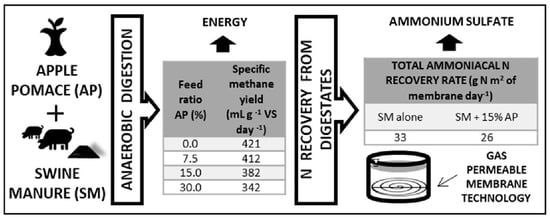
Energy and Nutrients from Apple Waste Using Anaerobic Digestion and Membrane Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Origin and Characterization of Apple Pomace, Manure, and Inoculum
2.2. Experimental Set-Up
2.2.1. Semi-Continuous Co-Digestion of Different Mixtures
2.2.2. Nitrogen Recovery from Digestate Using Gas-Permeable Membranes
2.3. Analytical Methods and Statistical Analysis
3. Results and Discussion
3.1. Semi-Continuous Co-Digestion of Different Mixtures
3.2. Nitrogen Recovery with GPM Technology
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shahbandeh, M. Apple Production Worldwide 2010–2020. 2022. Available online: https://www.statista.com/statistics/961248/production-of-apples-worldwide/ (accessed on 2 March 2022).
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of apple processing wastes as low-cost substrates for bioproduction of high value products: A review. Renew. Sust. Energ. Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Yates, M.; Gomez, M.R.; Martin-Luengo, M.A.; Ibáñez, V.Z.; Serrano, A.M.M. Multivalorization of apple pomace towards materials and chemicals. Waste to wealth. J. Clean. Prod. 2016, 143, 847–853. [Google Scholar] [CrossRef]
- Buckwell, A.; Nadeu, E. Nutrient Recovery and Reuse (NRR) in European Agriculture. A Review of the Issues, Opportunities, and Actions; RISE Foundation: Brussels, Belgium, 2016. [Google Scholar]
- Ampese, L.C.; Sganzerla, W.G.; Ziero, H.D.D.; Mudhoo, A.; Martins, G.; Forster-Carneiro, T. Research progress, trends, and updates on anaerobic digestion technology: A bibliometric analysis. J. Clean. Prod. 2022, 331, 130004. [Google Scholar] [CrossRef]
- Riaño, B.; Molinuevo-Salces, B.; Vanotti, M.B.; García-González, M.C. Ammonia Recovery from Digestate Using Gas-Permeable Membranes: A Pilot-Scale Study. Environments 2021, 8, 133. [Google Scholar] [CrossRef]
- European Commission. Farm to Fork Strategy; For a Fair, Healthy and Environmentally-Friendly Food System; European Commission: Brussels, Belgium, 2022. Available online: https://ec.europa.eu/food/system/files/2020-05/f2f_action-plan_2020_strategy-info_en.pdf (accessed on 1 May 2020).
- Labatut, R.A.; Angenent, L.T.; Scott, N.R. Biochemical methane potential and biodegradability of complex organic substrates. Bioresour. Technol. 2011, 201, 225–2264. [Google Scholar] [CrossRef]
- Bres, P.; Beily, M.E.; Young, B.J.; Gasulla, J.; Butti, M.; Crespo, D.; Candal, R.; Komilis, D. Performance of semi-continuous anaerobic co-digestion of poultry manure with fruit and vegetable waste and analysis of digestate quality: A bench scale study. Waste Manag. 2018, 82, 276–284. [Google Scholar] [CrossRef]
- Riaño, B.; Molinuevo-Salces, B.; Parralejo, A.; Royano, L.; González-Cortés, J.; García-González, M. Techno-economic evaluation of anaerobic co-digestion of pepper waste and swine manure. Biomass Conv. Bioref. 2021, 2190–6823. [Google Scholar] [CrossRef]
- Riggio, V.; Comino, E.; Rosso, M. Energy production from anaerobic co-digestion processing of cow slurry, olive pomace and apple pulp. Renew. Energ. 2015, 83, 1043–1049. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Hijosa-Valsero, M.; González-García, I.; Paniagua-García, A.I.; Hernández, D.; Garita-Cambronero, J.; Díez-Antolínez, R.; García-González, M.C. Valorization of Apple Pomaces for Biofuel Production: A Biorefinery Approach. Biomass Bioenergy 2020, 142, 105785. [Google Scholar] [CrossRef]
- Kafle, G.H.; Kim, S.H. Anaerobic treatment of apple waste with swine manure for biogas production: Batch and continuous operation. Appl. Energy 2013, 103, 61–72. [Google Scholar] [CrossRef]
- European Commission. Council Directive 91/676/EEC Concerning the Protection of Waters against Pollution Caused by Nitrates from Agricultural Sources; European Commission: Brussels, Belgium, 1991.
- Gontard, N.; Sonesson, U.; Birkved, M.; Majone, M.; Bolzonella, D.; Celli, A.; Angellier-Coussy, H.; Jang, G.-W.; Verniquet, A.; Jan Broeze, J.; et al. A research challenge vision regarding management of agricultural waste in a circular bio-based economy. Crit. Rev. Environ. Sci. Technol. 2018, 48, 614–654. [Google Scholar] [CrossRef]
- Romero-Güiza, M.S.; Mata-Alvarez, J.; Chimenos Rivera, J.M.; Astals-Garcia, S. Nutrient recovery technologies for anaerobic digestion systems: An overview. Rev. ION 2016, 29, 7–26. [Google Scholar] [CrossRef]
- Beckinghausen, A.; Odlare, M.; Thorin, E.; Schwede, S. From removal to recovery: An evaluation of nitrogen recovery techniques from wastewater. Appl. Energy 2020, 263, 114616. [Google Scholar] [CrossRef]
- Munasinghe-Arachchige, S.; Nirmalakhandant, N. Nitrogen-Fertilizer Recovery from the Centrate of Anaerobically Digested Sludge. Environ. Sci. Technol. Lett. 2020, 7, 450–459. [Google Scholar] [CrossRef]
- Pandey, B.; Chen, L. Technologies to recover nitrogen from livestock manure-A review. Sci. Total Environ. 2021, 784, 147098. [Google Scholar] [CrossRef] [PubMed]
- Vanotti, M.B.; Szogi, A.A. Use of gas-permeable membranes for the removal and recovery of ammonia from high strength livestock wastewater. In Proceedings of the Water Environment Federation Recovery and Management Conference, Miami, FL, USA, 9–12 January 2011. [Google Scholar]
- Vanotti, M.B.; Szogi, A.A. Systems and Methods for Reducing Ammonia Emissions from Liquid Effluents and for Recovering the Ammonia. U.S. Patent No. 9,005,333 B1, 14 April 2015. [Google Scholar]
- García-González, M.C.; Vanotti, M.B. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of waste strength and pH. Waste Manag. 2015, 38, 455–461. [Google Scholar] [CrossRef] [PubMed]
- García-González, M.C.; Vanotti, M.B.; Szogi, A.A. Recovery of ammonia from swine manure using gas-permeable membranes: Effect of aeration. J. Environ. Manag. 2015, 152, 19–26. [Google Scholar] [CrossRef] [PubMed]
- García-González, M.C.; Vanotti, M.B.; Szogi, A.A. Recovery of ammonia from anaerobically digested manure using gas-permeable membranes. Sci. Agri. 2016, 73, 434–438. [Google Scholar] [CrossRef]
- Molinuevo-Salces, B.; Riaño, B.; Vanotti, M.; García-González, M.C. Gas-permeable membrane technology coupled with anaerobic digestion for swine manure treatment. Front. Sustain. Food Syst. 2018, 2, 25. [Google Scholar] [CrossRef]
- Oliveira-Filho, J.; Daguerre-Martini, S.; Vanotti, M.; Saez-Tovar, J.; Rosal, A.; Perez-Murcia, M.; Bustamante, M.A.; Moral, R. Recovery of Ammonia in Raw and Co-digested Swine Manure Using Gas-Permeable Membrane Technology. Front. Sustain. Food Syst. 2018, 2, 30. [Google Scholar] [CrossRef] [Green Version]
- Hansen, K.; Angelidaki, I.; Ahring, B.K. Anaerobic digestion of swine manure: Inhibition by ammonia. Water Res. 1998, 32, 5–12. [Google Scholar] [CrossRef]
- APHA. Standard Methods for the Examination of Water and Wastewater, 21st ed.; American Public Health Association, American Water Works Association, Water Environment Federation: Washington, DC, USA, 2005. [Google Scholar]
- Ripley, W.; Converse, J.C. Improved Alkalimetric Monitoring for Anaerobic Digestion of High-Strength Wastes. J. Water Pollut. Control Fed. 1986, 58, 406–411. [Google Scholar]
- Pan, S.-Y.; Tsai, C.-Y.; Liu, C.-W.; Wang, S.-W.; Kim, H.; Fan, C. Anaerobic co-digestion of agricultural wastes toward circular bioeconomy. iScience 2021, 24, 102704. [Google Scholar] [CrossRef] [PubMed]
- Dhillon, G.S.; Kaur, S.; Sarma, S.J.; Brar, S.K. Integrated process for fungal citric acid fermentation using apple processing wastes and sequential extraction of chitosan from waste stream. Ind. Crops Prod. 2013, 50, 346–351. [Google Scholar] [CrossRef]
- Sudha, M.L. Apple Pomace (By-Product of Fruit Juice Industry) as a Flour Fortification Strategy. In Flour and Breads and their Fortification in Health and Disease Prevention; Academic Press: Cambridge, MA, USA, 2011; pp. 395–405. [Google Scholar] [CrossRef]
- Drosg, B.; Braun, R.; Bochmann, G.; Saedi, T.A. Analysis and characterisation of biogas feedstocks. In The Biogas Handbook; Woodhead Publishing Series in Energy; Woodhead Publishing: Sawston, UK, 2013; pp. 52–84. [Google Scholar] [CrossRef]







| R1 | R2 | |||||
|---|---|---|---|---|---|---|
| Parameters | Initial Period | Period I | Period II | Initial Period | Period I | Period II |
| Apple pomace (%) | 0 | 0 | 7.5 | 15 | 15 | 30 |
| pH | 7.19 (0.14) | 7.40 (0.36) | 6.98 (0.24) | 7.19 (0.14) | 7.40 (0.36) | 6.98 (0.24) |
| TA (mg CaCO3 L−1) | 11882 (1565) | 13626 (3268) | 8453 (2007) | 11882 (1565) | 13626 (3268) | 8453 (2007) |
| TCOD(g L −1) | 107.11 (60.54) | 92.00 (23.43) | 99.90 (25.23) | 89.58 (60.18) | 81.06 (17.32) | 90.09 (16.60) |
| SCOD (g L −1) | 32.51 (5.27) | 36.39 (4.81) | 37.81 (1.79) | 31.77 (3.42) | 32.26 (6.91) | 35.92 (6.77) |
| TS (g L −1) | 40.51 (0.41) | 42.79 (10.98) | 50.99 (12.69) | 43.68 (2.03) | 38.43 (11.34) | 44.95 (9.57) |
| VS (g L −1) | 28.95 (4.11) | 24.83 (6.53) | 36.74 (12.86) | 29.15 (1.92) | 21.91 (5.49) | 32.68 (9.75) |
| TVFA(mg TCOD L−1) | n.d. | 26243 (5839) | 31883 (1421) | n.d. | 19219 (394) | 26735 (6218) |
| TKN (mg N L −1) | 5121 (391) | 5085 (823) | 5048 (670) | 4665 (289) | 4519 (465) | 4235 (628) |
| TAN (mg N L −1) | 3806 (179) | 3793 (193) | 3874 (424) | 3286 (118) | 3325 (221) | 3182 (480) |
| R1 | R2 | |||
|---|---|---|---|---|
| Parameters | Period I | Period II | Period I | Period II |
| Apple pomace (%) | 0.0 | 7.5 | 15.0 | 30.0 |
| Biogas (mL day−1) | 3057 (1255) a | 3529 (542) b | 2828 (1135) a | 3030 (1130) a |
| Methane (CH4) (%) | 61.57 (4.28) a | 61.96 (1.23) a | 59.28 (2.04) ab | 57.96 (3.02) b |
| Specific methane yield (mL g VS−1 day −1) | 421.7 (153.6) a | 412.3 (62.6) a | 381.8 (134.1) ab | 341.9 (78.1) b |
| TCOD reduction (%) | 52.78 (13.43) a | 51.27 (11.50) a | 57.01 (12.07) a | 54.85 (15.10) a |
| SCOD reduction (%) | 48.57 (14.42) a | 58.94 (11.53) b | 67.13 (7.13) cd | 70.58 (7.79) d |
| VS reduction (%) | 32.29 (15.01) a | 44.27 (15.98) b | 31.05 (10.48) ab | 39.71 (14.51) ab |
| TVFA (mg TCOD L−1) | 5755 (18) a | 1767 (586) b | 1661 (119) b | 144 (9) c |
| IA/PA ratio | 0.23 (0.11) a | 0.15 (0.05) b | 0.22 (0.14) b | 0.25 (0.07) b |
| TAN in Manure (mg N) | TAN in the Acidic Solution (mg N) | TAN Removal Efficiency (%) | TAN Recovery Efficiency (%) | Average TAN Recovery Rate (g N m−2 day−1) | ||
|---|---|---|---|---|---|---|
| D-R1 | Initial | 5768 (0) | 0 (0) | 97.6 | 77.2 | 32.9 |
| Final | 133 (44) | 4383 (42) | ||||
| D-R2 | Initial | 4416 (73) | 0 (0) | 97.8 | 75.8 | 25.8 |
| Final | 98 (17) | 3434 (136) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-García, I.; Riaño, B.; Molinuevo-Salces, B.; García-González, M.C. Energy and Nutrients from Apple Waste Using Anaerobic Digestion and Membrane Technology. Membranes 2022, 12, 897. https://doi.org/10.3390/membranes12090897
González-García I, Riaño B, Molinuevo-Salces B, García-González MC. Energy and Nutrients from Apple Waste Using Anaerobic Digestion and Membrane Technology. Membranes. 2022; 12(9):897. https://doi.org/10.3390/membranes12090897
Chicago/Turabian StyleGonzález-García, Isabel, Berta Riaño, Beatriz Molinuevo-Salces, and María Cruz García-González. 2022. "Energy and Nutrients from Apple Waste Using Anaerobic Digestion and Membrane Technology" Membranes 12, no. 9: 897. https://doi.org/10.3390/membranes12090897