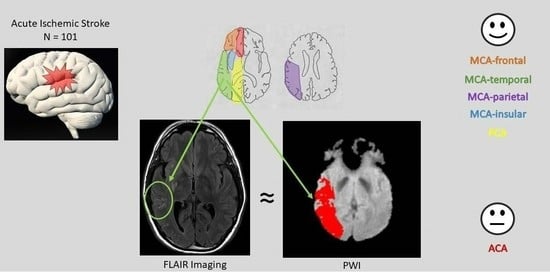
Location of Hyperintense Vessels on FLAIR Associated with the Location of Perfusion Deficits in PWI
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Imaging Acquisition
2.3. Perfusion Deficit Identification
2.3.1. FLAIR-Hyperintense Vessels
2.3.2. PWI Volume
3. Results
4. Discussion
4.1. Clinical Importance of Hypoperfusion
4.1.1. Stroke Diagnosis and Localization
4.1.2. Investigation of Neuroanatomical Function and Organization
4.2. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hillis, A.E.; Barker, P.B.; Beauchamp, N.J.; Gordon, B.; Wityk, R.J. MR Perfusion Imaging Reveals Regions of Hypoperfusion Associated with Aphasia and Neglect. Neurology 2000, 55, 782–788. [Google Scholar] [CrossRef]
- Hillis, A.E.; Wityk, R.J.; Barker, P.B.; Beauchamp, N.J.; Gailloud, P.; Murphy, K.; Cooper, O.; Metter, E.J. Subcortical Aphasia and Neglect in Acute Stroke: The Role of Cortical Hypoperfusion. Brain 2002, 125, 1094–1104. [Google Scholar] [CrossRef] [Green Version]
- Hillis, A.E.; Wityk, R.J.; Barker, P.B.; Caramazza, A. Neural Regions Essential for Writing Verbs. Nat. Neurosci. 2003, 6, 19–20. [Google Scholar] [CrossRef]
- Ramalho, J.; Semelka, R.C.; Ramalho, M.; Nunes, R.H.; AlObaidy, M.; Castillo, M. Gadolinium-Based Contrast Agent Accumulation and Toxicity: An Update. Am. J. Neuroradiol. 2016, 37, 1192–1198. [Google Scholar] [CrossRef] [Green Version]
- Hasebroock, K.M.; Serkova, N.J. Toxicity of MRI and CT Contrast Agents. Expert Opin. Drug Metab. Toxicol. 2009, 5, 403–416. [Google Scholar] [CrossRef]
- Kanda, T.; Ishii, K.; Kawaguchi, H.; Kitajima, K.; Takenaka, D. High Signal Intensity in the Dentate Nucleus and Globus Pallidus on Unenhanced T1-Weighted MR Images: Relationship with Increasing Cumulative Dose of a Gadolinium-Based Contrast Material. Radiology 2014, 270, 834–841. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z. Improving Cerebral Blood Flow Quantification for Arterial Spin Labeled Perfusion MRI by Removing Residual Motion Artifacts and Global Signal Fluctuations. Magn. Reson. Imaging 2012, 30, 1409–1415. [Google Scholar] [CrossRef] [Green Version]
- Xie, D.; Li, Y.; Yang, H.; Bai, L.; Wang, T.; Zhou, F.; Zhang, L.; Wang, Z. Denoising Arterial Spin Labeling Perfusion MRI with Deep Machine Learning. Magn. Reson. Imaging 2020, 68, 95–105. [Google Scholar] [CrossRef]
- Jezzard, P.; Chappell, M.A.; Okell, T.W. Arterial Spin Labeling for the Measurement of Cerebral Perfusion and Angiography. J. Cereb. Blood. Flow Metab. 2018, 38, 603–626. [Google Scholar] [CrossRef]
- Reyes, D.; Simpkins, A.N.; Hitomi, E.; Lynch, J.K.; Hsia, A.W.; Nadareishvili, Z.; Luby, M.; Latour, L.L.; Leigh, R. Estimating Perfusion Deficits in Acute Stroke Patients without Perfusion Imaging. Stroke 2022, 53, 3439–3445. [Google Scholar] [CrossRef]
- Toyoda, K.; Ida, M.; Fukuda, K. Fluid-Attenuated Inversion Recovery Intraarterial Signal: An Early Sign of Hyperacute Cerebral Ischemia. Am. J. Neuroradiol. 2001, 22, 1021–1029. [Google Scholar]
- Kamran, S.; Bates, V.; Bakshi, R.; Wright, P.; Kinkel, W.; Miletich, R. Significance of Hyperintense Vessels on FLAIR MRI in Acute Stroke. Neurology 2000, 55, 265–269. [Google Scholar] [CrossRef]
- Bunker, L.D.; Walker, A.; Meier, E.; Goldberg, E.; Leigh, R.; Hillis, A.E. Hyperintense Vessels on Imaging Account for Neurological Function Independent of Lesion Volume in Acute Ischemic Stroke. NeuroImage Clin. 2022, 34, 102991. [Google Scholar] [CrossRef]
- Akoglu, H. User’s Guide to Correlation Coefficients. Turk. J. Emerg. Med. 2018, 18, 91–93. [Google Scholar] [CrossRef]
- Astrup, J.; Siesjo, B.K.; Symon, L. Thresholds in Cerebral Ischemia—The Ischemic Penumbra. Stroke 1981, 12, 723–725. [Google Scholar] [CrossRef] [Green Version]
- Hillis, A.E.; Baron, J.-C. Editorial: The Ischemic Penumbra: Still the Target for Stroke Therapies? Front. Neurol. 2015, 6, 85. [Google Scholar] [CrossRef]
- Hillis, A.E.; Rorden, C.; Fridriksson, J. Brain Regions Essential for Word Comprehension: Drawing Inferences from Patients. Ann. Neurol. 2017, 81, 759–768. [Google Scholar] [CrossRef]
- Ross, E.D.; Monnot, M. Neurology of Affective Prosody and Its Functional-Anatomic Organization in Right Hemisphere. Brain Lang. 2008, 104, 51–74. [Google Scholar] [CrossRef]
- Tippett, D.C.; Godin, B.R.; Oishi, K.; Oishi, K.; Davis, C.; Gomez, Y.; Trupe, L.A.; Kim, E.H.; Hillis, A.E. Impaired Recognition of Emotional Faces after Stroke Involving Right Amygdala or Insula. Semin. Speech Lang. 2018, 39, 87–100. [Google Scholar] [CrossRef]
- Sheppard, S.M.; Meier, E.L.; Zezinka Durfee, A.; Walker, A.; Shea, J.; Hillis, A.E. Characterizing Subtypes and Neural Correlates of Receptive Aprosodia in Acute Right Hemisphere Stroke. Cortex 2021, 141, 36–54. [Google Scholar] [CrossRef]
- Leigh, R.; Oishi, K.; Hsu, J.; Lindquist, M.; Gottesman, R.F.; Jarso, S.; Crainiceanu, C.; Mori, S.; Hillis, A.E. Acute Lesions That Impair Affective Empathy. Brain 2013, 136, 2539–2549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nogueira, R.G.; Jadhav, A.P.; Haussen, D.C.; Bonafe, A.; Budzik, R.F.; Bhuva, P.; Yavagal, D.R.; Ribo, M.; Cognard, C.; Hanel, R.A.; et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018, 378, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Hillis, A.E.; Ulatowski, J.A.; Barker, P.B.; Torbey, M.; Ziai, W.; Beauchamp, N.J.; Oh, S.; Wityk, R.J. A Pilot Randomized Trial of Induced Blood Pressure Elevation: Effects on Function and Focal Perfusion in Acute and Subacute Stroke. Cereb. Dis. 2003, 16, 236–246. [Google Scholar] [CrossRef] [PubMed]
- Wityk, R.J.; Hillis, A.; Beauchamp, N.; Barker, P.B.; Rigamonti, D. Perfusion-Weighted Magnetic Resonance Imaging in Adult Moyamoya Syndrome: Characteristic Patterns and Change after Surgical Intervention: Case Report. Neurosurgery 2002, 51, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- Donnan, G.A.; Davis, S.M. Neuroimaging, the Ischaemic Penumbra, and Selection of Patients for Acute Stroke Therapy. Lancet Neurol. 2002, 1, 417–425. [Google Scholar] [CrossRef]
- Powers, W.J.; Rabinstein, A.A.; Ackerson, T.; Adeoye, O.M.; Bambakidis, N.C.; Becker, K.; Biller, J.; Brown, M.; Demaerschalk, B.M.; Hoh, B.; et al. Guidelines for the Early Management of Patients with Acute Ischemic Stroke: 2019 Update to the 2018 Guidelines for the Early Management of Acute Ischemic Stroke: A Guideline for Healthcare Professionals From the American Heart Association/American Stroke Association. Stroke 2019, 50, e344–e418. [Google Scholar] [CrossRef]
- Noguchi, K.; Ogawa, T.; Inugami, A.; Fujita, H.; Hatazawa, J.; Shimosegawa, E.; Okudera, T.; Uemura, K.; Seto, H. MRI of Acute Cerebral Infarction: A Comparison of FLAIR and T2-Weighted Fast Spin-Echo Imaging. Neuroradiology 1997, 39, 406–410. [Google Scholar] [CrossRef]
- Thomalla, G.; Rossbach, P.; Rosenkranz, M.; Siemonsen, S.; Krützelmann, A.; Fiehler, J.; Gerloff, C. Negative Fluid-Attenuated Inversion Recovery Imaging Identifies Acute Ischemic Stroke at 3 Hours or Less. Ann. Neurol. 2009, 65, 724–732. [Google Scholar] [CrossRef]
- Lee, K.Y.; Latour, L.L.; Luby, M.; Hsia, A.W.; Merino, J.G.; Warach, S. Distal Hyperintense Vessels on FLAIR. Neurology 2009, 72, 1134–1139. [Google Scholar] [CrossRef] [Green Version]
- Legrand, L.; Tisserand, M.; Turc, G.; Naggara, O.; Edjlali, M.; Mellerio, C.; Mas, J.-L.; Méder, J.-F.; Baron, J.-C.; Oppenheim, C. Do FLAIR Vascular Hyperintensities beyond the DWI Lesion Represent the Ischemic Penumbra? AJNR Am. J. Neuroradiol. 2015, 36, 269–274. [Google Scholar] [CrossRef] [Green Version]
- Hillis, A.E.; Barker, P.B.; Beauchamp, N.J.; Winters, B.D.; Mirski, M.; Wityk, R.J. Restoring Blood Pressure Reperfused Wernicke’s Area and Improved Language. Neurology 2001, 56, 670–672. [Google Scholar] [CrossRef] [PubMed]
- Hillis, A.E.; Kane, A.; Tuffiash, E.; Ulatowski, J.A.; Barker, P.B.; Beauchamp, N.J.; Wityk, R.J. Reperfusion of Specific Brain Regions by Raising Blood Pressure Restores Selective Language Functions in Subacute Stroke. Brain Lang. 2001, 79, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Hillis, A.E.; Barker, P.B.; Wityk, R.J.; Aldrich, E.M.; Restrepo, L.; Breese, E.L.; Work, M. Variability in Subcortical Aphasia Is Due to Variable Sites of Cortical Hypoperfusion. Brain Lang. 2004, 89, 524–530. [Google Scholar] [CrossRef] [PubMed]
- Kazui, S.; Sawada, T.; Naritomi, H.; Kuriyama, Y.; Yamaguchi, T. Angiographic Evaluation of Brain Infarction Limited to the Anterior Cerebral Artery Territory. Stroke 1993, 24, 549–553. [Google Scholar] [CrossRef] [Green Version]
- Bogousslavsky, J. Anterior Cerebral Artery Territory Infarction in the Lausanne Stroke Registry: Clinical and Etiologic Patterns. Arch. Neurol. 1990, 47, 144–150. [Google Scholar] [CrossRef]
- Stein, C.; Bunker, L.; Chu, B.; Leigh, R.; Faria, A.; Hillis, A.E. Various Tests of Left Neglect Are Associated with Distinct Territories of Hypoperfusion in Acute Stroke. Brain Commun. 2022, 4, fcac064. [Google Scholar] [CrossRef]
- National Institute of Neurological Disorders and Stroke. NIH Stroke Scale; National Institute of Neurological Disorders and Stroke, Department of Health and Human Services: Washington, DC, USA, 2011.
- Sato, S.; Toyoda, K.; Uehara, T.; Toratani, N.; Yokota, C.; Moriwaki, H.; Naritomi, H.; Minematsu, K. Baseline NIH Stroke Scale Score Predicting Outcome in Anterior and Posterior Circulation Strokes. Neurology 2008, 70, 2371–2377. [Google Scholar] [CrossRef]
- Woo, D.; Broderick, J.P.; Kothari, R.U.; Lu, M.; Brott, T.; Lyden, P.D.; Marler, J.R.; Grotta, J.C. Does the National Institutes of Health Stroke Scale Favor Left Hemisphere Strokes? Stroke 1999, 30, 2355–2359. [Google Scholar] [CrossRef] [Green Version]
- Fink, J.N.; Selim, M.H.; Kumar, S.; Silver, B.; Linfante, I.; Caplan, L.R.; Schlaug, G. Is the Association of National Institutes of Health Stroke Scale Scores and Acute Magnetic Resonance Imaging Stroke Volume Equal for Patients with Right- and Left-Hemisphere Ischemic Stroke? Stroke 2002, 33, 954–958. [Google Scholar] [CrossRef] [Green Version]


| Summary Statistics (n = 101) | |
|---|---|
| Age (Median (Range)) | 73 (58–83) |
| Sex (female, male) | 48, 53 |
| PWI volume (mL; M(SD)) | 37 (±56) |
| DWI volume (mL; M(SD)) † | 18 (±24) |
| NIH-FHV (median (range)) | 4 (1–6) |
| NIHSS (median (range)) | 8 (4–17) |
| Stroke Risk Factors | |
| HTN | n = 73 (72.3%) |
| HLD | n = 34 (33.7%) |
| DM | n = 20 (19.8%) |
| CAD | n = 15 (14.9%) |
| A-Fib | n = 31 (30.7%) |
| Smoking | n = 12 (11.9%) |
| Vascular Region | Chi-Square (Χ2) | p-Value | Cramér’s V |
|---|---|---|---|
| ACA | 5.29 | 0.02 | 0.23 |
| PCA | 12.06 | 0.001 * | 0.35 |
| MCA-F | 33.74 | 0.000 * | 0.58 |
| MCA-T | 36.58 | 0.000 * | 0.60 |
| MCA-P | 15.48 | 0.000 * | 0.39 |
| MCA-I | 28.55 | 0.000 * | 0.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bunker, L.D.; Hillis, A.E. Location of Hyperintense Vessels on FLAIR Associated with the Location of Perfusion Deficits in PWI. J. Clin. Med. 2023, 12, 1554. https://doi.org/10.3390/jcm12041554
Bunker LD, Hillis AE. Location of Hyperintense Vessels on FLAIR Associated with the Location of Perfusion Deficits in PWI. Journal of Clinical Medicine. 2023; 12(4):1554. https://doi.org/10.3390/jcm12041554
Chicago/Turabian StyleBunker, Lisa D., and Argye E. Hillis. 2023. "Location of Hyperintense Vessels on FLAIR Associated with the Location of Perfusion Deficits in PWI" Journal of Clinical Medicine 12, no. 4: 1554. https://doi.org/10.3390/jcm12041554