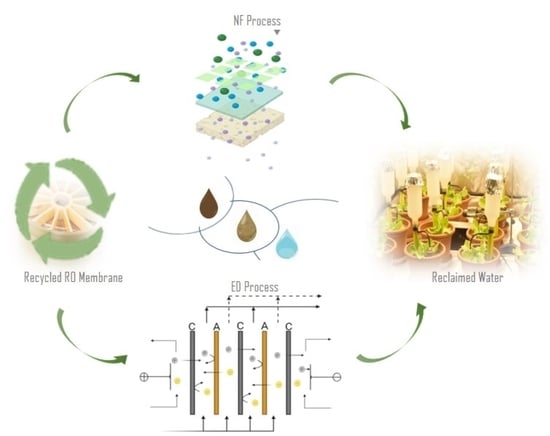
Validation of Recycled Nanofiltration and Anion-Exchange Membranes for the Treatment of Urban Wastewater for Crop Irrigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Sample
2.2. Chemical Reagents
2.3. Preparation of Membranes
2.3.1. Recycled Nanofiltration Membranes
2.3.2. Recycled Anion-Exchange Membranes
2.4. Membrane Performance in UWW Treatment
2.4.1. Nanofiltration Experiments
2.4.2. Electrodialysis Experiments
2.5. Irrigation of Lettuce
Statistical Analysis
2.6. Analytical Methods
3. Results
3.1. UWW Treatment by rNF Membranes
3.2. UWW Treatment by ED Applying rAEM
3.2.1. Determination of the Operating Voltage and LCD
3.2.2. rAEM Evaluation
3.3. Water Quality for Crop Irrigation
3.4. Lettuce Yield and Macronutrient Uptake in Dry Weight
4. Conclusions
- The rNF membrane showed a high selective rejection of divalent ions (i.e., SO42− (>96%); Ca2+ and Mg2+ (>93%)).
- Comparison between rAEM and commercial anion-exchange membranes (Ralex®) showed a suitable demineralization rate for irrigation of crops without compromising the power consumption.
- Both tested recycled membranes showed adequate potential in wastewater treatment for crop irrigation purposes in terms of conductivity and SAR value. No significant differences in individual macronutrients such as total N, P, Ca, and Mg of leaves of the lettuce of each treatment studied were observed.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Willis, R.M.; Stewart, R.A.; Williams, P.R.; Hacker, C.H.; Emmonds, S.C.; Capati, G. Residential Potable and Recycled Water End Uses in a Dual Reticulated Supply System. Desalination 2011, 272, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Racar, M.; Dolar, D.; Karadakić, K.; Čavarović, N.; Glumac, N.; Ašperger, D.; Košutić, K. Challenges of Municipal Wastewater Reclamation for Irrigation by MBR and NF/RO: Physico-Chemical and Microbiological Parameters, and Emerging Contaminants. Sci. Total Environ. 2020, 722, 137959. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, A.; Parida, V.K.; Majumder, A.; Gupta, B.; Gupta, A.K. Treatment of Saline Wastewater Using Physicochemical, Biological, and Hybrid Processes: Insights into Inhibition Mechanisms, Treatment Efficiencies and Performance Enhancement. J. Environ. Chem. Eng. 2021, 9, 105775. [Google Scholar] [CrossRef]
- Ayers, R.S.; Westcot, D.W. Water Quality for Agriculture; Food and Agriculture Organization of the United Nations: Rome, Italy, 1985; Volume 1, ISBN 9251022631. [Google Scholar]
- Ahmad, N.N.; Ang, W.L.; Leo, C.P.; Mohammad, A.W.; Hilal, N. Current Advances in Membrane Technologies for Saline Wastewater Treatment: A Comprehensive Review. Desalination 2021, 517, 115170. [Google Scholar] [CrossRef]
- Goodman, N.B.; Taylor, R.J.; Xie, Z.; Gozukara, Y.; Clements, A. A Feasibility Study of Municipal Wastewater Desalination Using Electrodialysis Reversal to Provide Recycled Water for Horticultural Irrigation. Desalination 2013, 317, 77–83. [Google Scholar] [CrossRef]
- Chojnacka, K.; Witek-Krowiak, A.; Moustakas, K.; Skrzypczak, D.; Mikula, K.; Loizidou, M. A Transition from Conventional Irrigation to Fertigation with Reclaimed Wastewater: Prospects and Challenges. Renew. Sustain. Energy Rev. 2020, 130, 109959. [Google Scholar] [CrossRef]
- Anis, S.F.; Hashaikeh, R.; Hilal, N. Microfiltration Membrane Processes: A Review of Research Trends over the Past Decade. J. Water Process Eng. 2019, 32, 100941. [Google Scholar] [CrossRef]
- Al Aani, S.; Mustafa, T.N.; Hilal, N. Ultrafiltration Membranes for Wastewater and Water Process Engineering: A Comprehensive Statistical Review over the Past Decade. J. Water Process Eng. 2020, 35, 101241. [Google Scholar] [CrossRef]
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration Membranes Review: Recent Advances and Future Prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Van Der Bruggen, B.; Koninckx, A.; Vandecasteele, C. Separation of Monovalent and Divalent Ions from Aqueous Solution by Electrodialysis and Nanofiltration. Water Res. 2004, 38, 1347–1353. [Google Scholar] [CrossRef]
- European Commission EUR-Lex—52019DC0640—EN—EUR-Lex. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1576150542719&uri=COM%3A2019%3A640%3AFIN (accessed on 3 May 2022).
- Rodríguez, J.J.; Jiménez, V.; Trujillo, O.; Veza, J. Reuse of Reverse Osmosis Membranes in Advanced Wastewater Treatment. Desalination 2002, 150, 219–225. [Google Scholar] [CrossRef]
- Veza, J.M.; Rodriguez-Gonzalez, J.J. Second Use for Old Reverse Osmosis Membranes: Wastewater Treatment. Desalination 2003, 157, 65–72. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Molina, S.; Rodríguez-Sáez, L.; Teli, S.B.; García-Calvo, E. Transformation of End-of-Life RO Membranes into NF and UF Membranes: Evaluation of Membrane Performance. J. Membr. Sci. 2015, 495, 305–315. [Google Scholar] [CrossRef]
- Molina, S.; García-Pacheco, R.; Rodríguez-Sáez, L.; García-Calvo, E.C.; Zarzo, D.; González, J.; Abajo, J. Transformation of End-of-Life RO Membranes into Recycled NF and UF Membranes, Surface Characterization. Idawc15 2015. Available online: https://www.researchgate.net/publication/305999265 (accessed on 3 May 2022).
- Ahmed, J.; Jamal, Y. A Pilot Application of Recycled Discarded RO Membranes for Low Strength Gray Water Reclamation. Environ. Sci. Pollut. Res. 2021, 28, 34042–34050. [Google Scholar] [CrossRef] [PubMed]
- García-Pacheco, R.; Landaburu-Aguirre, J.; Terrero-Rodríguez, P.; Campos, E.; Molina-Serrano, F.; Rabadán, J.; Zarzo, D.; García-Calvo, E. Validation of Recycled Membranes for Treating Brackish Water at Pilot Scale. Desalination 2018, 433, 199–208. [Google Scholar] [CrossRef]
- García-Pacheco, R.; Lawler, W.; Landaburu-Aguirre, J.; García-Calvo, E.; Le-Clech, P. 4.14 End-of-Life Membranes: Challenges and Opportunities. In Comprehensive Membrane Science and Engineering; Elsevier: Amsterdam, The Netherlands, 2017; pp. 293–310. [Google Scholar]
- Lejarazu-Larrañaga, A.; Molina, S.; Ortiz, J.M.; Navarro, R.; García-Calvo, E. Circular Economy in Membrane Technology: Using End-of-Life Reverse Osmosis Modules for Preparation of Recycled Anion Exchange Membranes and Validation in Electrodialysis. J. Membr. Sci. 2020, 593, 117423. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Ortiz, J.M.; Molina, S.; Zhao, Y.; García-Calvo, E. Nitrate-Selective Anion Exchange Membranes Prepared Using Discarded Reverse Osmosis Membranes as Support. Membranes 2020, 10, 377. [Google Scholar] [CrossRef] [PubMed]
- Strathmann, H. Electrodialysis, a Mature Technology with a Multitude of New Applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- Baker, R.W.; Wijmans, J.G.; Huang, Y. Permeability, Permeance and Selectivity: A Preferred Way of Reporting Pervaporation Performance Data. J. Membr. Sci. 2010, 348, 346–352. [Google Scholar] [CrossRef]
- Mowlid Nur, H.; Yüzer, B.; Aydin, M.İ.; Aydin, S.; Öngen, A.; Selçuk, H. Desalination and Fate of Nutrient Transport in Domestic Wastewater Using Electrodialysis Membrane Process. Desalin. Water Treat 2019, 172, 323–329. [Google Scholar] [CrossRef]
- Gherasim, C.V.; Křivčík, J.; Mikulášek, P. Investigation of Batch Electrodialysis Process for Removal of Lead Ions from Aqueous Solutions. Chem. Eng. J. 2014, 256, 324–334. [Google Scholar] [CrossRef]
- Merkel, A.; Ashrafi, A.M.; Ečer, J. Bipolar Membrane Electrodialysis Assisted PH Correction of Milk Whey. J. Membr. Sci. 2018, 555, 185–196. [Google Scholar] [CrossRef]
- Chemical and Physical Characteristics of Standard Soils According to GLP. Available online: https://www.lufa-speyer.de/ (accessed on 30 March 2022).
- Driscoll, W.C. Robustness of the ANOVA and Tukey-Kramer Statistical Tests. Comput. Ind. Eng. 1996, 31, 265–268. [Google Scholar] [CrossRef]
- American Public Health Association; American Water Works Association; Water Environment Federation. APHA Method 4500-NO3: Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1992; Volume 552, ISBN 0-87553-207-1. [Google Scholar]
- Oregon State University; Washington State University; University of Idaho; Hopkins, B.G.; Horneck, D.A.; Stevens, R.G.; Ellsworth, J.W.; Sullivan, D.M.; Pacific Northwest Cooperative Extension. Managing Irrigation Water Quality for Crop Production in the Pacific Northwest. Available online: https://ir.library.oregonstate.edu/concern/administrative_report_or_publications/w3763710v?locale=en (accessed on 14 February 2022).
- Xu, X.; He, Q.; Ma, G.; Wang, H.; Nirmalakhandan, N.; Xu, P. Selective Separation of Mono- and Di-Valent Cations in Electrodialysis during Brackish Water Desalination: Bench and Pilot-Scale Studies. Desalination 2018, 428, 146–160. [Google Scholar] [CrossRef]
- Cowan, D.A.; Brown, J.H. Effect of Turbulence on Limiting Current in Electrodialysis Cells. Ind. Eng. Chem. 1959, 51, 1445–1448. [Google Scholar] [CrossRef]
- Strathmann, H. Ion-Exchange Membrane Separation Prosses; Membrane Science and Technology Series; Elsevier: Amsterdam, The Netherlands, 2004; Volume 9, ISBN 044450236X. [Google Scholar]
- Lightfoot, E.N. Membrane Separations Technology: Principles and Applications. Chem. Eng. Sci. 1996, 51, 325–326. [Google Scholar] [CrossRef]
- Mohammadi, R.; Ramasamy, D.L.; Sillanpää, M. Enhancement of Nitrate Removal and Recovery from Municipal Wastewater through Single-and Multi-Batch Electrodialysis: Process Optimisation and Energy Consumption. Desalination 2021, 498, 114726. [Google Scholar] [CrossRef]
- Cifuentes-Araya, N.; Pourcelly, G.; Bazinet, L. Impact of Pulsed Electric Field on Electrodialysis Process Performance and Membrane Fouling during Consecutive Demineralization of a Model Salt Solution Containing a High Magnesium/Calcium Ratio. J. Colloid Interface Sci. 2011, 361, 79–89. [Google Scholar] [CrossRef]
- Lejarazu-Larrañaga, A.; Ortiz, J.M.; Molina, S.; Pawlowski, S.; Galinha, C.F.; Otero, V.; García-Calvo, E.; Velizarov, S.; Crespo, J.G. Nitrate Removal by Donnan Dialysis and Anion-Exchange Membrane Bioreactor Using Upcycled End-of-Life Reverse Osmosis Membranes. Membranes 2022, 12, 101. [Google Scholar] [CrossRef]
- Senán-Salinas, J.; Blanco, A.; García-Pacheco, R.; Landaburu-Aguirre, J.; García-Calvo, E. Prospective Life Cycle Assessment and Economic Analysis of Direct Recycling of End-of-Life Reverse Osmosis Membranes Based on Geographic Information Systems. J. Clean. Prod. 2021, 282, 124400. [Google Scholar] [CrossRef]
- Senán-Salinas, J.; García-Pacheco, R.; Landaburu-Aguirre, J.; García-Calvo, E. Recycling of End-of-Life Reverse Osmosis Membranes: Comparative LCA and Cost-Effectiveness Analysis at Pilot Scale. Resour. Conserv. Recycl. 2019, 150, 104423. [Google Scholar] [CrossRef]
- Dolar, D.; Racar, M.; Košutić, K. Municipal Wastewater Reclamation and Water Reuse for Irrigation by Membrane Processes. Chem. Biochem. Eng. Q. 2019, 33, 417–425. [Google Scholar] [CrossRef]
- Gündoğdu, M.; Jarma, Y.A.; Kabay, N.; Pek, T.; Yüksel, M. Integration of MBR with NF/RO Processes for Industrial Wastewater Reclamation and Water Reuse-Effect of Membrane Type on Product Water Quality. J. Water Process Eng. 2019, 29, 100574. [Google Scholar] [CrossRef]
- Petek, M.; Krvavica, L.; Karažija, T.; Žlabur, J.Š.; Ćustić, M.H. Macroelements Status in Lettuce Affected by Different Forms of Phosphorus Fertilization. Sci. Pap. Ser. B Hortic. 2020, LXIV, 227–234. [Google Scholar]
- López, A.; Javier, G.A.; Fenoll, J.; Hellín, P.; Flores, P. Chemical Composition and Antioxidant Capacity of Lettuce: Comparative Study of Regular-Sized (Romaine) and Baby-Sized (Little Gem and Mini Romaine) Types. J. Food Compos. Anal. 2014, 33, 39–48. [Google Scholar] [CrossRef]
- Broadley, M.R.; Seginer, I.; Burns, A.; Escobar-Gutiérrez, A.J.; Burns, I.G.; White, P.J. The Nitrogen and Nitrate Economy of Butterhead Lettuce (Lactuca sativa var. capitata L.). J. Exp. Bot. 2003, 54, 2081–2090. [Google Scholar] [CrossRef] [Green Version]
- Solaiman, Z.M.; Yang, H.; Archdeacon, D.; Tippett, O.; Tibi, M.; Whiteley, A.S. Humus-Rich Compost Increases Lettuce Growth, Nutrient Uptake, Mycorrhizal Colonisation, and Soil Fertility. Pedosphere 2019, 29, 170–179. [Google Scholar] [CrossRef]
- García-Pacheco, R. Nanofiltration and Ultrafiltration Membranes from End-of-Life Reverse Osmosis Membranes: A Study of Recycling. Ph.D. Thesis, Universidad de Alcalá, Madrid, Spain, 2017. [Google Scholar]






| Parameters | Standard Soil 5M | |
|---|---|---|
| Soil type | Sandy loam | |
| Sampling date | 20 July 2021 | |
| pH value (0.01 M CaCl2) | 7.4 ± 0.1 | |
| Organic carbon (% C) | 0.88 ± 0.18 | |
| Nitrogen (% N) | 0.11 ± 0.03 | |
| Max. water-holding capacity (g/100 g) | 41.8 ± 5.3 | |
| Weight per volume (g/1000 mL) | 1219 ± 88 | |
| Cation-exchange capacity (meq/100 g) | 8.5 ± 0.25 | |
| Particle size distribution (mm) according to USDA (%) | ||
| <0.002 | 0.002–0.05 | 0.05–2.0 |
| 11.9 ± 1 | 31.6 ± 3.2 | 56.5 ± 3.3 |
| Water Source | Conductivity (dS m−1) | Ionic Compound (ppm) | |||||
|---|---|---|---|---|---|---|---|
| Cl− | NO3− | Na+ | K+ | Ca2+ | Mg2+ | ||
| Synthetic UWW | 4.90 (±0.1) | 1224.8 | 68.21 | 694 | 47.20 | 209 | 102 |
| Permeate of rNF | 0.98 (±0.02) | 249 | 24.20 | 153 | 14.50 | 14.10 | 6.86 |
| Product of ED (rAEM–CMH-PES) | 1.93 (±0.1) | 390 | 22.90 | 320 | 23.60 | 52.90 | 29.20 |
| NTK | IN | K | Ca | Mg | |
|---|---|---|---|---|---|
| (%) | (%) | (%) | (%) | (%) | |
| TW | 1.03 | 0.11 | 4.10 | 1.71 | 0.25 |
| IRR | 1.20 | 0.12 | 4.42 | 1.48 | 0.28 |
| FRT | 1.24 | 0.11 | 4.91 | 1.68 | 0.29 |
| CV (%) | 0.22 | 0.22 | 1.16 | 0.48 | 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pompa-Pernía, A.; Molina, S.; Lejarazu-Larrañaga, A.; Landaburu-Aguirre, J.; García-Calvo, E. Validation of Recycled Nanofiltration and Anion-Exchange Membranes for the Treatment of Urban Wastewater for Crop Irrigation. Membranes 2022, 12, 746. https://doi.org/10.3390/membranes12080746
Pompa-Pernía A, Molina S, Lejarazu-Larrañaga A, Landaburu-Aguirre J, García-Calvo E. Validation of Recycled Nanofiltration and Anion-Exchange Membranes for the Treatment of Urban Wastewater for Crop Irrigation. Membranes. 2022; 12(8):746. https://doi.org/10.3390/membranes12080746
Chicago/Turabian StylePompa-Pernía, Anamary, Serena Molina, Amaia Lejarazu-Larrañaga, Junkal Landaburu-Aguirre, and Eloy García-Calvo. 2022. "Validation of Recycled Nanofiltration and Anion-Exchange Membranes for the Treatment of Urban Wastewater for Crop Irrigation" Membranes 12, no. 8: 746. https://doi.org/10.3390/membranes12080746