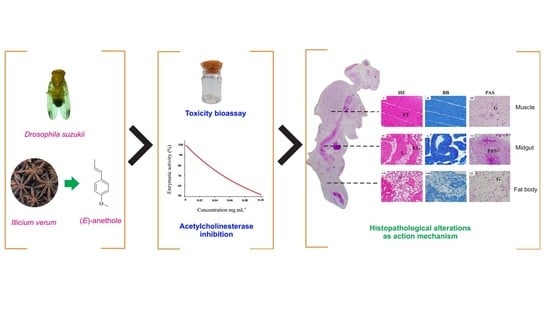
Toxicity, Histopathological Alterations and Acetylcholinesterase Inhibition of Illicium verum Essential Oil in Drosophila suzukii
Abstract
:1. Introduction
2. Material and Methods
2.1. Plant Material and Insect Population
2.2. Essential Oils Extraction and Chemical Characterization
2.3. Toxicity Assessment of Essential Oils against Drosophila suzukii
2.4. Acetylcholinesterase Inhibitory Activity of Essential Oils
2.5. Evaluation of the Effects of Exposure to Low Doses of Illicium verum Essential Oil on Females’ Histological Structures
2.5.1. Histopathological Analysis
2.5.2. Histochemical Analysis
2.5.3. Morphometric Analysis
2.6. Statistical Analysis
3. Results
3.1. Chemical Characterization of Extracted Essential Oils
3.2. Insecticidal Activity
3.3. Acetylcholinesterase Inhibitory Activity of Essential Oils
3.4. Histology of Thoracic Muscles, Midgut and Fat Body of Unexposed Adult Female of Drosophila suzukii
3.5. Histopathological Alterations Caused by Exposure to Illicium verum Essential Oil
3.5.1. Histological Alterations in the Midgut
3.5.2. Histological Alterations in the Fat Body
3.5.3. Histological Alterations in the Thoracic Muscle Fibers
3.5.4. Morphometric Analysis of the Histological Alterations
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cantrell, C.L.; Dayan, F.E.; Duke, S.O. Natural Products As Sources for New Pesticides. J. Nat. Prod. 2012, 75, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Cloonan, K.R.; Abraham, J.; Angeli, S.; Syed, Z.; Rodriguez-Saona, C. Advances in the Chemical Ecology of the Spotted Wing Drosophila (Drosophila suzukii) and Its Applications. J. Chem. Ecol. 2018, 44, 922–939. [Google Scholar] [CrossRef]
- Campolo, O.; Giunti, G.; Russo, A.; Palmeri, V.; Zappalà, L. Essential Oils in Stored Product Insect Pest Control. J. Food Qual. 2018, 2018, 6906105. [Google Scholar] [CrossRef] [Green Version]
- Haddi, K.; Turchen, L.M.; Viteri Jumbo, L.O.; Guedes, R.N.C.; Pereira, E.J.G.; Aguiar, R.W.S.; Oliveira, E.E. Rethinking Biorational Insecticides for Pest Management: Unintended Effects and Consequences. Pest Manag. Sci. 2020, 76, 2286–2293. [Google Scholar] [CrossRef] [PubMed]
- Isman, M.B. Botanical Insecticides, Deterrents, and Repellents in Modern Agriculture and an Increasingly Regulated World. Annu Rev Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [Green Version]
- Regnault-Roger, C.; Vincent, C.; Arnason, J.T. Essential Oils in Insect Control: Low-Risk Products in a High-Stakes World. Annu Rev Entomol. 2012, 57, 405–424. [Google Scholar] [CrossRef]
- Isman, M.B.; Grieneisen, M.L. Botanical Insecticide Research: Many Publications, Limited Useful Data. Trends Plant Sci. 2014, 19, 140–145. [Google Scholar] [CrossRef]
- Gonzales Correa, Y.D.C.; Faroni, L.R.A.; Haddi, K.; Oliveira, E.E.; Pereira, E.J.G. Locomotory and Physiological Responses Induced by Clove and Cinnamon Essential Oils in the Maize Weevil Sitophilus zeamais. Pestic BioChem. Physiol. 2015, 125, 31–37. [Google Scholar] [CrossRef]
- Freitas, R.C.P.; Faroni, L.R.D.; Haddi, K.; Viteri Jumbo, L.O.; Oliveira, E.E. Allyl Isothiocyanate Actions on Populations of Sitophilus zeamais Resistant to Phosphine: Toxicity, Emergence Inhibition and Repellency. J. Stored Prod. Res. 2016, 69, 257–264. [Google Scholar] [CrossRef]
- Massango, H.G.L.L.; Faroni, L.R.A.; Haddi, K.; Heleno, F.F.; Viteri Jumbo, L.O.; Oliveira, E.E. Toxicity and Metabolic Mechanisms Underlying the Insecticidal Activity of Parsley Essential Oil on Bean Weevil, Callosobruchus maculatus. J. Pest Sci. 2017, 90, 723–733. [Google Scholar] [CrossRef]
- Lourenço, A.M.; Haddi, K.; Ribeiro, B.M.; Corrêia, R.F.T.; Tomé, H.V.V.; Santos-Amaya, O.; Pereira, E.J.G.; Guedes, R.N.C.; Santos, G.R.; Oliveira, E.E.; et al. Essential Oil of Siparuna guianensis as an Alternative Tool for Improved Lepidopteran Control and Resistance Management Practices. Sci. Rep. 2018, 8, 7215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, J.C.M.; Haddi, K.; Oliveira, E.E.; Silva Andrade, B.; Nascimento, V.L.; Silva Melo, T.; Didonet, J.; Carvalho, J.C.T.; Cangussu, A.S.; Soares, I.M.; et al. Mosquiticidal and Repellent Potential of Formulations Containing Wood Residue Extracts of a Neotropical Plant, Tabebuia Heptaphylla. Ind. Crops Prod. 2019, 129, 424–433. [Google Scholar] [CrossRef]
- Ferreira, T.P.; Haddi, K.; Corrêa, R.F.T.; Zapata, V.L.B.; Piau, T.B.; Souza, L.F.N.; Santos, S.-M.G.; Oliveira, E.E.; Jumbo, L.O.V.; Ribeiro, B.M.; et al. Prolonged Mosquitocidal Activity of Siparuna guianensis Essential Oil Encapsulated in Chitosan Nanoparticles. PLoS Negl. Trop. Dis. 2019, 13, e0007624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaaya, E.; Rafaeli, A. Essential Oils as Biorational Insecticides–Potency and Mode of Action. In Insecticides design using advanced technologies; Ishaaya, I., Horowitz, A.R., Nauen, R., Eds.; Springer Science & Business Media: Berlin, Germany, 2007; pp. 249–261. [Google Scholar]
- Jankowska, M.; Rogalska, J.; Wyszkowska, J.; Stankiewicz, M. Molecular Targets for Components of Essential Oils in the Insect Nervous System-A Review. Molecules 2017, 23, 34. [Google Scholar] [CrossRef] [Green Version]
- Osman, S.; Swidan, M.; Kheirallah, D.; Nour, F. Histological Effects of Essential Oils, Their Monoterpenoids and Insect Growth Regulators on Midgut, Integument of Larvae and Ovaries of Khapra Beetle, Trogoderma granarium Everts. J. Biol. Sci. 2016, 16, 93–101. [Google Scholar] [CrossRef]
- Alves, K.F.; Caetano, F.H.; Pereira Garcia, I.J.; Santos, H.L.; Silva, D.B.; Siqueira, J.M.; Tanaka, A.S.; Alves, S.N. Baccharis dracunculifolia (Asteraceae) Essential Oil Toxicity to Culex quinquefasciatus (Culicidae). Environ. Sci. Pollut. Res. 2018, 25, 31718–31726. [Google Scholar] [CrossRef]
- Chaaban, A.; Richardi, V.S.; Carrer, A.R.; Brum, J.S.; Cipriano, R.R.; Martins, C.E.N.; Silva, M.A.N.; Deschamps, C.; Molento, M.B. Insecticide Activity of Curcuma longa (Leaves) Essential Oil and Its Major Compound α-Phellandrene against Lucilia cuprina Larvae (Diptera: Calliphoridae): Histological and Ultrastructural Biomarkers Assessment. Pestic. BioChem. Physiol. 2019, 153, 17–27. [Google Scholar] [CrossRef]
- Dutra, K.A.; Wanderley Teixeira, V.; Cruz, G.S.; Silva, C.T.S.; D Assunção, C.G.; Ferreira, C.G.M.; Monteiro, A.L.B.; Agra Neto, A.C.; Lapa Neto, C.J.C.; Teixeira, A.A.C.; et al. Morphological and Immunohistochemical Study of the Midgut and Fat Body of Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae) Treated with Essential Oils of the Genus Piper. Biotech. HistoChem. 2019, 94, 498–513. [Google Scholar] [CrossRef]
- França, L.P.; Amaral, A.C.F.; Ramos, A.d.S.; Ferreira, J.L.P.; Maria, A.C.B.; Oliveira, K.M.T.; Araujo, E.S.J.; Branches, A.D.S.; Silva, J.N.; Silva, N.G.; et al. Piper Capitarianum Essential Oil: A Promising Insecticidal Agent for the Management of Aedes aegypti and Aedes albopictus. Environ. Sci. Pollut. Res. Int. 2021, 28, 9760–9776. [Google Scholar] [CrossRef]
- Asplen, M.K.; Anfora, G.; Biondi, A.; Choi, D.-S.; Chu, D.; Daane, K.M.; Gibert, P.; Gutierrez, A.P.; Hoelmer, K.A.; Hutchison, W.D.; et al. Invasion Biology of Spotted Wing Drosophila (Drosophila suzukii): A Global Perspective and Future Priorities. J. Pest Sci. 2015, 88, 469–494. [Google Scholar] [CrossRef]
- Tait, G.; Mermer, S.; Stockton, D.; Lee, J.; Avosani, S.; Abrieux, A.; Anfora, G.; Beers, E.; Biondi, A.; Burrack, H.; et al. Drosophila suzukii (Diptera: Drosophilidae): A Decade of Research towards a Sustainable Integrated Pest Management Program. J. Econ. Entomol. 2021, 114, 1950–1974. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.C.; Bruck, D.J.; Dreves, A.J.; Ioriatti, C.; Vogt, H.; Baufeld, P. In Focus: Spotted Wing Drosophila, Drosophila suzukii, across Perspectives. Pest Manag. Sci. 2011, 67, 1349–1351. [Google Scholar] [CrossRef] [PubMed]
- Hamby, K.A.; Bellamy, D.E.; Chiu, J.C.; Lee, J.C.; Walton, V.M.; Wiman, N.G.; York, R.M.; Biondi, A. Biotic and Abiotic Factors Impacting Development, Behavior, Phenology, and Reproductive Biology of Drosophila Suzukii. J. Pest Sci. 2016, 89, 605–619. [Google Scholar] [CrossRef]
- Deprá, M.; Poppe, J.L.; Schmitz, H.J.; de Toni, D.C.; Valente, V.L.S. The First Records of the Invasive Pest Drosophila suzukii in the South American Continent. J. Pest Sci. 2014, 87, 379–383. [Google Scholar] [CrossRef]
- Walsh, D.B.; Bolda, M.P.; Goodhue, R.E.; Dreves, A.J.; Lee, J.; Bruck, D.J.; Walton, V.M.; O’Neal, S.D.; Zalom, F.G. Drosophila suzukii (Diptera: Drosophilidae): Invasive Pest of Ripening Soft Fruit Expanding Its Geographic Range and Damage Potential. J. Integr. Pest Manag. 2011, 2, G1–G7. [Google Scholar] [CrossRef]
- Karageorgi, M.; Bräcker, L.B.; Lebreton, S.; Minervino, C.; Cavey, M.; Siju, K.P.; Grunwald Kadow, I.C.; Gompel, N.; Prud’homme, B. Evolution of Multiple Sensory Systems Drives Novel Egg-Laying Behavior in the Fruit Pest Drosophila suzukii. Curr. Biol. 2017, 27, 847–853. [Google Scholar] [CrossRef] [Green Version]
- van Timmeren, S.; Isaacs, R. Control of Spotted Wing Drosophila, Drosophila suzukii, by Specific Insecticides and by Conventional and Organic Crop Protection Programs. Crop Prot. 2013, 54, 126–133. [Google Scholar] [CrossRef]
- Erland, L.; Rheault, M.; Mahmoud, S. Insecticidal and Oviposition Deterrent Effects of Essential Oils and Their Constituents against the Invasive Pest Drosophila suzukii (Matsumura) (Diptera: Drosophilidae). Crop Prot. 2015, 78, 20–26. [Google Scholar] [CrossRef] [Green Version]
- Renkema, J.M.; Wright, D.; Buitenhuis, R.; Hallett, R.H. Plant Essential Oils and Potassium Metabisulfite as Repellents for Drosophila suzukii (Diptera: Drosophilidae). Sci. Rep. 2016, 6, 21432. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Zhao, J.; Zhou, L.; Wang, J.; Gong, Y.; Chen, X.; Guo, Z.; Wang, Q.; Jiang, W. Antifungal Activity of the Essential Oil of Illicium verum Fruit and Its Main Component Trans-Anethole. Molecules 2010, 15, 7558–7569. [Google Scholar] [CrossRef]
- Gupta, A.D.; Bansal, V.K.; Babu, V.; Maithil, N. Chemistry, Antioxidant and Antimicrobial Potential of Nutmeg (Myristica fragrans Houtt). J. Genet. Eng. Biotechnol. 2013, 11, 25–31. [Google Scholar] [CrossRef] [Green Version]
- Muhsinah, A.B.; Maqbul, M.S.; Mahnashi, M.H.; Jalal, M.M.; Altayar, M.A.; Saeedi, N.H.; Alshehri, O.M.; Shaikh, I.A.; Khan, A.A.L.; Shakeel Iqubal, S.M.; et al. Antibacterial Activity of Illicium verum Essential Oil against MRSA Clinical Isolates and Determination of Its Phyto-Chemical Components. J. King Saud. Univ. Sci. 2022, 34, 101800. [Google Scholar] [CrossRef]
- Morales-Rabanales, Q.N.; Coyotl-Pérez, W.A.; Rubio-Rosas, E.; Cortes-Ramírez, G.S.; Sánchez Ramírez, J.F.; Villa-Ruano, N. Antifungal Properties of Hybrid Films Containing the Essential Oil of Schinus Molle: Protective Effect against Postharvest Rot of Tomato. Food Control 2022, 134, 108766. [Google Scholar] [CrossRef]
- Nikolic, V.; Nikolic, L.; Dinic, A.; Gajic, I.; Urosevic, M.; Stanojevic, L.; Stanojevic, J.; Danilovic, B. Chemical Composition, Antioxidant and Antimicrobial Activity of Nutmeg (Myristica fragrans Houtt.) Seed Essential Oil. J. Essent. Oil Bear. Plants 2021, 24, 218–227. [Google Scholar] [CrossRef]
- López, A.; Castro, S.; Andina, M.J.; Ures, X.; Munguía, B.; Llabot, J.M.; Elder, H.; Dellacassa, E.; Palma, S.; Domínguez, L. Insecticidal Activity of Microencapsulated Schinus Molle Essential Oil. Ind. Crops Prod. 2014, 53, 209–216. [Google Scholar] [CrossRef]
- Matos, L.F.; da Cruz Lima, E.; de Andrade Dutra, K.; Navarro, D.M.D.A.F.; Alves, J.L.R.; Silva, G.N. Chemical Composition and Insecticidal Effect of Essential Oils from Illicium verum and Eugenia Caryophyllus on Callosobruchus maculatus in Cowpea. Ind. Crops Prod. 2020, 145, 112088. [Google Scholar] [CrossRef]
- Gomes da Rocha Voris, D.; dos Santos Dias, L.; Alencar Lima, J.; dos Santos Cople Lima, K.; Pereira Lima, J.B.; dos Santos Lima, A.L. Evaluation of Larvicidal, Adulticidal, and Anticholinesterase Activities of Essential Oils of Illicium verum Hook. f., Pimenta Dioica (L.) Merr., and Myristica fragrans Houtt. against Zika Virus Vectors. Environ. Sci. Pollut. Res. Int. 2018, 25, 22541–22551. [Google Scholar] [CrossRef]
- Emiljanowicz, L.M.; Ryan, G.D.; Langille, A.; Newman, J. Development, Reproductive Output and Population Growth of the Fruit Fly Pest Drosophila suzukii (Diptera: Drosophilidae) on Artificial Diet. J. Econ. Entomol. 2014, 107, 1392–1398. [Google Scholar] [CrossRef] [Green Version]
- Andreazza, F.; Bernardi, D.; Marangon, R.; Scheunemann, T.; Botton, M.; Nava, D. Técnica de Criação de Drosophila Suzukii (Matsumura, 1931) (Diptera: Drosophilidae) Em Dieta Artificial; Boletim de Pesquisa e Desenvolvimento/Embrapa Clima Temperado. 2016. [Google Scholar]
- Mendonca, L.d.P.; Oliveira, E.E.; Andreazza, F.; Rezende, S.M.; Faroni, L.R.D.; Guedes, R.N.C.; Haddi, K. Host Potential and Adaptive Responses of Drosophila suzukii (Diptera: Drosophilidae) to Barbados Cherries. J. Econ. Entomol. 2019, 112, 3002–3006. [Google Scholar] [CrossRef]
- Brasil. Farmacopéia Brasileira, 5th ed.; Agência Nacional de Vigilância Sanitária: Brasília, Brazil, 2010.
- Pimentel, F.A.; Cardoso, M.D.G.; Salgado, A.P.S.; Aguiar, P.M.; Silva, V.D.F.; Morais, A.R.D.; Nelson, D.L. A Convenient Method for the Determination of Moisture in Aromatic Plants. Quim Nova 2006, 29, 373–375. [Google Scholar] [CrossRef]
- van den Dool, H.; Kratz, P. A Generalization of the Retention Index System Including Linear Temperature Programmed Gas—Liquid Partition Chromatography. J. Chromatogr A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R. Identification of Essential Oil Components by Gas Chromatography/Quadrupole Mass Spectroscopy. Carol. Stream 2005, 16, 65–120. [Google Scholar]
- Insecticide Resistance Action Committee (IRAC). Susceptibility Test Methods Series: Method 026. 2011. Available online: https://irac-online.org/methods/musca-domestica-adults/ (accessed on 23 July 2020).
- Ellman, G.L.; Courtney, K.D.; Andres, V.J.; Feather-Stone, R.M. A New and Rapid Colorimetric Determination of Acetylcholinesterase Activity. BioChem. Pharm. 1961, 7, 88–95. [Google Scholar] [CrossRef]
- Smirle, M.J.; Zurowski, C.L.; Ayyanath, M.-M.; Scott, I.M.; MacKenzie, K.E. Laboratory Studies of Insecticide Efficacy and Resistance in Drosophila suzukii (Matsumura) (Diptera: Drosophilidae) Populations from British Columbia, Canada. Pest Manag. Sci. 2017, 73, 130–137. [Google Scholar] [CrossRef]
- Blouquy, L.; Mottet, C.; Olivares, J.; Plantamp, C.; Siegwart, M.; Barrès, B. How Varying Parameters Impact Insecticide Resistance Bioassay: An Example on the Worldwide Invasive Pest Drosophila suzukii. PLoS ONE 2021, 16, e0247756. [Google Scholar] [CrossRef] [PubMed]
- Junqueira, L.C.; Junqueira, L. Basic Techniques of Cytology and Histology; Bookstore Santos: São Paulo, Brazil, 1983. [Google Scholar]
- Pearse, A.G.E. Histochemistry Theoretical and Applied; Churchill Livingstone: Edinburgh, UK, 1985. [Google Scholar]
- Robertson, J.; Jones, M.; Olguin, E.; Alberts, B. Bioassays with Arthropods, 3rd ed.; CRC: Boca Raton, FL, USA, 2017; ISBN 9781315373775. [Google Scholar]
- Li, Y.; Wang, Y.; Kong, W.; Yang, S.; Luo, J.; Yang, M. Illicium verum Essential Oil, a Potential Natural Fumigant in Preservation of Lotus Seeds from Fungal Contamination. Food Chem. Toxicol. 2020, 141, 111347. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, I.P.S.; Singh, B.; Singh, G.; de Heluani, C.S.; de Lampasona, M.P.; Catalan, C.A.N. Chemical Composition and Antioxidant Activity of Essential Oil and Oleoresins of Nutmeg (Myristica fragrans Houtt.) Fruits. Int. J. Food Prop 2013, 16, 1059–1070. [Google Scholar] [CrossRef] [Green Version]
- do Prado, A.C.; Garces, H.G.; Bagagli, E.; Rall, V.L.M.; Furlanetto, A.; Fernandes Junior, A.; Furtado, F.B. Schinus Molle Essential Oil as a Potential Source of Bioactive Compounds: Antifungal and Antibacterial Properties. J. Appl Microbiol. 2019, 126, 516–522. [Google Scholar] [CrossRef] [PubMed]
- Gobbo-Neto, L.; Lopes, N. Plantas Medicinais: Fatores de Influência No Conteúdo de Metabólitos Secundários. Quim. Nova 2007, 30, 374–381. [Google Scholar] [CrossRef] [Green Version]
- Blank, A.; Souza, E.; Paula, J.; Alves, P. Comportamento Fenotípico e Genotípico de Populações de Manjericão. Hortic Bras 2010, 28, 305–310. [Google Scholar] [CrossRef]
- Blenau, W.; Rademacher, E.; Baumann, A. Plant Essential Oils and Formamidines as Insecticides/Acaricides: What Are the Molecular Targets? Apidologie 2012, 43, 334–347. [Google Scholar] [CrossRef]
- Ikbal, C.; Pavela, R. Essential Oils as Active Ingredients of Botanical Insecticides against Aphids. J. Pest Sci. 2019, 92, 971–986. [Google Scholar] [CrossRef]
- Isman, M.B. Commercial Development of Plant Essential Oils and Their Constituents as Active Ingredients in Bioinsecticides. Phytochem. Rev. 2020, 19, 235–241. [Google Scholar] [CrossRef]
- Dewick, P.M. The Shikimate Pathway: Aromatic Amino Acids and Phenylpropanoids. Med. Nat. Prod. 2009, 137, 86. [Google Scholar]
- Solomons, T.W.G.; Fryhle, C.B.; Snyder, S.A. Organic Chemistry, 13th ed.; Wiley: Hoboken, NJ, USA, 2021. [Google Scholar]
- Hashem, A.S.; Awadalla, S.S.; Zayed, G.M.; Maggi, F.; Benelli, G. Pimpinella Anisum Essential Oil Nanoemulsions against Tribolium Castaneum-Insecticidal Activity and Mode of Action. Environ. Sci. Pollut. Res. Int. 2018, 25, 18802–18812. [Google Scholar] [CrossRef]
- Mattila, J.; Hietakangas, V. Regulation of Carbohydrate Energy Metabolism in Drosophila Melanogaster. Genetics 2017, 207, 1231–1253. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-K.; Wang, S.; Wang, S.-G.; Zhang, L.; Xu, Y.-X.; Guo, X.-J.; Zhang, F.; Tang, B. Effects of Starvation on the Carbohydrate Metabolism in Harmonia Axyridis (Pallas). Biol. Open 2017, 6, 1096–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, B.; Zhang, L.; Xiong, X.; Wang, H.; Wang, S. Advances in trehalose metabolism and its regulation of insect chitin synthesis. Sci. Agric. Sin. 2018, 51, 697–707. [Google Scholar]
- Canavoso, L.E.; Jouni, Z.E.; Karnas, K.J.; Pennington, J.E.; Wells, M.A. Fat Metabolism in Insects. Annu. Rev. Nutr. 2001, 21, 23–46. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.T.; Soulages, J.L.; Hariharasundaram, B.; Arrese, E.L. Activation of the Lipid Droplet Controls the Rate of Lipolysis of Triglycerides in the Insect Fat Body. J. Biol. Chem. 2005, 280, 22624–22631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrese, E.L.; Soulages, J.L. Insect Fat Body: Energy, Metabolism, and Regulation. Annu Rev Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roma, G.C.; Bueno, O.C.; Camargo-Mathias, M.I. Morpho-Physiological Analysis of the Insect Fat Body: A Review. Micron 2010, 41, 395–401. [Google Scholar] [CrossRef] [PubMed]
- McCue, M.D.; Guzman, R.M.; Passement, C.A.; Davidowitz, G. How and When Do Insects Rely on Endogenous Protein and Lipid Resources during Lethal Bouts of Starvation? A New Application for 13C-Breath Testing. PLoS ONE 2015, 10, e0140053. [Google Scholar] [CrossRef]
- Welch, K.C.J.; Péronnet, F.; Hatch, K.A.; Voigt, C.C.; McCue, M.D. Carbon Stable-Isotope Tracking in Breath for Comparative Studies of Fuel Use. Ann. N. Y. Acad. Sci. 2016, 1365, 15–32. [Google Scholar] [CrossRef]




| Illicium verum | Myristica fragrans | Schinus molle | ||||
|---|---|---|---|---|---|---|
| Mass (g) | 630 | 143 | 901 | |||
| Humidity (%) | 4.00 | 3.55 | 56.86 | |||
| Yield (%) | 2.49 ± 0.15 | 5.14 ± 0.11 | 1.08 ± 0.13 | |||
| Retention Time (min) | RIexp | RItab | Constituents | Percentage (%) | ||
| 6.223 | 925 | 924 | α-thujene | - | 1.16 | - |
| 6.466 | 933 | 932 | α-pinene | - | 17.77 | 4.39 |
| 7.610 | 972 | 969 | sabinene | - | 27.39 | 19.66 |
| 7.806 | 979 | 974 | β-pinene | - | 17.95 | 6.24 |
| 8.061 | 987 | 988 | myrcene | - | 0.61 | 0.52 |
| 8.815 | 1010 | 1002 | α-phelandrene | - | 0.54 | - |
| 9.079 | 1017 | 1014 | α-terpinene | - | 1.13 | - |
| 9.346 | 1024 | 1020 | p-cymene | - | 0.65 | 0.09 |
| 9.533 | 1029 | 1024 | limonene | 0.36 | 25.51 | 25.55 |
| 10.612 | 1057 | 1054 | γ-terpinene | - | 1.03 | - |
| 11.702 | 1085 | 1086 | terpinolene | - | 0.09 | - |
| 15.702 | 1180 | 1174 | terpinen-4-ol | - | 5.81 | - |
| 18.964 | 1255 | 1247 | p-anisaldehyde | 0.04 | - | - |
| 20.358 | 1287 | 1282 | (E)-anethole | 99.60 | - | - |
| 20.444 | 1289 | 1285 | safrole | - | 0.12 | - |
| 26.022 | 1419 | 1417 | trans- β-caryophyllene | - | - | 12.74 |
| 28.539 | 1480 | 1484 | germacrene D | - | - | 5.03 |
| 29.159 | 1495 | 1500 | bicyclogermacrene | - | - | 22.93 |
| 30.102 | 1519 | 1517 | myristicin | - | 0.23 | - |
| 32.354 | 1576 | 1577 | spathulenol | - | - | 2.84 |
| Total identified | 100 | 100 | 100 | |||
| Monoterpenic hydrocarbons | 0.36 | 93.84 | 56.45 | |||
| Sesquiterpenic hydrocarbons | - | - | 40.70 | |||
| Oxygenated monoterpenes | - | 5.81 | - | |||
| Oxygenated sesquiterpenes | - | - | 2.85 | |||
| Phenylpropanoids | 99.64 | 0.35 | - | |||
| Essential Oil | Number of Insects | LC20 (95% IF) | LC50 (95% IF) | LC95 (95% IF) | χ2 | p | TR LC50 (95% LC) |
|---|---|---|---|---|---|---|---|
| (μL mL−1) | (μL mL−1) | ||||||
| Illicium verum | 600 | 0.8 (0.6–1.0) | 1.9 (1.6–2.2) | 9.2 (7.3–12.4) | 0.3569 | 0.9859 | * |
| Myristica fragrans | 500 | 16.9 (15.1–18.6) | 26.5 (24.6–28.6) | 63.4 (54.5–78.1) | 2.5922 | 0.4589 | 13.9 (12.9–15.0) |
| Schinus molle | 500 | 19.3 (13.3–25.1) | 58.7 (49.3–68.8) | 518.5 (360.5–882.4) | 0.8475 | 0.8381 | 30.9 (25.9–36.2) |
| Essencial Oil | IC50 (mg mL−1) |
|---|---|
| Carvacrol | 0.029 ± 0.004 a |
| Illicium verum | 0.117 ± 0.002 b |
| Myristica fragrans | 0.057 ± 0.004 a |
| Schinus molle | 0.047 ± 0.002 a |
| Structures | Water + DMSO | LC20 | LC50 |
|---|---|---|---|
| Thickness of thoracic muscle fibers (μm) | 57.70 ± 5.65 a | 43.08 ± 2.16 b | 42.14 ± 0.94 b |
| Distance between thorax muscle fibers (μm) | 3.54 ± 0.27 a | 5.15 ± 0.13 b | 6.51 ± 0.12c |
| Thorax diameter (μm) | 760.80 ± 33.74 a | 737.92 ± 47.54 a | 718.18 ± 103.31 a |
| Thickness of the midgut epithelium (μm) | 37.05 ± 2.44 a | 43.34 ± 1.03 b | 47.16 ± 0.88 b |
| Average area of lipid droplets of fat body (μm2) | 42.54 ± 8.21 a | 23.25 ± 3.61 b | 16.15 ± 1.54c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Souza, L.; Cardoso, M.d.G.; Konig, I.F.M.; Ferreira, V.R.F.; Caetano, A.R.S.; Campolina, G.A.; Haddi, K. Toxicity, Histopathological Alterations and Acetylcholinesterase Inhibition of Illicium verum Essential Oil in Drosophila suzukii. Agriculture 2022, 12, 1667. https://doi.org/10.3390/agriculture12101667
de Souza L, Cardoso MdG, Konig IFM, Ferreira VRF, Caetano ARS, Campolina GA, Haddi K. Toxicity, Histopathological Alterations and Acetylcholinesterase Inhibition of Illicium verum Essential Oil in Drosophila suzukii. Agriculture. 2022; 12(10):1667. https://doi.org/10.3390/agriculture12101667
Chicago/Turabian Stylede Souza, Luciano, Maria das Graças Cardoso, Isaac Filipe Moreira Konig, Vanúzia Rodrigues Fernandes Ferreira, Alex Rodrigues Silva Caetano, Gabriela Aguiar Campolina, and Khalid Haddi. 2022. "Toxicity, Histopathological Alterations and Acetylcholinesterase Inhibition of Illicium verum Essential Oil in Drosophila suzukii" Agriculture 12, no. 10: 1667. https://doi.org/10.3390/agriculture12101667