Vertebrate Palaeoecology of the Pisco Formation (Miocene, Peru): Glimpses into the Ancient Humboldt Current Ecosystem
Abstract
:1. Introduction
2. Geological Framework
2.1. Geodynamic Setting

2.2. Stratigraphy of the Pisco Formation in the Lower Ica Valley
3. Compilation of the Fossil Vertebrate Dataset
3.1. Historical Rationale
3.2. Field Methods
4. Evolution of the Pisco Vertebrate Assemblage

5. Palaeoecological Reconstruction of the Pisco Vertebrate Assemblage
5.1. A Dynamic Palaeoecological and Palaeoenvironmental Landscape
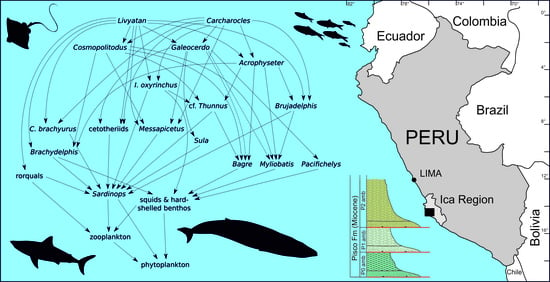
5.2. Was the Late Miocene EPB a Sardine-Based Microcosmos?
5.3. Carcharocles, Livyatan, and Other Overlords: What Ecological Role?
5.4. A Refugium of Mackerel Sharks
6. Conclusions and Perspectives
- (1)
- The P0 strata need to be investigated in greater detail to bridge the gap between the relatively well-known lower and upper Miocene assemblages of the EPB, and understand the impact of the Middle Miocene Climatic Optimum and Middle Miocene Climatic Transition. This will include prospecting additional sites such as Santa Rosa [59,96], the type locality of Incacetus broggi and Tiucetus rosae.
- (2)
- The rich shark assemblage from the ST-low1 interval of Cerro Colorado appears to have formed during a period of relative environmental stability, and as such provides an ideal opportunity to investigate feeding ecology and ontogenetic shifts in diet based on stable isotopes [143]. A similar approach could also be applied to toothed whales, e.g., with a view to corroborating the diet of extinct macroraptorial sperm whales and epipelagic beaked whales.
- (3)
- Despite its prominent ecological role, the deep-time history of the Pacific sardine remains largely obscure [157,158]. Abundant Sardinops-like fossils from the Neogene of Ecuador may help to rectify this, and elucidate the origin of the boosted fish productivity associated with the rise of the Humboldt Current Ecosystem.
- (4)
- Correlations between the EPB and the Sacaco area need to be improved to provide a clearer understanding of their respective ecological settings. At present, the radiometric dates from these two areas do not entirely match [96], with those from the lowermost strata of the Sacaco area being several decades old.
- (5)
- Comparisons of the Pisco Formation with coeval deposits elsewhere in the eastern Pacific and the Caribbean are currently sparse and ideally should focus on: (i) the Bahía Inglesa Formation of northern Chile (which shows obvious faunal similarities with both the EPB and the Sacaco area [159]), and (ii) the Miramar Formation and related deposits of northern Peru and Ecuador (which would elucidate the northern extent and impact of the ancient Humboldt Current).
- (6)
- Much systematic work remains to be done on the mysticetes, pinnipeds, and marine reptiles of the EPB, which ultimately will contribute to a more detailed understanding of the Pisco assemblages and their complex ecology.
- (7)
- Finally, a comprehensive assessment of the Palaeogene vertebrate assemblages of the EPB is still wanting. A broader knowledge of the fossil fishes and tetrapods of the Paracas and Otuma formations will elucidate the very roots of the present-day Humboldt Current Ecosystem.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Karstensen, J.; Ulloa, O. The Peru–Chile Current System. In Encyclopedia of Ocean Sciences, 2nd ed.; Steele, J.H., Turekian, K.K., Thorpe, S.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 385–392. [Google Scholar]
- Pfuhl, H.A.; McCave, I.N. Evidence for late Oligocene establishment of the Antarctic Circumpolar Current. Earth Planet. Sci. Lett. 2005, 235, 715–728. [Google Scholar] [CrossRef]
- Barker, P.F.; Filippelli, G.M.; Florindo, F.; Martin, E.E.; Scher, H.D. Onset and role of the Antarctic Circumpolar Current. Deep. Sea Res. Part II Top. Stud. Oceanogr. 2007, 54, 2388–2398. [Google Scholar] [CrossRef] [Green Version]
- Lagabrielle, Y.; Goddéris, Y.; Donnadieu, Y.; Malavieille, J.; Suarez, M. The tectonic history of Drake Passage and its possible impacts on global climate. Earth Planet. Sci. Lett. 2009, 279, 197–211. [Google Scholar] [CrossRef]
- Hill, D.J.; Haywood, A.M.; Valdes, P.J.; Francis, J.E.; Lunt, D.J.; Wade, B.S.; Bowman, V.C. Paleogeographic controls on the onset of the Antarctic circumpolar current. Geophys. Res. Lett. 2013, 40, 5199–5204. [Google Scholar] [CrossRef] [Green Version]
- Armijo, R.; Lacassin, R.; Coudurier-Curveur, A.; Carrizo, D. Coupled tectonic evolution of Andean orogeny and global climate. Earth-Sci. Rev. 2015, 143, 1–35. [Google Scholar] [CrossRef] [Green Version]
- Ryther, J.H. Photosynthesis and fish production in the sea. Science 1969, 166, 72–76. [Google Scholar] [CrossRef] [Green Version]
- Penven, P.; Echevin, V.; Pasapera, J.; Colas, F.; Tam, J. Average circulation, seasonal cycle, and mesoscale dynamics of the Peru Current System: A modeling approach. J. Geophys. Res. 2005, 110, C10021. [Google Scholar] [CrossRef]
- Chavez, F.P.; Messié, M. A comparison of eastern boundary upwelling ecosystems. Prog. Oceanogr. 2009, 83, 80–96. [Google Scholar] [CrossRef]
- Di Celma, C.; Malinverno, E.; Bosio, G.; Collareta, A.; Gariboldi, K.; Gioncada, A.; Molli, G.; Basso, D.; Varas-Malca, R.; Pierantoni, P.P.; et al. Sequence stratigraphy and paleontology of the upper Miocene Pisco Formation along the western side of the lower Ica Valley (Ica Desert, Peru). Riv. Ital. Di Paleontol. E Stratigr. 2017, 123, 255–273. [Google Scholar]
- Suess, E.; von Huene, R. Introduction, objectives, and principal results, Leg 112, Peru continental margin. Proc. Ocean Drill. Program Init. Rep. 1988, 112, 5–44. [Google Scholar]
- Dunbar, R.B.; Marty, R.C.; Baker, P.A. Cenozoic marine sedimentation in the Sechura and Pisco basins, Peru. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1990, 32, 235–261. [Google Scholar] [CrossRef]
- Bosio, G.; Malinverno, E.; Collareta, A.; Di Celma, C.; Gioncada, A.; Parente, M.; Berra, F.; Marx, F.G.; Vertino, A.; Urbina, M.; et al. Strontium Isotope Stratigraphy and the thermophilic fossil fauna from the middle Miocene of the East Pisco Basin (Peru). J. S. Am. Earth Sci. 2020, 97, 102399. [Google Scholar] [CrossRef]
- Bosio, G.; Malinverno, E.; Villa, I.M.; Di Celma, C.; Gariboldi, K.; Gioncada, A.; Barberini, V.; Urbina, M.; Bianucci, G. Tephrochronology and chronostratigraphy of the Miocene Chilcatay and Pisco formations (East Pisco Basin, Peru). Newsl. Stratigr. 2020, 53, 213–247. [Google Scholar] [CrossRef]
- de Muizon, C.; DeVries, T.J. Geology and paleontology of late Cenozoic marine deposits in the Sacaco area (Peru). Geol. Rundsch. 1985, 74, 547–563. [Google Scholar] [CrossRef]
- de Muizon, C. Les Vertébrés de la Formation Pisco (Pérou). Troisième partie: Les Odontocètes (Cetacea, Mammalia) du Miocène. Trav. De L’inst. Fr. D’études Andin. 1988, 42, 1–244. [Google Scholar]
- Brand, L.R.; Esperante, R.; Chadwick, A.V.; Poma, O.; Alomia, M. Fossil whale preservation implies high diatom accumulation rate in the Miocene-Pliocene Pisco Formation of Peru. Geology 2004, 32, 165–168. [Google Scholar] [CrossRef] [Green Version]
- Esperante, R.; Brand, L.R.; Chadwick, A.V.; Poma, O. Taphonomy and paleoenvironmental conditions of deposition of fossil whales in the diatomaceous sediments of the Miocene/Pliocene Pisco Formation, southern Peru—A new Fossil-Lagerstätte. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 417, 337–370. [Google Scholar] [CrossRef]
- Esperante, R.; Brand, L.R.; Nick, K.E.; Poma, O.; Urbina, M. Exceptional occurrence of fossil baleen in shallow marine sediments of the Neogene Pisco Formation, Southern Peru. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 257, 344–360. [Google Scholar] [CrossRef]
- Lambert, O.; Bianucci, G.; Post, K.; de Muizon, C.; Salas-Gismondi, R.; Urbina, M.; Reumer, J. The giant bite of a new raptorial sperm whale from the Miocene epoch of Peru. Nature 2010, 466, 105–108. [Google Scholar] [CrossRef]
- Lambert, O.; Collareta, A.; Landini, W.; Post, K.; Ramassamy, B.; Di Celma, C.; Urbina, M.; Bianucci, G. No deep diving: Evidence of predation on epipelagic fish for a stem beaked whale from the Late Miocene of Peru. Proc. R. Soc. B Biol. Sci. 2015, 282, 20151530. [Google Scholar] [CrossRef] [Green Version]
- Collareta, A.; Landini, W.; Lambert, O.; Post, K.; Tinelli, C.; Di Celma, C.; Panetta, D.; Tripodi, M.; Salvadori, P.; Caramella, D.; et al. Piscivory in a Miocene Cetotheriidae of Peru: First record of fossilized stomach content for an extinct baleen-bearing whale. Sci. Nat. 2015, 102, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Collareta, A.; Landini, W.; Chacaltana-Budiel, C.; Valdivia-Vera, W.; Altamirano-Sierra, A.; Urbina, M.; Bianucci, G. A well preserved skeleton of the fossil shark Cosmopolitodus hastalis from the late Miocene of Peru, featuring fish remains as fossilized stomach contents. Riv. Ital. Di Paleontol. E Stratigr. 2017, 123, 11–22. [Google Scholar]
- Collareta, A.; Di Celma, C.; Bosio, G.; Pierantoni, P.P.; Malinverno, E.; Lambert, O.; Marx, F.G.; Landini, W.; Urbina, M.; Bianucci, G. Distribution and paleoenvironmental framework of middle Miocene marine vertebrates along the western side of the lower Ica Valley (East Pisco Basin, Peru). J. Maps 2021, 17, 7–17. [Google Scholar] [CrossRef]
- Bianucci, G.; Di Celma, C.; Collareta, A.; Landini, W.; Post, K.; Tinelli, C.; de Muizon, C.; Bosio, G.; Gariboldi, K.; Gioncada, A.; et al. Fossil marine vertebrates of Cerro Los Quesos: Distribution of cetaceans, seals, crocodiles, seabirds, sharks, and bony fish in a late Miocene locality of the Pisco Basin, Peru. J. Maps 2016, 12, 1037–1046. [Google Scholar] [CrossRef] [Green Version]
- Bianucci, G.; Di Celma, C.; Landini, W.; Post, K.; Tinelli, C.; de Muizon, C.; Gariboldi, K.; Malinverno, E.; Cantalamessa, G.; Gioncada, A.; et al. Distribution of fossil marine vertebrates in Cerro Colorado, the type locality of the giant raptorial sperm whale Livyatan melvillei (Miocene, Pisco Formation, Peru). J. Maps 2016, 12, 543–557. [Google Scholar] [CrossRef] [Green Version]
- Gioncada, A.; Collareta, A.; Gariboldi, K.; Lambert, O.; Di Celma, C.; Bonaccorsi, E.; Urbina, M.; Bianucci, G. Inside baleen: Exceptional microstructure preservation in a late Miocene whale skeleton from Peru. Geology 2016, 44, 839–842. [Google Scholar] [CrossRef]
- Gioncada, A.; Gariboldi, K.; Collareta, A.; Di Celma, C.; Bosio, G.; Malinverno, E.; Lambert, O.; Pike, J.; Urbina, M.; Bianucci, G. Looking for the key to preservation of fossil marine vertebrates in the Pisco Formation of Peru: New insights from a small dolphin skeleton. Andean Geol. 2018, 45, 379–398. [Google Scholar] [CrossRef]
- Gioncada, A.; Petrini, R.; Bosio, G.; Gariboldi, K.; Collareta, A.; Malinverno, E.; Bonaccorsi, E.; Di Celma, C.; Pasero, M.; Urbina, M.; et al. Insights into the diagenetic environment of fossil marine vertebrates of the Pisco Formation (late Miocene, Peru) from mineralogical and Sr-isotope data. J. S. Am. Earth Sci. 2018, 81, 141–152. [Google Scholar] [CrossRef]
- Bosio, G.; Collareta, A.; Di Celma, C.; Lambert, O.; Marx, F.G.; de Muizon, C.; Gioncada, A.; Gariboldi, K.; Malinverno, E.; Varas-Malca, R.; et al. Taphonomy of marine vertebrates of the Pisco Formation (Miocene, Peru): Insights into the origin of an outstanding Fossil-Lagerstätte. PLoS ONE 2021, 16, e0254395. [Google Scholar]
- Boskovic, D.S.; Vidal, U.L.; Nick, K.E.; Esperante, R.; Brand, L.R.; Wright, K.R.; Sandberg, L.B.; Siviero, B.C. Structural and protein preservation in fossil whale bones from the Pisco Formation (middle-Upper miocene), Peru. Palaios 2021, 36, 155–164. [Google Scholar] [CrossRef]
- Marty, R.C. Statigraphy and Chemical Sedimentology of Cenozoic Biogenic Sediments from the Pisco and Sechura Basins. Ph.D. Thesis, Rice University, Houston, TX, USA, 1989; p. 268. [Google Scholar]
- Di Celma, C.; Malinverno, E.; Bosio, G.; Gariboldi, K.; Collareta, A.; Gioncada, A.; Landini, W.; Pierantoni, P.P.; Bianucci, G. Intraformational unconformities as a record of late Miocene eustatic falls of sea level in the Pisco Formation (southern Peru). J. Maps 2018, 14, 607–619. [Google Scholar] [CrossRef] [Green Version]
- Brand, L.R.; Urbina, M.; Chawick, A.; DeVries, T.J.; Esperante, R. A high resolution stratigraphic framework for the remarkable fossil cetacean assemblage of the Miocene/Pliocene Pisco Formation, Peru. J. S. Am. Earth Sci. 2011, 31, 414–425. [Google Scholar] [CrossRef]
- Thornburg, T.M.; Kulm, L.D. Sedimentary basins of the Peru continental margin: Structure, stratigraphy, and Cenozoic tectonics from 6 °S to 16 °S latitude. Nazca Plate Crustal Form. Andean Converg. 1981, 154, 393–422. [Google Scholar]
- Pilger, R.H. Plate reconstructions, aseismic ridges, and low-angle subduction beneath the Andes. Geol. Soc. Am. Bull. 1981, 92, 448–456. [Google Scholar] [CrossRef]
- Hsu, J.T. Quaternary uplift of the Peruvian coast related to the subduction of the Nazca Ridge: 13.5 to 15.6 degrees South latitude. Quat. Int. 1992, 15/16, 87–97. [Google Scholar] [CrossRef]
- Macharé, J.; Ortlieb, L. Plio-Quaternary vertical motions and the subduction of the Nazca Ridge, central coast of Peru. Tectonophysics 1992, 205, 97–108. [Google Scholar] [CrossRef]
- Hampel, A. The migration history of the Nazca Ridge along the Peruvian active margin: A re-evaluation and some geological implications. Earth Planet. Sci. Lett. 2002, 203, 665–679. [Google Scholar] [CrossRef]
- Saillard, M.; Hall, S.R.; Audin, L.; Faber, D.L.; Regard, V.; Herail, G. Andean coastal uplift and active tectonics in southern Peru: Be-10 surface exposure dating of differentially uplifted marine terrace sequences (San Juan de Marcona, similar to 15.4 degrees S). Geomorphology 2011, 128, 178–190. [Google Scholar] [CrossRef]
- DeVries, T.J. Oligocene deposition and Cenozoic sequence boundaries in the Pisco Basin (Peru). J. S. Am. Earth Sci. 1998, 11, 217–231. [Google Scholar] [CrossRef]
- DeVries, T.J.; Barron, J.A.; Urbina, M.; Ochoa, D.; Esperante, R.; Snee, L.W. The Miocene stratigraphy of the Laberinto area (Río Ica Valley) and its bearing on the geological history of the East Pisco Basin (south-central Peru). J. S. Am. Earth Sci. 2021, 111, 103458. [Google Scholar] [CrossRef]
- Malinverno, E.; Bosio, G.; Di Celma, C.; Gariboldi, K.; Gioncada, A.; Pierantoni, P.P.; Collareta, A.; Molli, G.; Bagnoli, G.; Sarti, G.; et al. (Bio)stratigraphic overview and paleoclimatic-paleoceanographic implications of the middle-upper Eocene deposits from the Ica River Valley (East Pisco Basin, Peru). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2021, 578, 110567. [Google Scholar] [CrossRef]
- Ochoa, D.; Salas-Gismondi, R.; DeVries, T.J.; Baby, P.; de Muizon, C.; Altamirano, A.; Barbosa-Espitia, A.; Foster, D.A.; Quispe, K.; Cardich, J.; et al. Late Neogene evolution of the Peruvian margin and its ecosystems: A synthesis from the Sacaco record. Int. J. Earth Sci. 2021, 110, 995–1025. [Google Scholar] [CrossRef]
- North American Commission on Stratigraphic Nomenclature. North American stratigraphic code. Am. Assoc. Pet. Geol. Bull. 2005, 89, 1547–1591. [Google Scholar]
- Ehret, D.J.; MacDaffen, B.J.; Jones, D.S.; DeVries, T.J.; Foster, D.A.; Salas-Gismondi, R. Origin of the white shark Carcharodon (Lamniformes: Lamnidae) based on recalibration of the upper Neogene Pisco Formation of Peru. Palaeontology 2012, 55, 1139–1153. [Google Scholar] [CrossRef]
- Gariboldi, K.; Bosio, G.; Malinverno, E.; Gioncada, A.; Di Celma, C.; Villa, I.M.; Urbina, M.; Bianucci, G. Biostratigraphy, geochronology and sedimentation rates of the upper Miocene Pisco Formation at two important marine vertebrate fossil-bearing sites of southern Peru. Newsl. Stratigr. 2017, 50, 417–444. [Google Scholar] [CrossRef]
- de Muizon, C. Les Vertébrés de la Formation Pisco (Pérou). Deuxième partie: Les Odontocètes (Cetacea, Mammalia) du Pliocène inférieur de Sud-Sacaco. Trav. De L’inst. Fr. D’études Andin. 1984, 27, 1–188. [Google Scholar]
- Marocco, R.; de Muizon, C. Le Bassin Pisco, bassin cénozoïque d’avant arc de la côte du Pérou central: Analyse géodynamique de son remplissage. Géodynamique 1988, 3, 3–19. [Google Scholar]
- Ehret, D.J.; Hubbel, G.; MacFadden, B.J. Exceptional preservation of the white shark Carcharodon (Lamniformes, Lamnidae) from the early Pliocene of Peru. J. Vertebr. Paleontol. 2009, 29, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Travis, R.B.; Gonzales, G.; Pardo, A. Hydrocarbon Potential of Coastal Basins of Peru. AAPG Bull. 1974, 58, 1460–1461. [Google Scholar]
- DeVries, T.J.; Schrader, H. Middle Miocene marine sediments in the Pisco basin (Peru). Boletín De La Soc. Geol. Del Perú 1997, 87, 1–13. [Google Scholar]
- Di Celma, C.; Malinverno, E.; Cantalamessa, G.; Gioncada, A.; Bosio, G.; Villa, I.M.; Gariboldi, K.; Rustichelli, A.; Pierantoni, P.P.; Landini, W.; et al. Stratigraphic framework of the late Miocene Pisco Formation at Cerro Los Quesos (Ica Desert, Peru). J. Maps 2016, 12, 1020–1028. [Google Scholar] [CrossRef] [Green Version]
- Di Celma, C.; Malinverno, E.; Gariboldi, K.; Gioncada, A.; Rustichelli, A.; Pierantoni, P.P.; Landini, W.; Bosio, G.; Tinelli, C.; Bianucci, G. Stratigraphic framework of the late Miocene to Pliocene Pisco Formation at Cerro Colorado. J. Maps 2016, 12, 515–529. [Google Scholar] [CrossRef]
- Bosio, G.; Gioncada, A.; Malinverno, E.; Di Celma, C.; Villa, I.M.; Cataldi, G.; Gariboldi, K.; Collareta, A.; Urbina, M.; Bianucci, G. Chemical and petrographic fingerprinting of volcanic ashes as a tool for high-resolution stratigraphy of the upper Miocene Pisco Formation (Peru). J. Geol. Soc. 2019, 176, 13–28. [Google Scholar] [CrossRef]
- Bianucci, G. Esplorazioni e nuove scoperte nel deserto del Perù: I cetacei fossili di Cerro Colorado e Cerro los Quesos. Quad. Del Mus. Di Stor. Nat. Di Livorno 2010, 23, 3–12. [Google Scholar]
- Lisson, C. Los fosfatos de Ocucaje. Boletín De Minas E Ind. De La Constr. 1898, 14, 33–34. [Google Scholar]
- Adams, G.I. An outline review of the geology of Peru. Annu. Rep. Smithson. Inst. 1908, 1908, 385–429. [Google Scholar]
- Colbert, E.H. A new fossil whale from the Miocene of Peru. Bull. Am. Musem Nat. Hist. 1908, 83, 195–216. [Google Scholar]
- Hoffstetter, R. Un gisement de vertèbrés tertiaires à Sacaco (Sud-Pèrou) témoin Néogène d’une migration de faunes australes au long de la còte occidentale sudaméricaine. Comptes Rendus Hebd. Des Séances De L’académie Des Sci. De Paris Série D 1968, 267, 1273–1276. [Google Scholar]
- de Muizon, C. Arctocephalus (Hydrarctos) lomasiensis, subgen. nov. et nov sp., un nouvel Otariidae du Mio-Pliocène de Sacaco. Bull. De L’inst. Fr. D’études Andin. 1978, 7, 169–189. [Google Scholar]
- de Muizon, C. Les Vertébrés de la Formation Pisco (Pérou). Première partie: Deux nouveaux Monachinae (Phocidae, Mammalia) du Pliocène de Sud-Sacaco. Trav. De L’inst. Fr. D’études Andin. 1981, 22, 1–160. [Google Scholar]
- de Muizon, C. Pliopontos littoralis un nouveau Platanistidae (Cetacea) du Pliocène de la côte péruvienne. Comptes Rendus De L’académie Des Sci. De Paris Ser. II 1983, 296, 625–628. [Google Scholar]
- de Muizon, C. Un nouveau Phocoenidae (Cetacea) du Pliocène inférieur du Pérou. Comptes Rendus De L’académie Des Sci. De Paris Ser. II 1983, 296, 1203–1206. [Google Scholar]
- de Muizon, C. Un Ziphiidae (Cetacea) nouveau du Pliocène inférieur du Pérou. Comptes Rendus De L’académie Des Sci. De Paris Ser. II 1983, 297, 85–88. [Google Scholar]
- de Muizon, C. Un nouveau Phocoenidae (Odontoceti, Mammalia) du Miocène supérieur de la Formation Pisco (Pérou). Comptes Rendus De L’académie Des Sci. De Paris Ser. II 1986, 303, 1509–1512. [Google Scholar]
- de Muizon, C. Walrus-like feeding adaptation in a new cetacean from the Pliocene of Peru. Nature 1993, 365, 745–748. [Google Scholar] [CrossRef]
- Pilleri, G. Beiträge Zur Paläontologie Der Cetaceen Perus; Hirnanatomisches Institut: Ostermundingen, Switzerland, 1989; 233p. [Google Scholar]
- Kindlimann, R. Selacios del Terciario Tardío de Sacaco, Departamento de Arequipa. Boletín De Lima 1990, 69, 91–95. [Google Scholar]
- de Muizon, C.; McDonald, H.G. An aquatic sloth from the Pliocene of Peru. Nature 1995, 375, 224–227. [Google Scholar] [CrossRef]
- Kraus, R. The cranium of Piscogavialis jugaliperforatus n.gen., n.sp. (Gavialidae, Crocodylia) from the Miocene of Peru. Paläontologische Z. 1998, 72, 389–405. [Google Scholar] [CrossRef]
- de Muizon, C.; Domning, D.P.; Parrish, M. Dimorphic tusks and adaptive strategies in a new species of walrus-like dolphin (Odobenocetopsidae) from the Pliocene of Peru. Comptes Rendus De L’académie Des Sci. De Paris Ser. II 1999, 329, 449–455. [Google Scholar] [CrossRef]
- Stucchi, M.; Urbina, M.; Giraldo, A. Una nueva especie de Spheniscidae del Mioceno tardío de la Formación Pisco. Boletín Del Inst. Fr. De Estud. Andin. 2003, 32, 361–375. [Google Scholar] [CrossRef]
- Stucchi, M.; Emslie, S.D.; Varas-Malca, R.M.; Urbina, M. A new late Miocene condor (Aves, Cathartidae) from Peru and the origin of South American Condors. J. Vertebr. Paleontol. 2015, 35, e972507. [Google Scholar] [CrossRef]
- Stucchi, M.; Varas-Malca, R.M.; Urbina-Schmitt, M. New Miocene sulid birds from Peru and considerations on their Neogene fossil record in the Eastern Pacific Ocean. Acta Palaeontol. Pol. 2016, 61, 417–427. [Google Scholar] [CrossRef] [Green Version]
- Bouetel, V.; de Muizon, C. The anatomy and relationships of Piscobalaena nana (Cetacea, Mysticeti), a Cetotheriidae s.s. from the early Pliocene of Peru. Geodiversitas 2006, 28, 319–395. [Google Scholar]
- Chávez-Hoffmeister, M.F.; Stucchi, M.; Urbina, M. El registro de Pelagornithidae (Aves: Pelecaniformes) y la avifauna neógena del Pacífico sudeste. Bull. De L’inst. Fr. D’études Andin. 2007, 33, 175–197. [Google Scholar] [CrossRef] [Green Version]
- Göhlich, U.B. The oldest fossil record of the extant penguin genus Spheniscus–A new species from the Miocene of Peru. Acta Palaeontol. Pol. 2007, 52, 285–298. [Google Scholar]
- Lambert, O.; Bianucci, G.; Post, K. A new beaked whale (Odontoceti, Ziphiidae) from the Middle Miocene of Peru. J. Vertebr. Paleontol. 2009, 29, 910–922. [Google Scholar] [CrossRef]
- Lambert, O.; Bianucci, G.; de Muizon, C. Macroraptorial sperm whales (Cetacea, Odontoceti, Physeteroidea) from the Miocene of Peru. Zool. J. Linn. Soc. 2017, 179, 404–474. [Google Scholar] [CrossRef]
- Lambert, O.; Bianucci, G.; Urbina, M.; Geisler, J.H. A new inioid (Cetacea, Odontoceti, Delphinida) from the Miocene of Peru and the origin of modern dolphin and porpoise families. Zool. J. Linn. Soc. 2017, 179, 919–946. [Google Scholar]
- Lambert, O.; Collareta, A.; Benites-Palomino, A.M.; Di Celma, C.; de Muizon, C.; Urbina, M.; Bianucci, G. A new small, mesorostrine inioid (Cetacea, Odontoceti, Delphinida) from four upper Miocene localities in the Pisco Basin, Peru. Pap. Palaeontol. 2021, 7, 1043–1064. [Google Scholar] [CrossRef]
- Bianucci, G.; Lambert, O.; Post, K. High concentration of long-snouted beaked whales (genus Messapicetus) from the Miocene of Peru. Palaeontology 2010, 53, 1077–1098. [Google Scholar] [CrossRef]
- Bianucci, G.; Di Celma, C.; Urbina, M.; Lambert, G. New beaked whales from the late Miocene of Peru and evidence for convergent evolution in stem and crown Ziphiidae (Cetacea, Odontoceti). PeerJ 2016, 4, e2479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parham, J.F.; Pyenson, N.D. New sea turtle from the Miocene of Peru and the iterative evolution of feeding ecomorphologies since the Cretaceous. J. Paleontol. 2010, 84, 231–247. [Google Scholar] [CrossRef]
- Lambert, O.; de Muizon, C. A new long-snouted species of the Miocene pontoporiid dolphin Brachydelphis and a review of the Mio-Pliocene marine mammal levels in the Sacaco Basin, Peru. J. Vertebr. Paleontol. 2013, 33, 709–721. [Google Scholar] [CrossRef]
- Marx, F.G.; Kohno, N. A new Miocene baleen whale from the Peruvian desert. R. Soc. Open Sci. 2016, 2, 160542. [Google Scholar] [CrossRef] [Green Version]
- Esperante, R.; Poma, O. Taphonomy and palaeopathology of two mysticete whales, upper Miocene Pisco Formation, Peru. Span. J. Palaeontol. 2015, 30, 1–14. [Google Scholar]
- Gariboldi, K.; Gioncada, A.; Bosio, G.; Malinverno, G.; Di Celma, C.; Tinelli, C.; Cantalamessa, G.; Landini, W.; Urbina, M.; Bianucci, G. The dolomite nodules enclosing fossil marine vertebrates in the East Pisco Basin, Peru: Field and petrographic insights into the Lagerstätte formation. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2015, 438, 81–95. [Google Scholar] [CrossRef]
- Landini, W.; Altamirano-Sierra, A.; Collareta, A.; Di Celma, C.; Urbina, M.; Bianucci, G. The late Miocene elasmobranch assemblage from Cerro Colorado (Pisco Formation, Peru). J. S. Am. Earth Sci. 2017, 73, 168–190. [Google Scholar] [CrossRef]
- Landini, W.; Collareta, A.; Pesci, F.; Di Celma, C.; Urbina, M.; Bianucci, G. A secondary nursery area for the copper shark Carcharhinus brachyurus from the late Miocene of Peru. J. S. Am. Earth Sci. 2017, 78, 164–174. [Google Scholar] [CrossRef]
- Bosio, G.; Bracchi, V.A.; Malinverno, E.; Collareta, A.; Coletti, G.; Gioncada, A.; Kočí, T.; Di Celma, C.; Bianucci, G.; Basso, D. Taphonomy of a Panopea Ménard de la Groye, 1807 shell bed from the Pisco Formation (Miocene, Peru). Comptes Rendus Palevol 2021, 20, 119–140. [Google Scholar]
- Bosio, G.; Gioncada, A.; Gariboldi, K.; Bonaccorsi, E.; Collareta, A.; Pasero, M.; Di Celma, C.; Malinverno, E.; Urbina, M.; Bianucci, G. Mineralogical and geochemical characterization of fossil bones from a Miocene marine Konservat-Lagerstätte. J. S. Am. Earth Sci. 2021, 105, 102924. [Google Scholar] [CrossRef]
- Di Celma, C.; Pierantoni, P.P.; Malinverno, E.; Collareta, A.; Lambert, O.; Landini, W.; Bosio, G.; Gariboldi, K.; Gioncada, A.; de Muizon, C.; et al. Allostratigraphy and paleontology of the lower Miocene Chilcatay Formation in the Zamaca area, East Pisco basin, southern Peru. J. Maps 2019, 15, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Marx, F.G.; Fitzgerald, E.M.; Fordyce, R.E. Like phoenix from the ashes: How modern baleen whales arose from a fossil “dark age”. Acta Palaeontol. Pol. 2019, 64, 231–238. [Google Scholar] [CrossRef]
- Marx, F.G.; Lambert, O.; de Muizon, C. A new Miocene baleen whale from Peru deciphers the dawn of cetotheriids. R. Sociey Open Sci. 2017, 4, 170560. [Google Scholar] [CrossRef] [Green Version]
- Bianucci, G.; Marx, F.G.; Collareta, A.; Di Stefano, A.; Landini, W.; Morigi, C.; Varola, A. Rise of the titans: Baleen whales became giants earlier than thought. Biol. Lett. 2019, 15, 20190175. [Google Scholar] [CrossRef] [Green Version]
- Lambert, O.; Bianucci, G.; Post, K. Tusk bearing beaked whales from the Miocene of Peru: Sexual dimorphism in fossil ziphiids? J. Mammal. 2010, 91, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Ramassamy, B.; Lambert, O.; Collareta, A.; Urbina, M.; Bianucci, G. Description of the skeleton of the fossil beaked whale Messapicetus gregarius: Searching potential proxies for deep-diving abilities. Foss. Rec. 2018, 21, 11–32. [Google Scholar] [CrossRef] [Green Version]
- Kočí, T.; Bosio, G.; Collareta, A.; Sanfilippo, R.; Ekrt, B.; Urbina, M.; Malinverno, E. First report on the cirratulid (Annelida, Polychaeta) reefs from the Miocene Chilcatay and Pisco Formations (East Pisco Basin, Peru). J. S. Am. Earth Sci. 2020, 107, 103042. [Google Scholar] [CrossRef]
- Lambert, O.; Bianucci, G.; Beatty, B.L. Bony outgrowths on the jaws of an extinct sperm whale support macroraptorial feeding in several stem physeteroids. Naturwissenschaften 2014, 101, 517–521. [Google Scholar] [CrossRef]
- Collareta, A.; Lambert, O.; de Muizon, C.; Benites-Palomino, A.M.; Urbina, M.; Bianucci, G. A new physeteroid from the late Miocene of Peru expands the diversity of extinct dwarf and pygmy sperm whales (Cetacea: Odontoceti: Kogiidae). Comptes Rendus Palevol 2020, 95, 79–100. [Google Scholar] [CrossRef]
- Marx, F.G.; Collareta, A.; Gioncada, A.; Post, K.; Lambert, O.; Bonaccorsi, E.; Urbina, M.; Bianucci, G. How whales used to filter: Exceptionally preserved baleen in a Miocene cetotheriid. J. Anat. 2017, 231, 212–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compagno, L.J.V. FAO Species Catalogue. Vol. 4. Sharks of the World. Part 1. Hexanchiformes to Lamniformes. FAO Fish. Synop. 1984, 125, 1–249. [Google Scholar]
- Bianucci, G.; Collareta, A.; Bosio, G.; Landini, W.; Gariboldi, K.; Gioncada, A.; Lambert, O.; Malinverno, E.; de Muizon, C.; Varas-Malca, R.; et al. Taphonomy and palaeoecology of the lower Miocene marine vertebrate assemblage of Ullujaya (Chilcatay Formation, East Pisco Basin, southern Peru). Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 511, 256–279. [Google Scholar] [CrossRef]
- Landini, W.; Collareta, A.; Di Celma, C.; Malinverno, E.; Urbina, M.; Bianucci, G. The early Miocene elasmobranch assemblage from Zamaca (Chilcatay Formation, Peru). J. S. Am. Earth Sci. 2019, 91, 352–371. [Google Scholar] [CrossRef]
- Apolín, J.; González, G.; Martínez, J.M. Seláceos del Mioceno Superior de Quebrada Pajaritos (Piura, Perú). In Actas XII Congreso Peruano De Geología; Sociedad Geológica del Perú: Lima, Peru, 2004; pp. 401–404. [Google Scholar]
- Schrader, H.; Ronning, P. Diatom biostratigraphy and coastal upwelling interpretation. In Cenozoic Geology of the Pisco Basin; Dunbar, R.B., Baker, P.A., Eds.; IGCP No. 156, Guidebook to Field Workshop; IGCP: Lima, Peru, 1988; pp. 135–140. [Google Scholar]
- Gariboldi, K. A note on diatom stratigraphic markers in upper Miocene sediments of the Pisco Formation, Peru, and description of Delphineis urbinai sp. nov. Diatom Res. 2016, 31, 285–301. [Google Scholar] [CrossRef]
- McCurry, M.R.; Pyenson, N.D. Hyper-longirostry and kinematic disparity in extinct toothed whales. Paleobiology 2019, 45, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Marocco, R.; de Muizon, C. Los vertebrados del Neogeno de la costa sur del Perú: Ambiente sedimentario y condiciones de fosilización. Bull. De L’inst. Fr. D’études Andin. 1988, 17, 105–117. [Google Scholar]
- Collareta, A.; Lambert, O.; de Muizon, C.; Urbina, M.; Bianucci, G. Koristocetus pescei gen. et sp. nov., a diminutive sperm whale (Cetacea: Odontoceti: Kogiidae) from the late Miocene of Peru. Foss. Rec. 2017, 20, 259–278. [Google Scholar] [CrossRef] [Green Version]
- Benites-Palomino, A.; Vélez-Juarbe, J.; Salas-Gismondi, R.; Urbina, M. Scaphokogia totajpe, sp. nov., a new bulky-faced pygmy sperm whale (Kogiidae) from the late Miocene of Peru. J. Vertebr. Paleontol. 2019, 39, e1728538. [Google Scholar] [CrossRef]
- Collareta, A.; Lambert, O.; Landini, W.; Di Celma, C.; Malinverno, E.; Varas-Malca, R.; Urbina, M.; Bianucci, G. Did the giant extinct shark Carcharocles megalodon target small prey? Bite marks on marine mammal remains from the late Miocene of Peru. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2017, 469, 84–91. [Google Scholar] [CrossRef]
- Tarquini, J.; Soibelzon, L.H.; Salas-Gismondi, R.; de Muizon, C. Cyonasua (Carnivora, Procyonidae) from late Miocene of Peru shed light on the early dispersal of carnivorans in South America. J. Vertebr. Paleontol. 2020, 40, e1834406. [Google Scholar] [CrossRef]
- Altamirano-Sierra, A. Primer registro de pelícano (Aves: Pelecanidae) para el Mioceno tardío de la formación Pisco, Perú. Bull. De L’inst. Fr. D’études Andin. 2013, 42, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Cliff, G.; Dudley, S.F.J. Sharks caught in the protective gill nets off Natal, South Africa. 6. The copper shark Carcharhinus brachyurus (Günther). S. Afr. J. Mar. Sci. 1992, 12, 663–674. [Google Scholar] [CrossRef] [Green Version]
- Crawford, R.J.M.; Goya, E.; Roux, J.P.; Zavalaga, C.B. Comparison of assemblages and some life-history traits of seabirds in the Humboldt and Benguela systems. Afr. J. Mar. Sci. 2006, 28, 553–560. [Google Scholar] [CrossRef]
- Dudley, S.F.; Cliff, G. Influence of the annual sardine run on catches of large sharks in the protective gillnets off KwaZulu-Natal, South Africa, and the occurrence of the sardine in shark diet. Afr. J. Mar. Sci. 2010, 32, 383–397. [Google Scholar] [CrossRef]
- Espinoza, P.; Bertrand, A. Revisiting Peruvian anchovy (Engraulis ringens) trophodynamics provides a new vision of the Humboldt Current system. Prog. Oceanogr. 2008, 79, 215–227. [Google Scholar] [CrossRef]
- Taylor, M.H.; Tam, J.; Blaskovic, V.; Espinoza, P.; Ballón, R.M.; Wosnitza-Mendo, C.; Argüelles, J.; Díaz, E.; Purca, S.; Ochoa, N.; et al. Trophic modeling of the Northern Humboldt Current Ecosystem, Part II: Elucidating ecosystem dynamics from 1995 to 2004 with a focus on the impact of ENSO. Prog. Oceanogr. 2008, 79, 366–378. [Google Scholar] [CrossRef] [Green Version]
- Espinoza, P.; Bertrand, A.; van der Lingen, C.D.; Garrido, S.; de Mendiola, B.R. Diet of sardine (Sardinops sagax) in the northern Humboldt Current System and comparison with the diets of clupeoids in this and other eastern boundary upwelling systems. Prog. Oceanogr. 2009, 83, 242–250. [Google Scholar] [CrossRef]
- Fréon, P.; Arístegui, J.; Bertrand, A.; Crawford, R.J.; Field, J.C.; Gibbons, M.J.; Tam, J.; Hutchings, L.; Masski, H.; Mullon, C.; et al. Functional group biodiversity in Eastern Boundary Upwelling Ecosystems questions the wasp-waist trophic structure. Prog. Oceanogr. 2009, 83, 97–106. [Google Scholar] [CrossRef]
- Galeotti, S.; Von der Heydt, A.; Huber, M.; Bice, D.; Dijkstra, H.; Jilbert, T.; Lanci, L.; Reichart, G.J. Evidence for active El Niño Southern Oscillation variability in the Late Miocene greenhouse climate. Geology 2010, 38, 419–422. [Google Scholar] [CrossRef] [Green Version]
- Weiss, T.L.; Denniston, R.F.; Wanamaker, A.D., Jr.; Villarini, G.; von der Heydt, A.S. El Niño–Southern Oscillation–like variability in a late Miocene Caribbean coral. Geology 2017, 45, 643–646. [Google Scholar] [CrossRef]
- Peri, E.; Falkingham, P.; Collareta, A.; Bianucci, G. Biting in the Miocene seas: Estimation of the bite force of the macroraptorial sperm whale Zygophyseter varolai using finite element analysis. Hist. Biol. 2021, in press. [Google Scholar] [CrossRef]
- Lluch-Belda, D.; Magallón, F.J.; Schwartzlose, R.A. Large fluctuations in the sardine fishery in the Gulf of California: Possible causes. CalCOFI Rep. 1986, 27, 136–140. [Google Scholar]
- Hammann, M.G.; Baumgartner, T.R.; Badan-Dangon, A. Coupling of the Pacific sardine (Sardinops sagax caeruleus) life cycle with the Gulf of California pelagic environment. CalCOFI Rep. 1988, 29, 102–109. [Google Scholar]
- Hammann, M.G.; Nevárez-Martínez, M.O.; Green-Ruíz, Y. Spawning habitat of the Pacific sardine (Sardinops sagax) in the Gulf of California: Egg and larval distribution 1956–1957 and 1971–1991. CalCOFI Rep. 1998, 39, 169–179. [Google Scholar]
- Cisneros-Mata, M.A.; Nevárez-Martínez, M.O.; Hammann, M.G. The rise and fall of the Pacific sardine, Sardinops sagax caeruleus Girard, in the Gulf of California, Mexico. CalCOFI Rep. 1995, 36, 136–143. [Google Scholar]
- Bianucci, G.; Landini, W. Killer sperm whale: A new basal physeteroid (Mammalia, Cetacea) from the Late Miocene of Italy. Zool. J. Linn. Soc. 2006, 148, 103–131. [Google Scholar] [CrossRef] [Green Version]
- Lambert, O.; Bianucci, G. How to break a sperm whale’s teeth: Dental damage in a large Miocene physeteroid from the North Sea Basin. J. Vertebr. Paleontol. 2019, 39, e1660987. [Google Scholar] [CrossRef]
- Peri, E.; Collareta, A.; Bianucci, G. A new record of Physeteroidea from the upper Miocene of the Pietra leccese (southern Italy): Systematics, paleoecology and taphonomy of a fossil macroraptorial sperm whale. Riv. Ital. Di Paleontol. E Stratigr. 2020, 126, 751–769. [Google Scholar]
- Corkeron, P.J.; Connor, R.C. Why do baleen whales migrate? Mar. Mammal Sci. 1999, 15, 1228–1245. [Google Scholar] [CrossRef]
- Rice, D.W. Sperm whale Physeter macrocephalus Linnaeus, 1758. In Handbook of Marine Mammals; Ridgway, S.H., Harrison, R., Eds.; Academic Press: London, UK, 1989; Volume 4, pp. 177–233. [Google Scholar]
- Caldwell, D.K.; Caldwell, M.C. Pygmy sperm whale Kogia breviceps (de Blainville, 1938); dwarf sperm whale Kogia simus Owen, 1866. In Handbook of Marine Mammals. River Dolphins and the Larger Toothed Whales; Ridgway, S.H., Harrison, R., Eds.; Academic Press: London, UK, 1989; Volume 4, pp. 235–260. [Google Scholar]
- Berta, A.; Lanzetti, A. Feeding in marine mammals: An integration of evolution and ecology through time. Palaeontol. Electron. 2020, 23, a40. [Google Scholar]
- Perez, V.J.; Leder, R.M.; Badaut, T. Body length estimation of Neogene macrophagous lamniform sharks (Carcharodon and Otodus) derived from associated fossil dentitions. Palaeontol. Electron. 2021, 24, a09. [Google Scholar]
- Shimada, K. The size of the megatooth shark, Otodus megalodon (Lamniformes: Otodontidae), revisited. Hist. Biol. 2021, 33, 904–911. [Google Scholar] [CrossRef]
- Purdy, R.W. Paleoecology of fossil white sharks. In Great White Sharks: The Biology of Carcharodon Carcharias; Klimley, A.P., Ainley, D.G., Eds.; Academic Press: San Diego, CA, USA, 1996; pp. 67–78. [Google Scholar]
- Godfrey, S.J.; Altman, J. A Miocene cetacean vertebra showing a partially healed compression fracture, the result of convulsions or failed predation by the giant white shark, Carcharodon megalodon. Jeffersoniana 2005, 16, 1–12. [Google Scholar]
- Aguilera, O.A.; García, L.; Cozzuol, M.A. Giant-toothed white sharks and cetacean trophic interaction from the Pliocene Caribbean Paraguaná Formation. Paläontol. Z. 2008, 82, 204–208. [Google Scholar] [CrossRef]
- Martin, J.E.; Tacail, T.; Adnet, S.; Girard, C.; Balter, V. Calcium isotopes reveal the trophic position of extant and fossil elasmobranchs. Chem. Geol. 2015, 415, 118–125. [Google Scholar] [CrossRef]
- Compagno, L.J.V. Alternative life-history styles of cartilaginous fishes in time and space. Environ. Biol. Fishes 1990, 28, 33–75. [Google Scholar] [CrossRef]
- Marx, F.G.; Fordyce, R.E. Baleen boom and bust: A synthesis of mysticete phylogeny, diversity and disparity. R. Soc. Open Sci. 2015, 2, 140434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slater, G.J.; Goldbogen, J.A.; Pyenson, N.D. Independent evolution of baleen whale gigantism linked to Plio-Pleistocene ocean dynamics. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170546. [Google Scholar] [CrossRef] [Green Version]
- McSweeney, F.; Buckeridge, J. The Fossils of the Urban Sanctuary: Rickett’s Point Victoria 3193; Greypath Productions: Cheltenham, Australia, 2017; 100p. [Google Scholar]
- Boessenecker, R.W.; Ehret, D.J.; Long, D.J.; Churchill, M.; Martin, E.; Boessenecker, S.J. The Early Pliocene extinction of the mega-toothed shark Otodus megalodon: A view from the eastern North Pacific. PeerJ 2019, 7, e6088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govender, R. Early Pliocene fossil cetaceans from Hondeklip Bay, Namaqualand, South Africa. Hist. Biol. 2021, 33, 574–593. [Google Scholar] [CrossRef]
- Pimiento, C.; MacFadden, B.J.; Clements, C.F.; Varela, S.; Jaramillo, C.; Velez-Juarbe, J.; Silliman, B.R. Geographical distribution patterns of Carcharocles megalodon over time reveal clues about extinction mechanisms. J. Biogeogr. 2016, 43, 1645–1655. [Google Scholar] [CrossRef]
- Pimiento, C.; Griffin, J.N.; Clements, C.F.; Silvestro, D.; Varela, S.; Uhen, M.D.; Jaramillo, C. The Pliocene marine megafauna extinction and its impact on functional diversity. Nat. Ecol. Evol. 2017, 1, 1100–1106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carrillo-Briceño, J.D.; Luz, Z.; Hendy, A.; Kocsis, L.; Aguilera, O.; Vennemann, T. Neogene Caribbean elasmobranchs: Diversity, paleoecology and paleoenvironmental significance of the Cocinetas Basin assemblage (Guajira Peninsula, Colombia). Biogeosciences 2019, 16, 33–56. [Google Scholar] [CrossRef] [Green Version]
- Sorenson, L.; Santini, F.; Alfaro, M.E. The effect of habitat on modern shark diversification. J. Evol. Biol. 2014, 27, 1536–1548. [Google Scholar] [CrossRef]
- Amiot, R.; Göhlich, U.B.; Lécuyer, C.; de Muizon, C.; Cappetta, H.; Fourel, F.; Héran, M.-A.; Martineau, F. Oxygen isotope compositions of phosphate from Middle Miocene–Early Pliocene marine vertebrates of Peru. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2008, 264, 85–92. [Google Scholar] [CrossRef]
- Teh, L.S.; Teh, L.C.; Sumaila, U.R. A global estimate of the number of coral reef fishers. PLoS ONE 2013, 8, e65397. [Google Scholar] [CrossRef]
- Cione, A.L.; Cabrera, D.A.; Barla, M.J. Oldest record of the great white shark (Lamnidae, Carcharodon; Miocene) in the Southern Atlantic. Geobios 2012, 45, 167–172. [Google Scholar] [CrossRef]
- Parrish, R.H.; Serra, R.; Grant, W.S. The monotypic sardines, Sardina and Sardinops: Their taxonomy, distribution, stock structure, and zoogeography. Can. J. Fish. Aquat. Sci. 1989, 46, 2019–2036. [Google Scholar] [CrossRef] [Green Version]
- Oyanadel-Urbina, P.; De Gracia, C.; Carrillo-Briceño, J.D.; Nielsen, S.N.; Flores, H.; Casteletto, V.; Kriwet, J.; Rivadeneira, M.; Villafaña, J.A. Neogene Bony Fishes from the Bahía Inglesa Formation, Northern Chile. Ameghiniana 2021, 58, 345–368. [Google Scholar] [CrossRef]
- Pyenson, N.D.; Gutstein, C.S.; Parham, J.F.; Le Roux, J.P.; Chavarría, C.C.; Little, H.; Metallo, A.; Rossi, V.; Valenzuela-Toro, A.M.; Velez-Juarbe, J.; et al. Repeated mass strandings of Miocene marine mammals from Atacama Region of Chile point to sudden death at sea. Proc. R. Soc. B Biol. Sci. 2014, 281, 20133316. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Collareta, A.; Lambert, O.; Marx, F.G.; de Muizon, C.; Varas-Malca, R.; Landini, W.; Bosio, G.; Malinverno, E.; Gariboldi, K.; Gioncada, A.; et al. Vertebrate Palaeoecology of the Pisco Formation (Miocene, Peru): Glimpses into the Ancient Humboldt Current Ecosystem. J. Mar. Sci. Eng. 2021, 9, 1188. https://doi.org/10.3390/jmse9111188
Collareta A, Lambert O, Marx FG, de Muizon C, Varas-Malca R, Landini W, Bosio G, Malinverno E, Gariboldi K, Gioncada A, et al. Vertebrate Palaeoecology of the Pisco Formation (Miocene, Peru): Glimpses into the Ancient Humboldt Current Ecosystem. Journal of Marine Science and Engineering. 2021; 9(11):1188. https://doi.org/10.3390/jmse9111188
Chicago/Turabian StyleCollareta, Alberto, Olivier Lambert, Felix G. Marx, Christian de Muizon, Rafael Varas-Malca, Walter Landini, Giulia Bosio, Elisa Malinverno, Karen Gariboldi, Anna Gioncada, and et al. 2021. "Vertebrate Palaeoecology of the Pisco Formation (Miocene, Peru): Glimpses into the Ancient Humboldt Current Ecosystem" Journal of Marine Science and Engineering 9, no. 11: 1188. https://doi.org/10.3390/jmse9111188