Methyl Benzoate as a Promising, Environmentally Safe Insecticide: Current Status and Future Perspectives
Abstract
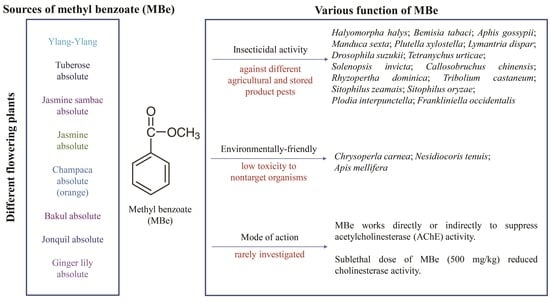
:1. Introduction
2. Natural Function and Sources of Methyl Benzoate
3. Extraction, Biosynthesis Pathway, and Chemical Properties of Methyl Benzoate
4. Insecticidal Effects of Methyl Benzoate
4.1. Contact Toxicants
4.2. Fumigant Toxicity
4.3. Repellents, Oviposition Deterrents, Attractants, and Developmental Disruptors
5. Toxicity of Methyl Benzoate to Natural Enemies, Pollinators, Plants, and Mammals
6. Sublethal Effects of Methyl Benzoate
7. Lack of Knowledge of Molecular Target(s) and the Mode of Action of Methyl Benzoate
8. Future Perspectives
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Culliney, T.W. Crop losses to arthropods. In Integrated Pest Management: Pesticide Problems; Pimentel, D., Peshin, R., Eds.; Springer: Dordrecht, The Netherlands, 2014; Volume 3, pp. 201–225. [Google Scholar]
- Damalas, C.A.; Koutroubas, S.D. Botanical pesticides for eco-friendly pest management. In Pesticides in Crop Production; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2020; pp. 181–193. [Google Scholar]
- Ebadollahi, A.; Ziaee, M.; Palla, F. Essential oils extracted from different species of the lamiaceae plant family as prospective bioagents against several detrimental pests. Molecules 2020, 25, 1556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, M.; Chang, C.-H.; Tao, L.; Lu, C. Residential exposure to pesticide during childhood and childhood cancers: A meta-analysis. Pediatrics 2015, 136, 719–729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damalas, C.A.; Eleftherohorinos, I.G. Pesticide exposure, safety issues, and risk assessment indicators. Int. J. Environ. Res. Public Health 2011, 8, 1402–1419. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D. REVIEW: An overview of the environmental risks posed by neonicotinoid insecticides. J. Appl. Ecol. 2013, 50, 977–987. [Google Scholar] [CrossRef]
- Naqqash, M.N.; Gökçe, A.; Bakhsh, A.; Salim, M. Insecticide resistance and its molecular basis in urban insect pests. Parasitol. Res. 2016, 115, 1363–1373. [Google Scholar] [CrossRef]
- Zikankuba, V.L.; Mwanyika, G.; Ntwenya, J.E.; James, A. Pesticide regulations and their malpractice implications on food and environment safety. Cogent. Food. Agric. 2019, 5, 1601544. [Google Scholar] [CrossRef]
- Ahmed, M.; Peiwen, Q.; Gu, Z.; Liu, Y.; Sikandar, A.; Hussain, D.; Javeed, A.; Shafi, J.; Iqbal, M.F.; An, R.; et al. Insecticidal activity and biochemical composition of Citrullus colocynthis, Cannabis indica and Artemisia argyi extracts against cabbage aphid (Brevicoryne brassicae L.). Sci. Rep. 2020, 10, 522. [Google Scholar] [CrossRef]
- Campos, E.V.R.; Proença, P.L.F.; Oliveira, J.L.; Bakshi, M.; Abhilash, P.C.; Fraceto, L.F. Use of botanical insecticides for sustainable agriculture: Future perspectives. Ecol. Indic. 2019, 105, 483–495. [Google Scholar] [CrossRef] [Green Version]
- Dougoud, J.; Toepfer, S.; Bateman, M.; Jenner, W.H. Efficacy of homemade botanical insecticides based on traditional knowledge—A review. Agron. Sustain. Dev. 2019, 39, 37. [Google Scholar] [CrossRef] [Green Version]
- Falkowski, M.; Jahn-Oyac, A.; Odonne, G.; Flora, C.; Estevez, Y.; Touré, S.; Boulogne, I.; Robinson, J.-C.; Béreau, D.; Petit, P.; et al. Towards the optimization of botanical insecticides research: Aedes aegypti larvicidal natural products in French Guiana. Acta Trop. 2020, 201, 105179. [Google Scholar] [CrossRef]
- Feng, Y.; Chen, J.; Zhang, A. Commercially available natural benzyl esters and their synthetic analogs exhibit different toxicities against insect pests. Sci. Rep. 2018, 8, 7902. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Zhang, A. A floral fragrance methyl benzoate is an efficient green pesticide. Sci. Rep. 2017, 7, 42168. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Maggi, F.; Iannarelli, R.; Benelli, G. Plant extracts for developing mosquito larvicides: From laboratory to the field, with insights on the modes of action. Acta Trop. 2019, 193, 236–271. [Google Scholar] [CrossRef] [PubMed]
- Ruttanaphan, T.; de Sousa, G.; Pengsook, A.; Pluempanupat, W.; Huditz, H.-I.; Bullangpoti, V.; Le Goff, G. A novel insecticidal molecule extracted from Alpinia galanga with potential to control the pest insect Spodoptera frugiperda. Insects 2020, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. A renaissance for botanical insecticides? Pest Manag. Sci. 2015, 71, 1587–1590. [Google Scholar] [CrossRef]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects—A review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar]
- Pavela, R.; Benelli, G. Essential oils as ecofriendly biopesticides? challenges and constraints. Trends Plant Sci. 2016, 21, 1000–1007. [Google Scholar] [CrossRef]
- Isman, M.B. Bridging the gap: Moving botanical insecticides from the laboratory to the farm. Ind. Crops Prod. 2017, 110, 10–14. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial development of plant essential oils and their constituents as active ingredients in bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Magierowicz, K.; Górska-Drabik, E.; Golan, K. Effects of plant extracts and essential oils on the behavior of Acrobasis advenella (Zinck.) caterpillars and females. J. Plant Dis. Prot. 2020, 127, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Campos, E.V.R.; De Oliveira, J.L.; Pascoli, M.; De Lima, R.; Fraceto, L.F. Neem oil and crop protection: From now to the future. Front. Plant Sci. 2016, 7, 1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhary, S.; Kanwar, R.K.; Sehgal, A.; Cahill, D.M.; Barrow, C.J.; Sehgal, R.; Kanwar, J.R. Progress on Azadirachta indica based biopesticides in replacing synthetic toxic pesticides. Front. Plant Sci. 2017, 8, 610. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical insecticides in the twenty-first century—Fulfilling their promise? Annu. Rev. Entomol. 2020, 65, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Pavela, R. Essential oils for the development of eco-friendly mosquito larvicides: A review. Ind. Crops Prod. 2015, 76, 174–187. [Google Scholar] [CrossRef]
- Bhavya, M.L.; Chandu, A.G.S.; Devi, S.S. Ocimum tenuiflorum oil, a potential insecticide against rice weevil with anti-acetylcholinesterase activity. Ind. Crops Prod. 2018, 126, 434–439. [Google Scholar] [CrossRef]
- Ma, S.; Jia, R.; Guo, M.; Qin, K.; Zhang, L. Insecticidal activity of essential oil from Cephalotaxus sinensis and its main components against various agricultural pests. Ind. Crops Prod. 2020, 150, 112403. [Google Scholar] [CrossRef]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential oils in insect control: Low-risk products in a high-stakes world. Annu. Rev. Entomol. 2011, 57, 405–424. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential oil chemical characterization and investigation of some biological activities: A critical review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [Green Version]
- Eslahi, H.; Fahimi, N.; Sardarian, A.R. Chemical composition of essential oils. In Essential Oils in Food Processing; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2017; pp. 119–171. [Google Scholar]
- Arena, J.S.; Merlo, C.; Defagó, M.T.; Zygadlo, J.A. Insecticidal and antibacterial effects of some essential oils against the poultry pest Alphitobius diaperinus and its associated microorganisms. J. Pest Sci. 2020, 93, 403–414. [Google Scholar] [CrossRef]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef]
- Marrone, P.G. Pesticidal natural products—Status and future potential. Pest Manag. Sci. 2019, 75, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Pavela, R.; Govindarajan, M. The essential oil from Zanthoxylum monophyllum a potential mosquito larvicide with low toxicity to the non-target fish Gambusia affinis. J. Pest Sci. 2017, 90, 369–378. [Google Scholar] [CrossRef]
- Isman, M.B.; Miresmailli, S.; Machial, C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011, 10, 197–204. [Google Scholar] [CrossRef]
- Peng, Y.; Bishop, K.S.; Quek, S.Y. Compositional analysis and aroma evaluation of feijoa essential oils from New Zealand grown cultivars. Molecules 2019, 24, 2053. [Google Scholar] [CrossRef] [Green Version]
- Nematollahi, N.; Kolev, S.D.; Steinemann, A. Volatile chemical emissions from essential oils. Air Qual. Atmos. Health 2018, 11, 949–954. [Google Scholar] [CrossRef]
- Hardy, P.J.; Michael, B.J. Volatile components of feijoa fruits. Phytochemistry 1970, 9, 1355–1357. [Google Scholar] [CrossRef]
- Yang, Y.; Isman, M.B.; Tak, J.-H. Insecticidal activity of 28 essential oils and a commercial product containing Cinnamomum cassia bark essential oil against Sitophilus zeamais Motschulsky. Insects 2020, 11, 474. [Google Scholar] [CrossRef]
- Cheong, M.-W.; Loke, X.-Q.; Liu, S.-Q.; Pramudya, K.; Curran, P.; Yu, B. Characterization of volatile compounds and aroma profiles of Malaysian pomelo (Citrus grandis (L.) Osbeck) blossom and peel. J. Essent. Oil Res. 2011, 23, 34–44. [Google Scholar] [CrossRef]
- Tisserand, R.; Young, R. Essential Oil Safety: A Guide for Health Care Professionals, 2nd ed.; Churchill Livingstone: Edinburgh, UK, 2013. [Google Scholar]
- Cheong, M.-W.; Liu, S.-Q.; Yeo, J.; Chionh, H.-K.; Pramudya, K.; Curran, P.; Yu, B. Identification of aroma-active compounds in Malaysian pomelo (Citrus grandis (L.) Osbeck) peel by gas chromatography-olfactometry. J. Essent. Oil Res. 2011, 23, 34–42. [Google Scholar] [CrossRef]
- Dudareva, N.; Murfitt, L.M.; Mann, C.J.; Gorenstein, N.; Kolosova, N.; Kish, C.M.; Bonham, C.; Wood, K. Developmental regulation of methyl benzoate biosynthesis and emission in snapdragon flowers. Plant Cell 2000, 12, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Bruton, R.; Park, A.; Zhang, A. Identification of attractive blend for spotted wing drosophila, Drosophila suzukii, from apple juice. J. Pest Sci. 2018, 91, 1251–1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.-C.; Wang, Y.; Portilla, M.; Parys, K.; Li, W. Risk and toxicity assessment of a potential natural insecticide, methyl benzoate, in honey bees (Apis mellifera L.). Insects 2019, 10, 382. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafiz, M.M.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Lethal and sublethal effects of methyl benzoate on the predatory bug Nesidiocoris tenuis. Insects 2020, 11, 377. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Insecticidal efficacy of three benzoate derivatives against Aphis gossypii and its predator Chrysoperla carnea. Ecotoxicol. Environ. Saf. 2019, 184, 109653. [Google Scholar] [CrossRef]
- Choudhary, M.I.; Naheed, N.; Abbaskhan, A.; Musharraf, S.G.; Siddiqui, H.; Atta-ur-Rahman. Phenolic and other constituents of fresh water fern Salvinia molesta. Phytochemistry 2008, 69, 1018–1023. [Google Scholar] [CrossRef]
- Knudsen, J.; Tollsten, L. Trend in floral scent chemistry in pollination syndromes: Floral scent composition in moth-pollinated taxa. Bot. J. Linn. Soc. 1993, 113, 263–284. [Google Scholar] [CrossRef]
- Effmert, U.; Saschenbrecker, S.; Ross, J.; Negre, F.; Fraser, C.M.; Noel, J.P.; Dudareva, N.; Piechulla, B. Floral benzenoid carboxyl methyltransferases: From in vitro to in planta function. Phytochemistry 2005, 66, 1211–1230. [Google Scholar] [CrossRef]
- Shi, S.; Duan, G.; Li, D.; Wu, J.; Liu, X.; Hong, B.; Yi, M.; Zhang, Z. Two-dimensional analysis provides molecular insight into flower scent of Lilium “Siberia”. Sci. Rep. 2018, 8, 5352. [Google Scholar] [CrossRef] [Green Version]
- Shaw, G.J.; Ellingham, P.J.; Birch, E.J. Volatile constituents of feijoa—Headspace analysis of intact fruit. J. Sci. Food Agric. 1983, 34, 743–747. [Google Scholar] [CrossRef]
- Young, H.; Paterson, V.J.; Burns, D.J.W. Volatile aroma constituents of kiwifruit. J. Sci. Food Agric. 1983, 34, 81–85. [Google Scholar] [CrossRef]
- Bartley, J.; Schwede, A. Production of volatile compounds in ripening kiwi fruit (Actinidia chinensis). J. Agric. Food Chem. 1989, 37, 1023–1025. [Google Scholar] [CrossRef]
- Binder, R.G.; Flath, R.A. Volatile components of pineapple guava. J. Agric. Food. Chem. 1989, 37, 734–736. [Google Scholar] [CrossRef]
- Froehlich, O.; Duque, C.; Schreier, P. Volatile constituents of curuba (Passiflora mollissima) fruit. J. Agric. Food. Chem. 1989, 37, 421–425. [Google Scholar] [CrossRef]
- Knudsen, J.T.; Tollsten, L.; Bergström, L.G. Floral scents—A checklist of volatile compounds isolated by head-space techniques. Phytochemistry 1993, 33, 253–280. [Google Scholar] [CrossRef]
- Levin, R.A.; Raguso, R.A.; McDade, L.A. Fragrance chemistry and pollinator affinities in Nyctaginaceae. Phytochemistry 2001, 58, 429–440. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Guan, J.; Ferrer, J.-L.; Engle, N.; Chern, M.; Ronald, P.; Tschaplinski, T.J.; Chen, F. Biosynthesis and emission of insect-induced methyl salicylate and methyl benzoate from rice. Plant Physiol. Biochem. 2010, 48, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Silva, D.B.; Weldegergis, B.T.; Van Loon, J.J.A.; Bueno, V.H.P. Qualitative and quantitative differences in herbivore-induced plant volatile blends from tomato plants infested by either Tuta absoluta or Bemisia tabaci. J. Chem. Ecol. 2017, 43, 53–65. [Google Scholar] [CrossRef] [Green Version]
- Schiestl, F.P.; Roubik, D.W. Odor compound detection in male euglossine bees. J. Chem. Ecol. 2003, 29, 253–257. [Google Scholar] [CrossRef]
- Murfitt, L.M.; Kolosova, N.; Mann, C.J.; Dudareva, N. Purification and characterization of S-adenosyl-L-methionine: Benzoic acid carboxyl methyltransferase, the enzyme responsible for biosynthesis of the volatile ester methyl benzoate in flowers of Antirrhinum majus. Arch. Biochem. Biophys. 2000, 382, 145–151. [Google Scholar] [CrossRef]
- Kolosova, N.; Gorenstein, N.; Kish, C.M.; Dudareva, N. Regulation of circadian methyl benzoate emission in diurnally and nocturnally emitting plants. Plant Cell 2001, 13, 2333–2347. [Google Scholar] [CrossRef] [Green Version]
- Negre, F.; Kish, C.M.; Boatright, J.; Underwood, B.; Shibuya, K.; Wagner, C.; Clark, D.G.; Dudareva, N. Regulation of methyl benzoate emission after pollination in snapdragon and petunia flowers. Plant Cell 2003, 15, 2992–3006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinrich, B. Bumblebee Economics; Harvard University Press: Cambridge, MA, USA, 2004. [Google Scholar]
- El-Sayed, A.M. The Pherobase: Database of Insect Pheromones and Semiochemicals. Available online: http://www.pherobase.com (accessed on 25 January 2022).
- Bickers, D.R.; Calow, P.; Greim, H.A.; Hanifin, J.M.; Rogers, A.E.; Saurat, J.-H.; Glenn Sipes, I.; Smith, R.L.; Tagami, H. The safety assessment of fragrance materials. Regul. Toxicol. Pharmacol. 2003, 37, 218–273. [Google Scholar] [CrossRef]
- European-Commission. List of Preservatives Allowed in Cosmetic Products. Available online: https://data.europa.eu/data/datasets/cosmetic-ingredient-database-list-of-preservatives-allowed-in-cosmetic products?locale=en (accessed on 25 January 2022).
- George, A.B. Fenaroli’s Handbook of Flavor Ingredients; CRC Press: Boca Raton, FL, USA, 2010. [Google Scholar]
- Atkinson, R. A structure-activity relationship for the estimation of rate constants for the gas-phase reactions of OH radicals with organic compounds. Int. J. Chem. Kinet. 1987, 19, 799–828. [Google Scholar] [CrossRef]
- Yue, Y.; Wang, L.; Yu, R.; Chen, F.; He, J.; Li, X.; Yu, Y.; Fan, Y. Coordinated and high-level expression of biosynthetic pathway genes is responsible for the production of a major floral scent compound methyl benzoate in Hedychium coronarium. Front. Plant Sci. 2021, 12, 650582. [Google Scholar] [CrossRef]
- Widhalm, J.R.; Dudareva, N. A familiar ring to it: Biosynthesis of plant benzoic acids. Mol. Plant 2015, 8, 83–97. [Google Scholar] [CrossRef] [Green Version]
- Bolte, K.; Rensing, S.A.; Maier, U.-G. The evolution of eukaryotic cells from the perspective of peroxisomes: Phylogenetic analyses of peroxisomal beta-oxidation enzymes support mitochondria-first models of eukaryotic cell evolution. Bioessays 2015, 37, 195–203. [Google Scholar] [CrossRef]
- Adebesin, F.; Widhalm, J.R.; Lynch, J.H.; McCoy, R.M.; Dudareva, N. A peroxisomal thioesterase plays auxiliary roles in plant β-oxidative benzoic acid metabolism. Plant J. 2018, 93, 905–916. [Google Scholar] [CrossRef] [Green Version]
- Boatright, J.; Negre, F.; Chen, X.; Kish, C.M.; Wood, B.; Peel, G.; Orlova, I.; Gang, D.; Rhodes, D.; Dudareva, N. Understanding in vivo benzenoid metabolism in petunia petal tissue. Plant Physiol. 2004, 135, 1993–2011. [Google Scholar] [CrossRef] [Green Version]
- Verdonk, J.C.; Ric de Vos, C.H.; Verhoeven, H.A.; Haring, M.A.; van Tunen, A.J.; Schuurink, R.C. Regulation of floral scent production in petunia revealed by targeted metabolomics. Phytochemistry 2003, 62, 997–1008. [Google Scholar] [CrossRef]
- Qualley, A.V.; Widhalm, J.R.; Adebesin, F.; Kish, C.M.; Dudareva, N. Completion of the core β-oxidative pathway of benzoic acid biosynthesis in plants. Proc. Natl. Acad. Sci. USA 2012, 109, 16383–16388. [Google Scholar] [CrossRef] [Green Version]
- Klempien, A.; Kaminaga, Y.; Qualley, A.; Nagegowda, D.A.; Widhalm, J.R.; Orlova, I.; Shasany, A.K.; Taguchi, G.; Kish, C.M.; Cooper, B.R.; et al. Contribution of CoA ligases to benzenoid biosynthesis in petunia flowers. Plant Cell 2012, 24, 2015–2030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kravets-Bekker, A.A.; Ivanova, O.P. Toxicological characteristics of methyl benzoate and potassium benzoate. Fakt. Vnesh. Sredy Ikh Znac. Zdorovya Naseleniya Russ. 1970, 75, 125–129. [Google Scholar]
- Mostafiz, M.M.; Jhan, P.K.; Shim, J.-K.; Lee, K.-Y. Methyl benzoate exhibits insecticidal and repellent activities against Bemisia tabaci (Gennadius) (Hemiptera: Aleyrodidae). PLoS ONE 2018, 13, e0208552. [Google Scholar] [CrossRef] [PubMed]
- Mostafiz, M.M.; Shim, J.-K.; Hwang, H.-S.; Bunch, H.; Lee, K.-Y. Acaricidal effects of methyl benzoate against Tetranychus urticae Koch (Acari: Tetranychidae) on common crop plants. Pest Manag. Sci. 2020, 76, 2347–2354. [Google Scholar] [CrossRef]
- Park, C.G.; Shin, E.; Kim, J. Insecticidal activities of essential oils, Gaultheria fragrantissima and Illicium verum, their components and analogs against Callosobruchus chinensis adults. J. Asia-Pac. Entomol. 2016, 19, 269–273. [Google Scholar] [CrossRef]
- Oliveira, M.R.V.; Henneberry, T.J.; Anderson, P. History, History, current status, and collaborative research projects for Bemisia tabaci. Crop Prot. 2001, 20, 709–723. [Google Scholar] [CrossRef] [Green Version]
- Stansly, P.A.; Naranjo, S.E. Bemisia: Bionomics and Management of a Global Pest; Springer: Dordrecht, The Netherlands, 2010. [Google Scholar]
- Chen, J.; Rashid, T.; Feng, G.; Feng, Y.; Zhang, A.; Grodowitz, M.J. Insecticidal activity of methyl benzoate analogs against red imported fire ants, Solenopsis invicta (Hymenoptera: Formicidae). J. Econ. Entomol. 2019, 112, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Larson, N.R.; Nega, M.; Zhang, A.; Feldlaufer, M. Toxicity of methyl benzoate and analogs to adult Aedes aegypti. J. Am. Mosq. Control. Assoc. 2021, 37, 83–86. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Ryu, J.; Akintola, A.A.; Choi, K.S.; Hwang, U.W.; Hassan, E.; Lee, K.-Y. Larvicidal activity of methyl benzoate, a volatile organic compound, against the mosquitoes Aedes Albopictus and Culex Pipiens (Diptera: Culicidae). J. Med. Entomol. 2022, tjab230. [Google Scholar] [CrossRef]
- Ebert, T.; Cartwright, B. Biology and ecology of Aphis gossypii Glover (Homoptera: Aphididae). Southwest. Entomol. 1997, 22, 116–153. [Google Scholar]
- Blackman, R.L.; Eastop, V.F. Aphids on the World’s Crops: An Identification and Information Guide, 2nd ed.; John Wiley & Sons Ltd.: Chichester, UK, 2000. [Google Scholar]
- Takafuji, A.; Ozawa, A.; Nemoto, H.; Gotoh, T. Spider mites of Japan: Their biology and control. Exp. Appl. Acarol. 2000, 24, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.-S.; Song, M.-H.; Ahn, K.-S.; Lee, K.-Y.; Kim, J.-W.; Kim, G.-H. Monitoring of acaricide resistance in two-spotted spider mite (Tetranychus urticae) populations from rose greenhouses in Korea. J. Asia-Pac. Entomol. 2003, 6, 91–96. [Google Scholar] [CrossRef]
- Morrison, L.W.; Porter, S.D.; Daniels, E.; Korzukhin, M.D. Potential global range expansion of the invasive fire ant, Solenopsis invicta. Biol. Invasions 2004, 6, 183–191. [Google Scholar] [CrossRef]
- Vinson, S.B. Impact of the invasion of the imported fire ant. Insect Sci. 2013, 20, 439–455. [Google Scholar] [CrossRef]
- Anukiruthika, T.; Jian, F.; Jayas, D.S. Movement and behavioral response of stored product insects under stored grain environments—A review. J. Stored Prod. Res. 2021, 90, 101752. [Google Scholar] [CrossRef]
- Nayak, M.K.; Daglish, G.J.; Phillips, T.W.; Ebert, P.R. Resistance to the fumigant phosphine and its management in insect pests of stored products: A global perspective. Annu. Rev. Entomol. 2020, 65, 333–350. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.-W.; Kang, J.; Park, I.-K. Fumigant toxicity of apiaceae essential oils and their constituents against Sitophilus oryzae and their acetylcholinesterase inhibitory activity. J. Asia-Pac. Entomol. 2013, 16, 443–448. [Google Scholar] [CrossRef]
- Zhang, Z.; Yang, T.; Zhang, Y.; Wang, L.; Xie, Y. Fumigant toxicity of monoterpenes against fruitfly, Drosophila melanogaster. Ind. Crops Prod. 2016, 81, 147–151. [Google Scholar] [CrossRef]
- Morrison, W.R.; Larson, N.L.; Brabec, D.; Zhang, A. Methyl benzoate as a putative alternative, environmentally friendly fumigant for the control of stored product insects. J. Econ. Entomol. 2019, 112, 2458–2468. [Google Scholar] [CrossRef]
- Larson, N.R.; Zhang, A.; Feldlaufer, M.F. Fumigation activities of methyl benzoate and Its derivatives against the common bed bug (Hemiptera: Cimicidae). J. Med. Entomol. 2020, 57, 187–191. [Google Scholar] [CrossRef]
- Yang, X.; Liu, Y.-B.; Feng, Y.; Zhang, A. Methyl benzoate fumigation for control of post-harvest pests and its effects on apple quality. J. Appl. Entomol. 2020, 144, 191–200. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Hassan, E.; Acharya, R.; Shim, J.-K.; Lee, K.-Y. Methyl benzoate is superior to other natural fumigants for controlling the Indian meal moth (Plodia interpunctella). Insects 2021, 12, 23. [Google Scholar] [CrossRef] [PubMed]
- Thorvilson, H.G.; Phillips, S.A., Jr.; Sorensen, A.A. An innovative thermo-fumigation technique for control of red imported fire ants (Hymenoptera: Formicidae). J. Agric. Entomol. 1989, 6, 31–36. [Google Scholar]
- Doggett, S.L.; Miller, D.M.; Lee, C.-Y. Advances in the Biology and Management of Modern Bed Bugs, 1st ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2018. [Google Scholar]
- Doggett, S.L.; Dwyer, D.E.; Peñas, P.F.; Russell, R.C. Natural compounds for controlling Drosophila suzukii. a review. Agron. Sustain. Dev. 2012, 25, 164–192. [Google Scholar]
- Dam, D.; Molitor, D.; Beyer, M. Natural compounds for controlling Drosophila suzukii—A review. Agron. Sustain. Dev. 2019, 39, 53. [Google Scholar] [CrossRef]
- Kwon, Y.; Kim, S.H.; Ronderos, D.S.; Lee, Y.; Akitake, B.; Woodward, O.M.; Guggino, W.B.; Smith, D.P.; Montell, C. Drosophila TRPA1 channel is required to avoid the naturally occurring insect repellent citronellal. Curr. Biol. 2010, 20, 1672–1678. [Google Scholar] [CrossRef] [Green Version]
- Larson, N.R.; Strickland, J.; Zhang, A.; Feldlaufer, M.F. Behavioral activity of methyl benzoate against the common bed bug (Hemiptera: Cimicidae). J. Entomol. Sci. 2020, 55, 344–349. [Google Scholar] [CrossRef]
- Zhang, Q.-H.; Schneidmiller, R.G.; Hoover, D.R.; Zhou, G.; Margaryan, A.; Bryant, P. Essential oils as spatial repellents for the brown marmorated stink bug, Halyomorpha halys (Stål) (Hemiptera: Pentatomidae). J. Appl. Entomol. 2014, 138, 490–499. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Matviishyn, T.M.; Husak, V.V.; Storey, J.M.; Storey, K.B. Pesticide toxicity: A mechanistic approach. EXCLI J. 2018, 17, 1101–1136. [Google Scholar]
- Zacharia, J.T. Identity, Physical and Chemical Properties of Pesticides. In Pesticides in the Modern World: Trends in Pesticides Analysis; Stoytcheva, M., Ed.; IntechOpen: Rijeka, Croatia, 2011. [Google Scholar]
- Rodriguez-Saona, C.; Wanumen, A.C.; Salamanca, J.; Holdcraft, R.; Kyryczenko-Roth, V. Toxicity of insecticides on various life stages of two tortricid pests of cranberries and on a non-target predator. Insects 2016, 7, 15. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, F.L.; Bacci, L.; Fernandes, M.S. Impact and selectivity of insecticides to predators and parasitoids. EntomoBrasilis 2010, 3, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Bolognesi, C.; Merlo, F.D. Pesticides: Human health effects. In Encyclopedia of Environmental Health, 2nd ed.; Nriagu, J., Ed.; Elsevier: Oxford, UK, 2019; pp. 118–132. [Google Scholar]
- Khan, M.Z.; Law, F.C.P. Adverse effects of pesticides and related chemicals on enzyme and hormone systems of fish, amphibians and reptiles: A review. Proc. Pak. Acad. Sci. 2005, 42, 315–323. [Google Scholar]
- Krieger, R.I. Handbook of Pesticide Toxicology, 2nd ed.; Hayes’ Handbook of Pesticide Toxicology; Academic Press: Cambridge, MA, USA, 2010. [Google Scholar]
- Graham, B.E.; Kuizenga, M.H. Toxicity studies on benzyl benzoate and related benzyl compounds. J. Pharmacol. Exp. Ther. 1945, 84, 358–362. [Google Scholar] [PubMed]
- Jenner, P.M.; Hagan, E.C.; Taylor, J.M.; Cook, E.L.; Fitzhugh, O.G. Food flavourings and compounds of related structure I. Acute oral toxicity. Food Cosmet. Toxicol. 1964, 2, 327–343. [Google Scholar] [CrossRef]
- Desneux, N.; Decourtye, A.; Delpuech, J.-M. The sublethal effects of pesticides on beneficial arthropods. Annu. Rev. Entomol. 2007, 52, 81–106. [Google Scholar] [CrossRef]
- De França, S.M.; Breda, M.O.; Barbosa, D.R.; Araujo, A.M.; Guedes, C.A. The sublethal effects of insecticides in insects. In Biological Control of Pest and Vector Insects; IntechOpen: London, UK, 2017; pp. 23–39. [Google Scholar]
- Müller, C. Impacts of sublethal insecticide exposure on insects—Facts and knowledge gaps. Basic Appl. Ecol. 2018, 30, 1–10. [Google Scholar] [CrossRef]
- Mostafiz, M.M.; Alam, M.B.; Chi, H.; Hassan, E.; Shim, J.-K.; Lee, K.-Y. Effects of sublethal doses of methyl benzoate on the life history traits and acetylcholinesterase (AChE) activity of Aphis gossypii. Agronomy 2020, 10, 1313. [Google Scholar] [CrossRef]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular targets for components of essential oils in the insect nervous system—A review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef] [Green Version]
- Pang, Y.-P.; Brimijoin, S.; Ragsdale, D.W.; Zhu, K.Y.; Suranyi, R. Novel and viable acetylcholinesterase target site for developing effective and environmentally safe insecticides. Curr. Drug Targets 2012, 13, 471–482. [Google Scholar] [CrossRef] [Green Version]
- Dassanayake, M.K.; Chong, C.H.; Khoo, T.-J.; Figiel, A.; Szumny, A.; Choo, C.M. Synergistic field crop pest management properties of plant-derived essential oils in combination with synthetic pesticides and bioactive molecules: A review. Foods 2021, 10, 2016. [Google Scholar] [CrossRef]
- Colović, M.B.; Krstić, D.Z.; Lazarević-Pašti, T.D.; Bondžić, A.M.; Vasić, V.M. Acetylcholinesterase inhibitors: Pharmacology and toxicology. Curr. Neuropharmacol. 2013, 11, 315–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gnagey, A.L.; Forte, M.; Rosenberry, T.L. Isolation and characterization of acetylcholinesterase from Drosophila. J. Biol. Chem. 1987, 262, 13290–13298. [Google Scholar] [CrossRef]
- Bourguet, D.; Roig, A.; Toutant, J.-P.; Arpagaus, M. Analysis of molecular forms and pharmacological properties of acetylcholinesterase in several mosquito species. Neurochem. Int. 1997, 31, 65–72. [Google Scholar] [CrossRef]
- Marcel, V.; Palacios, L.; Pertuy, C.; Masson, P.; Fournier, D. Two invertebrate acetylcholinesterases show activation followed by inhibition with substrate concentration. Biochem. J. 1998, 329, 329–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trang, A.; Khandhar, P.B. Physiology, Acetylcholinesterase. In StatPearls; StatPearls Publishing: St. Petersburg, FL, USA, 2019. [Google Scholar]
- Lee, S.E.; Lee, B.H.; Choi, W.S.; Park, B.S.; Kim, J.G.; Campbell, B.C. Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the rice weevil, Sitophilus oryzae (L). Pest Manag. Sci. 2001, 57, 548–553. [Google Scholar] [CrossRef]
- Park, C.G.; Jang, M.; Yoon, K.A.; Kim, J. Insecticidal and acetylcholinesterase inhibitory activities of lamiaceae plant essential oils and their major components against Drosophila suzukii (Diptera: Drosophilidae). Ind. Crops Prod. 2016, 89, 507–513. [Google Scholar] [CrossRef]
- Anderson, J.A.; Coats, J.R. Acetylcholinesterase inhibition by nootkatone and carvacrol in arthropods. Pestic. Biochem. Phys. 2012, 102, 124–128. [Google Scholar] [CrossRef] [Green Version]
- Evans, P.D. Biogenic amines in the insect nervous system. In Advances in Insect Physiology; Berridge, M.J., Treherne, J.E., Wigglesworth, V.B., Eds.; Academic Press: Cambridge, MA, USA, 1980; Volume 15, pp. 317–473. [Google Scholar]
- Atwood, H.L.; Klose, M.K. Neuromuscular transmission modulation at invertebrate neuromuscular junctions. In Encyclopedia of Neuroscience; Squire, L.R., Ed.; Academic Press: Oxford, UK, 2009; pp. 671–690. [Google Scholar]
- Farooqui, T. Octopamine-mediated neuromodulation of insect senses. Neurochem. Res. 2007, 32, 1511–1529. [Google Scholar] [CrossRef]
- Nathanson, J.A. Octopamine receptors, adenosine 3′,5′-monophosphate, and neural control of firefly flashing. Science 1979, 203, 65–68. [Google Scholar] [CrossRef]
- Enan, E. Insecticidal activity of essential oils: Octopaminergic sites of action. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2001, 130, 325–337. [Google Scholar] [CrossRef]
- Pandey, C.; Li, W.; Wang, Y.; Jiang, S. Octopamine levels in Blattella germanica L. tissues by capillary gas chromatography with electron capture detection. Int. J. Mol. Sci. 2005, 6, 188–197. [Google Scholar] [CrossRef]
- Sattelle, D.B. GABA receptors of insects. In Advances in Insect Physiology; Evans, P.D., Wigglesworth, V.B., Eds.; Academic Press: Cambridge, MA, USA, 1990; Volume 22, pp. 1–113. [Google Scholar]
- Sigel, E.; Steinmann, M.E. Structure, function, and modulation of GABA(A) receptors. J. Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Ari, Y.; Khalilov, I.; Kahle, K.T.; Cherubini, E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist 2012, 18, 467–486. [Google Scholar] [CrossRef] [PubMed]
- Tong, F.; Coats, J.R. Effects of monoterpenoid insecticides on [3H]-TBOB binding in house fly GABA receptor and 36Cl− uptake in American cockroach ventral nerve cord. Pestic. Biochem. Physiol. 2010, 98, 317–324. [Google Scholar] [CrossRef]
- Yu, H.-Z.; Xu, J.-P.; Wang, X.-Y.; Ma, Y.; Yu, D.; Fei, D.-Q.; Zhang, S.-Z.; Wang, W.-L. Identification of four ATP-binding cassette transporter genes in Cnaphalocrocis medinalis and their expression in response to insecticide treatment. J. Insect Sci. 2017, 17, 44. [Google Scholar] [CrossRef] [Green Version]
- Merzendorfer, H. Chitin synthesis inhibitors: Old molecules and new developments. Insect Sci. 2013, 20, 121–138. [Google Scholar] [CrossRef]
- Liao, M.; Xiao, J.J.; Zhou, L.J.; Liu, Y.; Wu, X.W.; Hua, R.M.; Wang, G.R.; Cao, H.Q. Insecticidal activity of Melaleuca alternifolia essential oil and RNA-Seq analysis of Sitophilus zeamais transcriptome in response to oil fumigation. PLoS ONE 2016, 11, e0167748. [Google Scholar] [CrossRef] [Green Version]
- Liao, M.; Xiao, J.J.; Zhou, L.J.; Yao, X.; Tang, F.; Hua, R.M.; Wu, X.W.; Cao, H.Q. Chemical composition, insecticidal and biochemical effects of Melaleuca alternifolia essential oil on the Helicoverpa armigera. J. Appl. Entomol. 2017, 141, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Opdyke, D.L. Monographs on fragrance raw materials. Food. Cosmet. Toxicol. 1979, 17, 357–390. [Google Scholar] [CrossRef]
- Pongsavee, M. Effect of sodium benzoate preservative on micronucleus induction, chromosome break, and Ala40Thr superoxide dismutase gene mutation in lymphocytes. Biomed. Res. Int. 2015, 2015, 103512. [Google Scholar] [CrossRef]
- Yadav, A.; Kumar, A.; Das, M.; Tripathi, A. Sodium benzoate, a food preservative, affects the functional and activation status of splenocytes at non cytotoxic dose. Food Chem. Toxicol. 2016, 88, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Nathan, P.W.; Sears, T.A. Action of methyl hydroxybenzoate on nervous conduction. Nature 1961, 192, 668–669. [Google Scholar] [CrossRef] [PubMed]
- Tsay, H.-J.; Wang, Y.-H.; Chen, W.-L.; Huang, M.-Y.; Chen, Y.-H. Treatment with sodium benzoate leads to malformation of zebrafish larvae. Neurotoxicol. Teratol. 2007, 29, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Siegwart, M.; Graillot, B.; Blachere Lopez, C.; Besse, S.; Bardin, M.; Nicot, P.C.; Lopez-Ferber, M. Resistance to bio-insecticides or how to enhance their sustainability: A review. Front. Plant Sci. 2015, 6, 381. [Google Scholar] [CrossRef] [Green Version]
- Enan, E.E. Molecular and pharmacological analysis of an octopamine receptor from American cockroach and fruit fly in response to plant essential oils. Arch. Insect Biochem. Physiol. 2005, 59, 161–171. [Google Scholar] [CrossRef]
- Tong, F.; Coats, J.R. Quantitative structure-activity relationships of monoterpenoid binding activities to the housefly GABA receptor. Pest Manag. Sci. 2012, 68, 1122–1129. [Google Scholar] [CrossRef] [Green Version]
- Bell, C.H. Pest Control of stored food products: Insects and Mites. In Hygiene in Food Processing, 2nd ed.; Lelieveld, H.L.M., Holah, J.T., Napper, D., Eds.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Cambridge, UK, 2014; pp. 494–538. [Google Scholar]


| Groups | Species | Developmental Stages | LC50 | Units | References |
|---|---|---|---|---|---|
| Pests | Halyomorpha halys (Hemiptera: Pentatomidae) | Egg | 0.02 | mg/cm2 | Feng and Zhang [14] |
| 1st-instar nymph | 1.03 | μL/vial | |||
| 2nd-instar nymph | 1.01 | μL/vial | |||
| 3rd-instar nymph | 1.23 | μL/vial | |||
| 4th-instar nymph | 2.39 | μL/vial | |||
| 5th-instar nymph | 1.77 | μL/vial | |||
| Bemisia tabaci (Hemiptera: Aleyrodidae) | Egg | 0.3 | % | Mostafiz et al. [81] | |
| 4th-instar nymph | 0.2 | % | |||
| Adult | 0.2 | % | |||
| Aphis gossypii (Hemiptera: Aphididae) | 3rd-instar nymph | 0.18 | % | Mostafiz et al. [48] | |
| Adult | 0.32 | % | |||
| Manduca sexta (Lepidoptera: Sphingidae) | Egg | 0.015 | mg/cm2 | Feng and Zhang [14] | |
| Plutellaxylostella (Lepidoptera: Plutellidae) | Egg | 0.001 | mg/cm2 | ||
| Lymantria dispar (Lepidoptera: Erebidae) | Larvae | 0.114 | mg/cm2 | Feng et al. [13] | |
| Drosophila suzukii (Diptera: Drosophilidae) | Larvae | 1 | % | Feng and Zhang [14] | |
| Pupae | 1 | % | |||
| Adult | 1 | % | |||
| Tetranychus urticae (Trombidiformes: Tetranychidae) | Egg | 0.27 | % | Mostafiz et al. [82] | |
| Adult | 0.38 | % | |||
| Solenopsis invicta (Hymenoptera: Formicidae) | Worker | 93.65 | μg/ant | Chen et al. [86] | |
| Callosobruchus chinensis (Coleoptera: Chrysomelidae) | Adult | 44.81 | μg/beetle | Park et al. [83] | |
| Aedes aegypti (Diptera: Culicidae) | Adult | 45.6 | μg/mosquito | Larson et al. [87] | |
| Aedes albopictus (Diptera: Culicidae) | 4th-instar larvae | 61 | ppm | Mostafiz et al. [88] | |
| Culex pipiens (Diptera: Culicidae) | 4th-instar larvae | 185 | ppm | ||
| Predators | Chrysoperla carnea (Neuroptera: Chrysopidae) | 1st-instar larvae | >1 | % | Mostafiz et al. [48] |
| 2nd-instar larvae | >1 | % | |||
| Adult | >1 | % | |||
| Nesidiocoris tenuis (Hemiptera: Miridae) | Adult | >1 | % | Mostafiz et al. [47] |
| Species | Developmental Stages | LC50 | Units | References |
|---|---|---|---|---|
| Callosobruchus chinensis (Coleoptera: Chrysomelidae) | Adult | 5.36 | mg/L | Park et al. [83] |
| Rhyzopertha dominica (Coleoptera: Bostrichidae) | Adult | <1080 | mg/L | Morrison et al. [99] |
| Tribolium castaneum (Coleoptera: Tenebrionidae) | Adult | <1080 | mg/L | Morrison et al. [99] |
| Sitophilus zeamais (Coleoptera: Curculionidae) | Adult | <1080 | mg/L | Morrison et al. [99] |
| Sitophilus oryzae (Coleoptera: Curculionidae) | Adult | - | - | Yang et al. [101] |
| Trogoderma variabile (Coleoptera: Dermestidae) | Larvae | >1080 | mg/L | Morrison et al. [99] |
| Plodia interpunctella (Lepidoptera: Pyralidae) | Adult | 0.1 | μL/L | Mostafiz et al. [102] |
| Frankliniella occidentalis (Thysanoptera: Thripidae) | Larva and adult | - | - | Yang et al. [101] |
| Nasonovia ribisnigri (Hemiptera: Aphididae) | Nymph and adult | - | - | Yang et al. [101] |
| Rhizoglyphus spp. (Sarcoptiformes: Acaridae) | Adult | Yang et al. [101] | ||
| Solenopsis invicta (Hymenoptera: Formicidae) | Worker | 0.77 | μg/mL | Chen et al. [86] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafiz, M.M.; Hassan, E.; Lee, K.-Y. Methyl Benzoate as a Promising, Environmentally Safe Insecticide: Current Status and Future Perspectives. Agriculture 2022, 12, 378. https://doi.org/10.3390/agriculture12030378
Mostafiz MM, Hassan E, Lee K-Y. Methyl Benzoate as a Promising, Environmentally Safe Insecticide: Current Status and Future Perspectives. Agriculture. 2022; 12(3):378. https://doi.org/10.3390/agriculture12030378
Chicago/Turabian StyleMostafiz, Md. Munir, Errol Hassan, and Kyeong-Yeoll Lee. 2022. "Methyl Benzoate as a Promising, Environmentally Safe Insecticide: Current Status and Future Perspectives" Agriculture 12, no. 3: 378. https://doi.org/10.3390/agriculture12030378