The Short-Term Opening of Cyclosporin A-Independent Palmitate/Sr2+-Induced Pore Can Underlie Ion Efflux in the Oscillatory Mode of Functioning of Rat Liver Mitochondria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Animals
2.3. Isolation of Rat Liver Mitochondria
2.4. Estimation of the Functional Parameters of Mitochondria
2.5. Determination of Free Fatty Acids upon Mitochondrial Oscillations by Gas Chromatography
2.6. Detection of the Group IV Cytosolic Phospholipase A2 in Isolated Rat Liver Mitochondria by Immunoelectron Microscopy
2.7. Data Processing
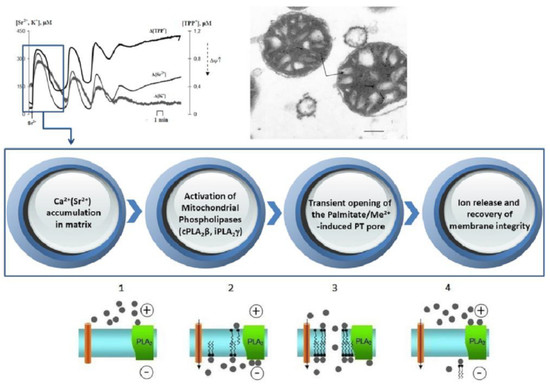
3. Results
3.1. Generation of Spontaneous Oscillations in Ion Fluxes and Respiration Rate of Rat Liver Mitochondria
3.2. Accumulation of Free Fatty Acids and Effects of Inhibitors of PLA2 on the Long-Term Oscillatory Mode of Mitochondrial Functioning
3.3. Ultrastructural Localization of the Group IV Ca2+(Sr2+)-Dependent Cytosolic Phospholipase A2 in Isolated Rat Liver Mitochondria
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wacquier, B.; Combettes, L.; Dupont, G. Cytoplasmic and mitochondrial calcium signaling: A two-way relationship. Cold Spring Harb. Perspect. Biol. 2020, 12, a038802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saris, N.-E.L.; Carafoli, E. A historical review of cellular calcium handling, with emphasis on mitochondria. Biochemistry 2005, 70, 187–194. [Google Scholar] [CrossRef] [PubMed]
- Rizzuto, R.; De Stefani, D.; Raffaello, A.; Mammucari, C. Mitochondria as sensors and regulators of calcium signalling. Nat. Rev. Mol. Cell. Biol. 2012, 13, 566–578. [Google Scholar] [CrossRef]
- Aon, M.A.; Cortassa, S.; O’Rourke, B. Mitochondrial oscillations in physiology and pathophysiology. Adv. Exp. Med. Biol. 2008, 641, 98–117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wacquier, B.; Combettes, L.; Van Nhieu, G.T.; Dupont, G. Interplay between intracellular Ca2+ oscillations and Ca2+-stimulated mitochondrial metabolism. Sci. Rep. 2016, 6, 19316. [Google Scholar] [CrossRef]
- Gylkhandanyan, A.V.; Evtodienko, Y.V.; Zhabotinsky, A.M.; Kondrashova, M.N. Continuous Sr2+-induced oscillations of the ionic fluxes in mitochondria. FEBS Lett. 1976, 66, 644–647. [Google Scholar] [CrossRef] [Green Version]
- Evtodienko, Y.V.; Zinchenko, V.P.; Holmuhamedov, E.L.; Gylkhandanyan, A.V.; Zhabotinsky, A.M. The stoichiometry of ion fluxes during Sr2+-induced oscillations in mitochondria. Biochim. Biophys. Acta 1980, 589, 157–161. [Google Scholar] [CrossRef]
- Holmuhamedov, E.L.; Teplova, V.V.; Chukhlova, E.A. Excitability of inner mitochondrial membrane. II. Reversible Sr2+-induced Sr2+ release from mitochondria. Biol. Membr. 1991, 8, 612–620. [Google Scholar]
- Teplova, V.V.; Sidash, S.S.; Makarov, P.R.; Evdotienko, Y.V. Characteristics of reversible and irreversible Ca2+-induced Ca2+ release from mitochondria in permeabilized Ehrlich ascites carcinoma cells. Biokhimiia 1995, 60, 944–950. [Google Scholar]
- Holmuhamedov, E.L.; Teplova, V.V.; Chukhlova, E.A.; Evtodienko, Y.V.; Ulrich, R.G. Strontium excitability of the inner mitochondrial membrane: Regenerative strontium-induced strontium release. Biochem. Mol. Biol. Int. 1995, 36, 39–49. [Google Scholar]
- Sidash, S.S.; Evtodienko, I.V.; Kholmokhamedov, E.L.; Teplova, V.V. Characteristics of Sr2+-induced permeability increase in the inner mitochondrial membrane. Membr. Cell Biol. 1995, 8, 447–454. [Google Scholar]
- Mironova, G.D.; Saris, N.E.; Belosludtseva, N.V.; Agafonov, A.V.; Elantsev, A.B.; Belosludtsev, K.N. Involvement of palmitate/Ca2+(Sr2+)-induced pore in the cycling of ions across the mitochondrial membrane. Biochim. Biophys. Acta 2015, 1848, 488–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chance, B.; Ghosh, A.; Pye, E. Biological and Biochemical Oscillators, 1st ed.; Academic Press: New York, NY, USA; London, UK, 1973; p. 534. [Google Scholar]
- Garbincius, J.F.; Elrod, J.W. Mitochondrial calcium exchange in physiology and disease. Physiol. Rev. 2022, 102, 893–992. [Google Scholar] [CrossRef] [PubMed]
- Elustondo, P.A.; Nichols, M.; Robertson, G.S.; Pavlov, E.V. Mitochondrial Ca2+ uptake pathways. J. Bioenerg. Biomembr. 2017, 49, 113–119. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Dubinin, M.V.; Belosludtseva, N.V.; Mironova, G.D. Mitochondrial Ca2+ transport: Mechanisms, molecular structures, and role in cells. Biochemistry 2019, 84, 593–607. [Google Scholar] [CrossRef]
- Grant, C.W.; Usachev, Y.M. MCU (mitochondrial Ca2+ uniporter) makes the calcium go round. J. Biol. Chem. 2022, 298, 101604. [Google Scholar] [CrossRef]
- Buckman, J.F.; Reynolds, I.J. Spontaneous changes in mitochondrial membrane potential in cultured neurons. J. Neurosci. 2001, 21, 5054–5065. [Google Scholar] [CrossRef] [Green Version]
- Vergun, O.; Votyakova, T.V.; Reynolds, I.J. Spontaneous changes in mitochondrial membrane potential in single isolated brain mitochondria. Biophys. J. 2003, 85, 3358–3366. [Google Scholar] [CrossRef] [Green Version]
- Aon, M.A.; Cortassa, S.; Marbán, E.; O’Rourke, B. Synchronized whole cell oscillations in mitochondrial metabolism triggered by a local release of reactive oxygen species in cardiac myocytes. J. Biol. Chem. 2003, 278, 44735–44744. [Google Scholar] [CrossRef] [Green Version]
- Mironova, G.D.; Belosludtsev, K.N.; Belosludtseva, N.V.; Gritsenko, E.N.; Khodorov, B.I.; Saris, N.-E.L. Mitochondrial Ca2+cycle mediated by the palmitate-activated cyclosporin A-insensitive pore. J. Bioenerg. Biomembr. 2007, 39, 167–174. [Google Scholar] [CrossRef]
- Mironova, G.D.; Gritsenko, E.; Gateau-Roesch, O.; Levrat, C.; Agafonov, A.; Belosludtsev, K.; Prigent, A.F.; Muntean, D.; Dubois, M.; Ovize, M. Formation of palmitic acid/Ca2+complexes in the mitochondrial membrane: A possible role in the cyclosporin-insensitive permeability transition. J. Bioenerg. Biomembr. 2004, 36, 171–178. [Google Scholar] [CrossRef] [PubMed]
- Agafonov, A.; Gritsenko, E.; Belosludtsev, K.; Kovalev, A.; Gateau-Roesch, O.; Saris, N.-E.L.; Mironova, G.D. A permeability transition in liposomes induced by the formation of Ca2+/palmitic acid complexes. Biochim. Biophys. Acta 2003, 1609, 153–160. [Google Scholar] [CrossRef] [Green Version]
- Agafonov, A.V.; Gritsenko, E.N.; Shlyapnikova, E.A.; Kharakoz, D.P.; Belosludtseva, N.V.; Lezhnev, E.I.; Saris, N.-E.L.; Mironova, G.D. Ca2+-induced phase separation in the membrane of palmitate-containing liposomes and its possible relation to membrane permeabilization. J. Membr. Biol. 2007, 215, 57–68. [Google Scholar] [CrossRef] [PubMed]
- Mironova, G.D.; Gateau-Roesch, O.; Levrat, C.; Gritsenko, E.; Pavlov, E.; Lazareva, A.V.; Limarenko, E.; Rey, C.; Louisot, P.; Saris, N.-E.L. Palmitic and stearic acids bind Ca2+ with high affinity and form nonspecific channels in black-lipid membranes. Possible relation to Ca2+-activated mitochondrial pores. J. Bioenerg. Biomembr. 2001, 33, 319–331. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Mironova, G.D. Possible mechanism for formation and regulation of the palmitate-induced cyclosporin A-insensitive mitochondrial pore. Biochemistry 2005, 70, 815–821. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Saris, N.E.; Belosludtseva, N.V.; Trudovishnikov, A.S.; Lukyanova, L.D.; Mironova, G.D. Physiological aspects of the mitochondrial cyclosporin A-insensitive palmitate/Ca2+-induced pore: Tissue specificity, age profile and dependence on the animal’s adaptation to hypoxia. J. Bioenerg. Biomembr. 2009, 41, 395–401. [Google Scholar] [CrossRef]
- Sultan, A.; Sokolove, P.M. Free fatty acid effects on mitochondrial permeability: An overview. Arch. Biochem. Biophys. 2001, 386, 52–61. [Google Scholar] [CrossRef]
- Sultan, A.; Sokolove, P.M. Palmitic acid opens a novel cyclosporin A-insensitive pore in the inner mitochondrial membrane. Arch. Biochem. Biophys. 2001, 386, 37–51. [Google Scholar] [CrossRef]
- Mironova, G.D.; Pavlov, E.V. Mitochondrial cyclosporine A-independent palmitate/Ca2+-induced permeability transition pore (PA-mPT Pore) and its role in mitochondrial function and protection against calcium overload and glutamate toxicity. Cells 2021, 10, 125. [Google Scholar] [CrossRef]
- Antonov, V.F.; Shevchenko, E.V. Lipid pores and stability of cell membranes. Prog. Biophys. Mol. Biol. 1996, 65, 319–325. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar] [CrossRef]
- Aiuchi, T.; Matsunaga, M.; Nakaya, K.; Nakamura, Y. Calculation of membrane potential in synaptosomes with use of a lipophilic cation (tetraphenylphosphonium). Chem. Pharm. Bull. 1989, 37, 3333–3337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Belosludtsev, K.N.; Belosludtseva, N.V.; Talanov, E.Y.; Tenkov, K.S.; Starinets, V.S.; Agafonov, A.A.; Pavlik, L.L.; Dubinin, M.V. Effect of bedaquiline on the functions of rat liver mitochondria. Biochim. Biophys. Acta Biomembr. 2019, 1861, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Garlid, K.D.; Paucek, P. The mitochondrial potassium cycle. IUBMB Life 2001, 52, 153–158. [Google Scholar] [CrossRef]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef]
- Kargapolov, A.V. Changes in the phospholipid content of intact mitochondria under mitochondrial swelling in hypotonic sucrose solutions. Biokhimiia 1979, 44, 293–296. [Google Scholar]
- Saris, N.-E.L. Stimulation of phospholipase A2 activity in mitochondria by magnesium and polyamines. Magnes. Res. 1994, 7, 5–10. [Google Scholar]
- Murakami, M.; Sato, H.; Taketomi, Y. Updating phospholipase A2 biology. Biomolecules 2020, 10, 1457. [Google Scholar] [CrossRef]
- Kravenska, Y.; Checchetto, V.; Szabo, I. Routes for potassium ions across mitochondrial membranes: A biophysical point of view with special focus on the ATP-sensitive K+ channel. Biomolecules 2021, 11, 1172. [Google Scholar] [CrossRef]
- Zoratti, M.; Szabò, I. The mitochondrial permeability transition. Biochim. Biophys. Acta 1995, 1241, 139–176. [Google Scholar] [CrossRef]
- Mironova, G.D.; Belosludtsev, K.N.; Surin, A.M.; Trudovishnikov, A.S.; Belosludtseva, N.V.; Pinelis, V.G.; Krasilynikova, I.A.; Khodorov, B.I. Mitochondrial lipid pore in the mechanism of glutamate-induced calcium disturbance of neurons. Biochem. Suppl. Ser. A Membr. Cell Biol. 2012, 6, 45–55. [Google Scholar] [CrossRef]
- Ghosh, M.; Loper, R.; Gelb, M.H.; Leslie, C.C. Identification of the expressed form of human cytosolic phospholipase A2β(cPLA2β). cPLA2β3 is a novel variant localized to mitochondria and early endosomes. J. Biol. Chem. 2006, 281, 16615–16624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Taketomi, Y.; Miki, Y.; Sato, H.; Hirabayashi, T.; Yamamoto, K. Recent progress in phospholipase A2 research: From cells to animals to humans. Prog. Lipid Res. 2011, 50, 152–192. [Google Scholar] [CrossRef]
- Moon, S.H.; Jenkins, C.M.; Liu, X.; Guan, S.; Mancuso, D.J.; Gross, R.W. Activation of mitochondrial calcium-independent phospholipase A2γ (iPLA2γ) by divalent cations mediating arachidonate release and production of downstream eicosanoids. J. Biol. Chem. 2012, 287, 14880–14895. [Google Scholar] [CrossRef] [Green Version]
- Horvath, S.E.; Daum, G. Lipids of mitochondria. Prog. Lipid Res. 2013, 52, 590–614. [Google Scholar] [CrossRef] [PubMed]
- Schonfeld, P.; Wieckowski, M.R.; Wojtczak, L. Long-chain fatty acid-promoted swelling of mitochondria: Further evidence for the protonophoric effect of fatty acids in the inner mitochondrial membrane. FEBS Lett. 2000, 471, 108–112. [Google Scholar] [CrossRef] [Green Version]
- Kocherginsky, N. Biomimetic membranes with aqueous nanochannels. Phase transitions and oscillations. Membr. Membr. Technol. 2021, 3, 442–447. [Google Scholar] [CrossRef]
- Kocherginsky, N. Biomimetic membranes without proteins but with aqueous nanochannels and facilitated transport. Membr. Membr. Technol. 2021, 3, 434–441. [Google Scholar] [CrossRef]
- Belosludtsev, K.N.; Trudovishnikov, A.S.; Belosludtseva, N.V.; Agafonov, A.V.; Mironova, G.D. Palmitic acid induces the opening of a Ca2+-dependent pore in the plasma membrane of red blood cells: The possible role of the pore in erythrocyte lysis. J. Membr. Biol. 2010, 237, 13–19. [Google Scholar] [CrossRef]
- Wang, F.; Li, A.; Meng, T.G.; Wang, L.-Y.; Wang, L.-J.; Hou, Y.; Schatten, H.; Sun, Q.-Y.; Ou, X.-H. Regulation of [Ca2+]i oscillations and mitochondrial activity by various calcium transporters in mouse oocytes. Reprod. Biol. Endocrinol. 2020, 18, 87. [Google Scholar] [CrossRef]
- Cortassa, S.; Aon, M.A.; Winslow, R.L.; O’Rourke, B. A mitochondrial oscillator dependent on reactive oxygen species. Biophys. J. 2004, 87, 2060–2073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giorgi, C.; Marchi, S.; Pinton, P. The machineries, regulation and cellular functions of mitochondrial calcium. Nat. Rev. Mol. Cell Biol. 2018, 19, 713–730. [Google Scholar] [CrossRef] [PubMed]
- Briston, T.; Selwood, D.L.; Szabadkai, G.; Duchen, M.R. Mitochondrial permeability transition: A molecular lesion with multiple drug targets. Trends Pharmacol. Sci. 2019, 40, 50–70. [Google Scholar] [CrossRef] [PubMed]
- Morciano, G.; Naumova, N.; Koprowski, P.; Valente, S.; Sardão, V.A.; Potes, Y.; Rimessi, A.; Wieckowski, M.R.; Oliveira, P.J. The mitochondrial permeability transition pore: An evolving concept critical for cell life and death. Biol. Rev. Camb. Philos. Soc. 2021, 96, 2489–2521. [Google Scholar] [CrossRef]
- Korshunov, S.S.; Skulachev, V.P.; Starkov, A.A. High protonic potential actuates a mechanism of production of reactive oxygen species in mitochondria. FEBS Lett. 1997, 416, 15–18. [Google Scholar] [CrossRef] [Green Version]
- Kotova, E.A.; Antonenko, Y.N. Fifty years of research on protonophores: Mitochondrial uncoupling as a basis for therapeutic action. Acta Nat. 2022, 14, 4–13. [Google Scholar] [CrossRef]
- Demine, S.; Renard, P.; Arnould, T. Mitochondrial uncoupling: A key controller of biological processes in physiology and diseases. Cells 2019, 8, 795. [Google Scholar] [CrossRef] [Green Version]
- Wiswedel, I.; Barnstorf, U.; Augustin, W.; Holmuhamedov, E.; Medvedev, B.; Evtodienko, Y. Involvement of periodic deacylation-acylation cycles of mitochondrial phospholipids during Sr2+-induced oscillatory ion transport in rat liver mitochondria. Biochim. Biophys. Acta 1982, 688, 597–604. [Google Scholar] [CrossRef]
- Samanta, K.; Mirams, G.R.; Parekh, A.B. Sequential forward and reverse transport of the Na+/Ca2+ exchanger generates Ca2+ oscillations within mitochondria. Nat. Commun. 2018, 9, 156. [Google Scholar] [CrossRef]







| PLA2 Inhibitor | Concentration Required to Suppress Mitochondrial Oscillations |
|---|---|
| Aristolochic acid | 25 µM |
| Trifluoperazine dihydrochloride | 10 µM |
| Arachidonyl trifluoromethyl ketone (AACOCF3) | 15 µM |
| 4-(4-Octadecylphenyl)-4-oxobutenoic acid | 1 µM |
| Bromoenol lactone | 15 µM |
| 4′-bromophenacyl bromide | 40 µM |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belosludtseva, N.V.; Pavlik, L.L.; Belosludtsev, K.N.; Saris, N.-E.L.; Shigaeva, M.I.; Mironova, G.D. The Short-Term Opening of Cyclosporin A-Independent Palmitate/Sr2+-Induced Pore Can Underlie Ion Efflux in the Oscillatory Mode of Functioning of Rat Liver Mitochondria. Membranes 2022, 12, 667. https://doi.org/10.3390/membranes12070667
Belosludtseva NV, Pavlik LL, Belosludtsev KN, Saris N-EL, Shigaeva MI, Mironova GD. The Short-Term Opening of Cyclosporin A-Independent Palmitate/Sr2+-Induced Pore Can Underlie Ion Efflux in the Oscillatory Mode of Functioning of Rat Liver Mitochondria. Membranes. 2022; 12(7):667. https://doi.org/10.3390/membranes12070667
Chicago/Turabian StyleBelosludtseva, Natalia V., Lyubov L. Pavlik, Konstantin N. Belosludtsev, Nils-Erik L. Saris, Maria I. Shigaeva, and Galina D. Mironova. 2022. "The Short-Term Opening of Cyclosporin A-Independent Palmitate/Sr2+-Induced Pore Can Underlie Ion Efflux in the Oscillatory Mode of Functioning of Rat Liver Mitochondria" Membranes 12, no. 7: 667. https://doi.org/10.3390/membranes12070667