.R.D.S.; Ervolino-Silva, A.C.; Garcia-Junior, I.R.; Okamoto, R. Sonochemical time standardization for bioactive materials used in periimplantar defects filling. Ultrason. Sonochem. 2019, 56, 437–446. [Google Scholar] [CrossRef]
- Lee, E.M.R.; Borges, R.; Marchi, J.; Eduardo, C.D.P.; Marques, M.M. Bioactive glass and high-intensity lasers as a promising treatment for dentin hypersensitivity: An in vitro study. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 108, 939–947. [Google Scholar] [CrossRef]
- Andersson, O.H.; Kangasniemi, I.O. Calcium phosphate formation at the surface of bioactive glass in vitro. J. Biomed. Mater. Res. 1991, 25, 1019–1030. [Google Scholar] [CrossRef]
- Bahari Javan, N.; Rezaie Shirmard, L.; Jafary Omid, N.; Akbari Javar, H.; Rafiee Tehrani, M.; Abedin Dorkoosh, F. Preparation, statistical optimisation and in vitro characterisation of poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/poly (lactic-co-glycolic acid) blend nanoparticles for prolonged delivery of teriparatide. J. Microencapsul. 2016, 33, 460–474. [Google Scholar] [CrossRef]
- Fiume, E.; Barberi, J.; Verné, E.; Baino, F. Bioactive glasses: From parent 45S5 composition to scaffold-assisted tissue-healing therapies. J. Funct. Biomater. 2018, 9, 24. [Google Scholar] [CrossRef]
- Gomes-Ferreira, P.H.S.; Micheletti, C.; Frigério, P.B.; de Souza Batista, F.R.; Monteiro, N.G.; Bim-Júnior, O.; Lisboa-Filho, P.N.; Grandfield, K.; Okamoto, R. PTH 1-34-functionalized bioactive glass improves peri-implant bone repair in orchiectomized rats: Microscale and ultrastructural evaluation. Biomater. Adv. 2022, 134, 112688. [Google Scholar] [CrossRef]
- Cai, K.; Frant, M.; Bossert, J.; Hildebrand, G.; Liefeith, K.; Jandt, K.D. Surface functionalized titanium thin films: Zeta-potential, protein adsorption and cell proliferation. Colloids Surf. B Biointerfaces 2006, 50, 1–8. [Google Scholar] [CrossRef]
- Lin, S.; Van den Bergh, W.; Baker, S.; Jones, J.R. Protein interactions with nanoporous sol-gel derived bioactive glasses. Acta Biomater. 2011, 7, 3606–3615. [Google Scholar] [CrossRef]
- Stoddart, M.J. Cell viability assays: Introduction. Methods Mol. Biol. 2011, 740, 1–6. [Google Scholar] [CrossRef]
- Westhauser, F.; Karadjian, M.; Essers, C.; Senger, A.-S.; Hagmann, S.; Schmidmaier, G.; Moghaddam, A. Osteogenic differentiation of mesenchymal stem cells is enhanced in a 45S5-supplemented β-TCP composite scaffold: An in-vitro comparison of Vitoss and Vitoss BA. PLoS ONE 2019, 14, e0212799. [Google Scholar] [CrossRef] [PubMed]
- Furusawa, T.; Mizunuma, K. Osteoconductive properties and efficacy of resorbable bioactive glass as a bone-grafting material. Implant Dent. 1997, 6, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Vanderschueren, D.; Vandenput, L.; Boonen, S.; Lindberg, M.K.; Bouillon, R.; Ohlsson, C. Androgens and bone. Endocr. Rev. 2004, 25, 389–425. [Google Scholar] [CrossRef] [PubMed]
- Reible, B.; Schmidmaier, G.; Prokscha, M.; Moghaddam, A.; Westhauser, F. Continuous stimulation with differentiation factors is necessary to enhance osteogenic differentiation of human mesenchymal stem cells in-vitro. Growth Factors 2017, 35, 179–188. [Google Scholar] [CrossRef]
- Tsigkou, O.; Jones, J.R.; Polak, J.M.; Stevens, M.M. Differentiation of fetal osteoblasts and formation of mineralized bone nodules by 45S5 Bioglass conditioned medium in the absence of osteogenic supplements. Biomaterials 2009, 30, 3542–3550. [Google Scholar] [CrossRef]
- Che, L.; Wang, Y.; Sha, D.; Li, G.; Wei, Z.; Liu, C.; Yuan, Y.; Song, D. A biomimetic and bioactive scaffold with intelligently pulsatile teriparatide delivery for local and systemic osteoporosis regeneration. Bioact. Mater. 2022, 19, 75–87. [Google Scholar] [CrossRef]
- Rodrigues, C.; Naasani, L.I.S.; Zanatelli, C.; Paim, T.C.; Azevedo, J.G.; de Lima, J.C.; Fernandes, M.D.C.; Buchner, S.; Wink, M.R. Bioglass 45S5: Structural characterization of short range order and analysis of biocompatibility with adipose-derived mesenchymal stromal cells in vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 103, 109781. [Google Scholar] [CrossRef]
- Reible, B.; Schmidmaier, G.; Moghaddam, A.; Westhauser, F. Insulin-like growth factor-1 as a possible alternative to bone morphogenetic protein-7 to induce osteogenic differentiation of human mesenchymal stem cells in vitro. Int. J. Mol. Sci. 2018, 19, 1674. [Google Scholar] [CrossRef]
- Blick, S.K.; Dhillon, S.; Keam, S.J. Teriparatide: A review of its use in osteoporosis. Drugs 2008, 68, 2709–2737. [Google Scholar] [CrossRef]
- Roggia, C.; Gao, Y.; Cenci, S.; Weitzmann, M.N.; Toraldo, G.; Isaia, G.; Pacifici, R. Up-regulation of TNF-producing T cells in the bone marrow: A key mechanism by which estrogen deficiency induces bone loss in vivo. Proc. Natl. Acad. Sci. USA 2001, 98, 13960–13965. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Pacifici, R. Estrogen deficiency and bone loss: An inflammatory tale. J. Clin. Investig. 2006, 116, 1186–1194. [Google Scholar] [CrossRef] [Green Version]
- Weitzmann, M.N.; Roggia, C.; Toraldo, G.; Weitzmann, L.; Pacifici, R. Increased production of IL-7 uncouples bone formation from bone resorption during estrogen deficiency. J. Clin. Investig. 2002, 110, 1643–1650. [Google Scholar] [CrossRef]
- Weitzmann, M.N.; Pacifici, R. Role of the immune system in postmenopausal bone loss. Curr. Osteoporos. Rep. 2005, 3, 92–97. [Google Scholar] [CrossRef]
- Hao, F.; Gu, Y.; Tan, X.; Deng, Y.; Wu, Z.-T.; Xu, M.-J.; Wang, W.-Z. Estrogen replacement reduces oxidative stress in the rostral ventrolateral medulla of ovariectomized rats. Oxid. Med. Cell. Longev. 2016, 2016, 2158971. [Google Scholar] [CrossRef]
- Ramalho-Ferreira, G.; Faverani, L.P.; Momesso, G.A.C.; Luvizuto, E.R.; de Oliveira Puttini, I.; Okamoto, R. Effect of antiresorptive drugs in the alveolar bone healing: A histometric and immunohistochemical study in ovariectomized rats. Clin. Oral Investig. 2017, 21, 1485–1494. [Google Scholar] [CrossRef]
- Huebsch, N.; Mooney, D.J. Inspiration and application in the evolution of biomaterials. Nature 2009, 462, 426–432. [Google Scholar] [CrossRef]
- Day, R.M.; Boccaccini, A.R.; Shurey, S.; Roether, J.A.; Forbes, A.; Hench, L.L.; Gabe, S.M. Assessment of polyglycolic acid mesh and bioactive glass for soft-tissue engineering scaffolds. Biomaterials 2004, 25, 5857–5866. [Google Scholar] [CrossRef]
- Auersvald, C.M.; Santos, F.R.; Nakano, M.M.; Leoni, G.B.; Neto, M.D.D.S.; Scariot, R.; Giovanini, A.F.; Deliberador, T.M. The local administration of parathyroid hormone encourages the healing of bone defects in the rat calvaria: Micro-computed tomography, histological and histomorphometric evaluation. Arch. Oral Biol. 2017, 79, 14–19. [Google Scholar] [CrossRef]
- Malik, D.K.; Baboota, S.; Ahuja, S.; Hasan, S.; Ali, J. Recent advances in protein and peptide drug delivery systems. Curr. Drug Deliv. 2007, 4, 141–151. [Google Scholar] [CrossRef]
- Frigério, P.B.; Gomes-Ferreira, P.H.S.; de Souza Batista, F.R.; Moura, J.; Garcia Júnior, I.R.; Botticelli, D.; Lisboa-Filho, P.N.; Okamoto , R. Effect of Topical PTH 1-34 Functionalized to Biogran® in the Process of Alveolar Repair in Rats Submitted to Orchiectomy. Materials 2021, 15, 207. [Google Scholar] [CrossRef]
- Moore, A.E.; Blake, G.M.; Taylor, K.A.; Rana, A.E.; Wong, M.; Chen, P.; Fogelman, I. Assessment of regional changes in skeletal metabolism following 3 and 18 months of teriparatide treatment. J. Bone Miner. Res. 2010, 25, 960–967. [Google Scholar] [CrossRef]
- Ohata, T.; Maruno, H.; Ichimura, S. Changes over time in callus formation caused by intermittently administering PTH in rabbit distraction osteogenesis models. J. Orthop. Surg. Res. 2015, 10, 88. [Google Scholar] [CrossRef] [PubMed]
- Aleksyniene, R.; Thomsen, J.S.; Eckardt, H.; Bundgaard, K.G.; Lind, M.; Hvid, I. Threedimensional microstructural properties of regenerated mineralizing tissue after PTH (1–34) treatment in a rabbit tibial lengthening model. J. Musculoskelet. Neuronal Interact 2009, 9, 268–277. [Google Scholar] [PubMed]
- Boskey, A.L.; Gadaleta, S.; Gundberg, C.; Doty, S.B.; Ducy, P.; Karsenty, G. Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone 1998, 23, 187–196. [Google Scholar] [CrossRef]
- Hauschka, P.V.; Frenkel, J.; DeMuth, R.; Gundberg, C.M. Presence of osteocalcin and related higher molecular weight 4-carboxyglutamic acid-containing proteins in developing bone. J. Biol. Chem. 1983, 258, 176–182. [Google Scholar] [CrossRef] [PubMed]
- Aldini., N.N.; Fini, M.; Giavaresi, G.; Giardino, R.; Greggi, T.; Parisini, P. Pedicular fixation in the osteoporotic spine: A pilot in vivo study on long-term ovariectomized sheep. J Orthop Res. 2002, 20, 1217–1224. [Google Scholar] [CrossRef]
- Weng, S.J.; Yan, D.Y.; Tang, J.H.; Shen, Z.J.; Wu, Z.Y.; Xie, Z.J.; Yang, J.-Y.; Bai, B.-L.; Chen, L.; Boodhun, V.; et al. Combined treatment with cinnamaldehyde and β-TCP had an additive effect on bone formation and angiogenesis in critical size calvarial defect in ovariectomized rats. Biomed. Pharmacother. 2019, 109, 573–581. [Google Scholar] [CrossRef]
- Tera, T.M.; Prado, R.F.; De Marco, A.C.; Santamaria, M.P.; Jardini, M.A. The RANK/ RANKL/ OPG interaction in the repair of autogenous bone grafts in female rats with estrogen deficiency. Braz. Oral Res. 2014, 28. [Google Scholar] [CrossRef]
- Yolcu, Y.; Alvi, M.A.; Wanderman, N.; Carlson, B.; Sebastian, A.; Bydon, M.; Freedman, B. Effect of teriparatide use on bone mineral density and spinal fusion: A narrative review of animal models. Int. J. Neurosci. 2019, 129, 814–820. [Google Scholar] [CrossRef]
- Seebach, C.; Skripitz, R.; Andreassen, T.T.; Aspenberg, P. Intermittent parathyroid hormone (1-34) enhances mechanical strength and density of new bone after distraction osteogenesis in rats. J. Orthop. Res. 2004, 22, 472–478. [Google Scholar] [CrossRef]
- Andreassen, T.T.; Fledelius, C.; Ejersted, C.; Oxlund, H. Increases in callus formation and mechanical strength of healing fractures in old rats treated with parathyroid hormone. Acta Orthop. Scand. 2001, 72, 304–307. [Google Scholar] [CrossRef] [Green Version]
- Ejersted, C.; Andreassen, T.; Oxlund, H.; Jørgensen, P.; Bak, B.; Häggblad, J.; Tørring, O.; Nilsson, M. Human parathyroid hormone (1–34) and (1–84) increase the mechanical strength and thickness of cortical bone in rats. J. Bone Miner. Res. 1993, 9, 1097–1101. [Google Scholar] [CrossRef]
- Skripitz, R.; Andreassen, T.T.; Aspenberg, P. Parathyroid hormone (1–34) increases the density of rat cancellous bone in a bone chamber. J. Bone Jt. Surgery. 2000, 82-B, 138–141. [Google Scholar] [CrossRef]
- Leiblein, M.; Henrich, D.; Fervers, F.; Kontradowitz, K.; Marzi, I.; Seebach, C. Do antiosteoporotic drugs improve bone regeneration in vivo? Eur. J. Trauma Emerg. Surg. 2020, 46, 287–299. [Google Scholar] [CrossRef]
- Kadiroğlu, E.T.; Akbalık, M.E.; Karaöz, E.; Kanay, B.E.; Dağ, A.; Ketani, M.A.; Eroğlu, E.G.; Uysal, E.; Tuncer, M.C. Calvarial bone defects in ovariectomised rats treated with mesenchymal stem cells and demineralised freeze-dried bone allografts. Folia Morphol. 2020, 79, 720–735. [Google Scholar] [CrossRef] [Green Version]













| EDS Groups | O (Wt%) | Na (Wt%) | Ca (Wt%) | Si (Wt%) | Au (Wt%) | P (Wt%) | C (Wt%) |
|---|---|---|---|---|---|---|---|
| 45S5 bioglass | 47.8 ± 0.2 | 11.7 ± 0.1 | 12.7 ± 0.1 | 11.3 ± 0.1 | 0 | 1.6 ± 0 | 0 |
| BG | 45.2 ± 0.2 | 10.5 ± 0.1 | 13.9 ± 0.1 | 9.8 ± 0.1 | 2.5 ± 0.2 | 1.4 ± 0 | 16.6 ± 0.2 |
| BGT | 49.8 ± 0.2 | 24.4 ± 0.2 | 9.7 ± 0.1 | 6.5 ± 0.1 | 6.2 ± 0.3 | 3.4 ± 0.1 | 0 |
| OC | RankL | |||
|---|---|---|---|---|
| 2 Weeks | 6 Weeks | 2 Weeks | 6 Weeks | |
| CS | (++) | (++) | (+) | (++) |
| CO | (++) | (++) | (++) | (+) |
| BGO | (+) | (+) | (+) | (+) |
| BGTO | (+) | (++) | (++) | (+) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Araújo, J.C.R.; Sobral Silva, L.A.; de Barros Lima, V.A.; Bastos Campos, T.M.; Lisboa Filho, P.N.; Okamoto, R.; de Vasconcellos, L.M.R. The Local Release of Teriparatide Incorporated in 45S5 Bioglass Promotes a Beneficial Effect on Osteogenic Cells and Bone Repair in Calvarial Defects in Ovariectomized Rats. J. Funct. Biomater. 2023, 14, 93. https://doi.org/10.3390/jfb14020093
de Araújo JCR, Sobral Silva LA, de Barros Lima VA, Bastos Campos TM, Lisboa Filho PN, Okamoto R, de Vasconcellos LMR. The Local Release of Teriparatide Incorporated in 45S5 Bioglass Promotes a Beneficial Effect on Osteogenic Cells and Bone Repair in Calvarial Defects in Ovariectomized Rats. Journal of Functional Biomaterials. 2023; 14(2):93. https://doi.org/10.3390/jfb14020093
Chicago/Turabian Stylede Araújo, Juliani Caroline Ribeiro, Leonardo Alvares Sobral Silva, Vinicius Almeida de Barros Lima, Tiago Moreira Bastos Campos, Paulo Noronha Lisboa Filho, Roberta Okamoto, and Luana Marotta Reis de Vasconcellos. 2023. "The Local Release of Teriparatide Incorporated in 45S5 Bioglass Promotes a Beneficial Effect on Osteogenic Cells and Bone Repair in Calvarial Defects in Ovariectomized Rats" Journal of Functional Biomaterials 14, no. 2: 93. https://doi.org/10.3390/jfb14020093