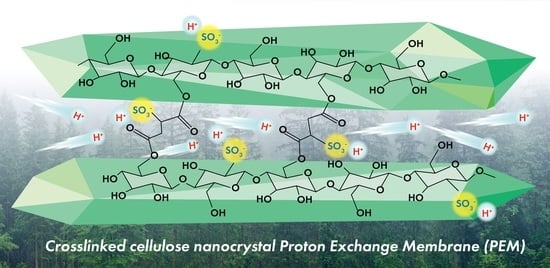
Cellulose Nanocrystals Crosslinked with Sulfosuccinic Acid as Sustainable Proton Exchange Membranes for Electrochemical Energy Applications
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanocellulose Membrane Preparation
2.2. Mechanical Properties
2.3. Proton Conductivity
2.4. Ion Exchange Capacity
2.5. Membrane Characterization
3. Results
3.1. Appearance and Morphology
3.2. Elemental Composition and Chemical Structure
3.2.1. Elemental Mapping
3.2.2. Infrared Spectroscopy
3.2.3. X-ray Diffraction
3.2.4. X-ray Photoelectron Spectroscopy
3.3. Mechanical Properties
3.4. Water Stability and Ion Exchange Capacity
3.5. Proton Conductivity
3.6. Activation Energies
3.7. Retention of Conductivity after Boiling
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Staffell, I.; Scamman, D.; Velazquez Abad, A.; Balcombe, P.; Dodds, P.E.; Ekins, P.; Shah, N.; Ward, K.R. The role of hydrogen and fuel cells in the global energy system. Energy Environ. Sci. 2019, 12, 463–491. [Google Scholar] [CrossRef] [Green Version]
- Jiao, K.; Xuan, J.; Du, Q.; Bao, Z.; Xie, B.; Wang, B.; Zhao, Y.; Fan, L.; Wang, H.; Hou, Z.; et al. Designing the next generation of proton-exchange membrane fuel cells. Nature 2021, 595, 361–369. [Google Scholar] [CrossRef] [PubMed]
- Carmo, M.; Fritz, D.L.; Mergel, J.; Stolten, D. A comprehensive review on PEM water electrolysis. Int. J. Hydr. Energy 2013, 38, 4901–4934. [Google Scholar] [CrossRef]
- Klose, C.; Saatkamp, T.; Münchinger, A.; Bohn, L.; Titvinidze, G.; Breitwieser, M.; Kreuer, K.; Vierrath, S. All-Hydrocarbon MEA for PEM Water Electrolysis Combining Low Hydrogen Crossover and High Efficiency. Adv. Energy Mater. 2020, 10, 1903995. [Google Scholar] [CrossRef]
- Weber, A.Z.; Balasubramanian, S.; Das, P.K. Proton Exchange Membrane Fuel Cells. Adv. Chem. Eng. 2012, 41, 65–144. [Google Scholar] [CrossRef]
- Kreuer, K. On the development of proton conducting polymer membranes for hydrogen and methanol fuel cells. J. Membr. Sci. 2001, 185, 29–39. [Google Scholar] [CrossRef]
- Rao, A.S.; Rashmi, K.; Manjunatha, D.; Jayarama, A.; Shastrimath, V.V.D.; Pinto, R. Methanol crossover reduction and power enhancement of methanol fuel cells with polyvinyl alcohol coated Nafion membranes. Mater. Today Proc. 2020, 35, 344–351. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Nale, A.; Pagot, G.; Vezzù, K.; Zawodzinski, T.A.; Meda, L.; Gambaro, C.; Di Noto, V. An efficient barrier toward vanadium crossover in redox flow batteries: The bilayer [Nafion/(WO3)x] hybrid inorganic-organic membrane. Electrochim. Acta 2021, 378, 138133. [Google Scholar] [CrossRef]
- Peckham, T.J.; Holdcroft, S. Structure-Morphology-Property Relationships of Non-Perfluorinated Proton-Conducting Membranes. Adv. Mater. 2010, 22, 4667–4690. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Vivekanandhan, S.; Pin, J.-M.; Misra, M. Composites from renewable and sustainable resources: Challenges and innovations. Science 2018, 362, 536–542. [Google Scholar] [CrossRef] [Green Version]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef] [PubMed]
- Selyanchyn, O.; Selyanchyn, R.; Lyth, S.M. A Review of Proton Conductivity in Cellulosic Materials. Front. Energy Res. 2020, 8, 596164. [Google Scholar] [CrossRef]
- Calle-Gil, R.; Castillo-Martínez, E.; Carretero-González, J. Cellulose Nanocrystals in Sustainable Energy Systems. Adv. Sustain. Syst. 2022, 6, 2100395. [Google Scholar] [CrossRef]
- Usov, I.; Nystrom, G.; Adamcik, J.; Handschin, S.; Schütz, C.; Fall, A.; Bergström, L.; Mezzenga, R. Understanding nanocellulose chirality and structure–properties relationship at the single fibril level. Nat. Commun. 2015, 6, 7564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Y.; Liang, Y.; Mei, C.; Cai, L.; Nadda, A.; Van Le, Q.; Peng, Y.; Lam, S.S.; Sonne, C.; Xia, C. Advanced nanocellulose-based gas barrier materials: Present status and prospects. Chemosphere 2021, 286, 131891. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.; Selyanchyn, R.; Nishihara, M.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. High Temperature Proton Conduction in Nanocellulose Membranes: Paper Fuel Cells. Chem. Mater. 2016, 28, 4805–4814. [Google Scholar] [CrossRef]
- Vilela, C.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Nanocellulose-based materials as components of polymer electrolyte fuel cells. J. Mater. Chem. A 2019, 7, 20045–20074. [Google Scholar] [CrossRef]
- Bayer, T.; Cunning, B.V.; Šmíd, B.; Selyanchyn, R.; Fujikawa, S.; Sasaki, K.; Lyth, S.M. Spray deposition of sulfonated cellulose nanofibers as electrolyte membranes in fuel cells. Cellulose 2021, 28, 1355–1367. [Google Scholar] [CrossRef]
- Kang, I.-S.; Yang, C.Q.; Wei, W.; Lickfield, G.C. Mechanical Strength of Durable Press Finished Cotton Fabrics. Text. Res. J. 1998, 68, 865–870. [Google Scholar] [CrossRef]
- Chen, C.-C.; Wang, C.-C. Crosslinking of cotton cellulose with succinic acid in the presence of titanium dioxide nano-catalyst under UV irradiation. J. Sol-Gel Sci. Technol. 2006, 40, 31–38. [Google Scholar] [CrossRef]
- Hassan, M.; Tucker, N.; Le Guen, M. Thermal, mechanical and viscoelastic properties of citric acid-crosslinked starch/cellulose composite foams. Carbohydr. Polym. 2019, 230, 115675. [Google Scholar] [CrossRef] [PubMed]
- Quellmalz, A.; Mihranyan, A. Citric Acid Cross-Linked Nanocellulose-Based Paper for Size-Exclusion Nanofiltration. ACS Biomater. Sci. Eng. 2015, 1, 271–276. [Google Scholar] [CrossRef] [PubMed]
- Belay, M.; Tyeb, S.; Rathore, K.; Kumar, M.; Verma, V. Synergistic effect of bacterial cellulose reinforcement and succinic acid crosslinking on the properties of agar. Int. J. Biol. Macromol. 2020, 165, 3115–3122. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.B.; Lee, C.-S.; Jun, J.-H.; Kim, D.S.; Lee, Y.M. Crosslinked poly(vinyl alcohol) membranes containing sulfonic acid group: Proton and methanol transport through membranes. J. Membr. Sci. 2004, 238, 143–151. [Google Scholar] [CrossRef]
- Mohanapriya, S.; Rambabu, G.; Bhat, S.D.; Raj, V. Hybrid membranes for polymer electrolyte fuel cells operating under various relative humidity values. J. Solid State Electrochem. 2017, 21, 3437–3448. [Google Scholar] [CrossRef]
- Seo, J.A.; Kim, J.C.; Koh, J.K.; Ahn, S.H.; Kim, J.H. Preparation and characterization of crosslinked cellulose/sulfosuccinic acid membranes as proton conducting electrolytes. Ionics 2009, 15, 555–560. [Google Scholar] [CrossRef]
- Sriruangrungkamol, A.; Chonkaew, W. Modification of nanocellulose membrane by impregnation method with sulfosuccinic acid for direct methanol fuel cell applications. Polym. Bull. 2020, 78, 3705–3728. [Google Scholar] [CrossRef]
- Reid, M.; Villalobos, M.; Cranston, E.D. Benchmarking Cellulose Nanocrystals: From the Laboratory to Industrial Production. Langmuir 2016, 33, 1583–1598. [Google Scholar] [CrossRef]
- Kedzior, S.A.; Kiriakou, M.; Niinivaara, E.; Dubé, M.A.; Fraschini, C.; Berry, R.M.; Cranston, E.D. Incorporating Cellulose Nanocrystals into the Core of Polymer Latex Particles via Polymer Grafting. ACS Macro Lett. 2018, 7, 990–996. [Google Scholar] [CrossRef]
- Cooper, K. Progress Toward Accurate Through-Plane Membrane Resistance and Conductivity Measurement. ECS Meet. Abstr. 2009, MA2009-02, 973. [Google Scholar] [CrossRef]
- Li, D.; Henschen, J.; Ek, M. Esterification and hydrolysis of cellulose using oxalic acid dihydrate in a solvent-free reaction suitable for preparation of surface-functionalised cellulose nanocrystals with high yield. Green Chem. 2017, 19, 5564–5567. [Google Scholar] [CrossRef] [Green Version]
- Bayer, T.; Selyanchyn, R.; Fujikawa, S.; Sasaki, K.; Lyth, S. Spray-painted graphene oxide membrane fuel cells. J. Membr. Sci. 2017, 541, 347–357. [Google Scholar] [CrossRef]
- Larkin, P.J. Illustrated IR and Raman Spectra Demonstrating Important Functional Groups. In Infrared and Raman Spectroscopy, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 153–210. [Google Scholar] [CrossRef]
- Sun, C.; Negro, E.; Vezzù, K.; Pagot, G.; Cavinato, G.; Nale, A.; Bang, Y.H.; Di Noto, V. Hybrid inorganic-organic proton-conducting membranes based on SPEEK doped with WO3 nanoparticles for application in vanadium redox flow batteries. Electrochim. Acta 2019, 309, 311–325. [Google Scholar] [CrossRef]
- French, A.D. Idealized powder diffraction patterns for cellulose polymorphs. Cellulose 2013, 21, 885–896. [Google Scholar] [CrossRef]
- Moulder, J.; Stickle, W.; Sobol, P.; Bomben, K. Handbook of X-ray Photoelectron Spectroscopy; ULVAC-PHI Inc.: Chigasaki, Japan, 1995. [Google Scholar]
- Shen, L.; Xu, H.; Kong, L.; Yang, Y. Non-Toxic Crosslinking of Starch Using Polycarboxylic Acids: Kinetic Study and Quantitative Correlation of Mechanical Properties and Crosslinking Degrees. J. Polym. Environ. 2015, 23, 588–594. [Google Scholar] [CrossRef]
- Guccini, V.; Carlson, A.; Yu, S.; Lindbergh, G.; Lindström, R.W.; Salazar-Alvarez, G. Highly proton conductive membranes based on carboxylated cellulose nanofibres and their performance in proton exchange membrane fuel cells. J. Mater. Chem. A 2019, 7, 25032–25039. [Google Scholar] [CrossRef] [Green Version]
- Vilela, C.; Silva, A.C.; Domingues, E.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.; Santos, S.A.; Freire, C.S. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2019, 230, 115604. [Google Scholar] [CrossRef]
- Ohma, A.; Shinohara, K.; Iiyama, A.; Yoshida, T.; Daimaru, A. Membrane and Catalyst Performance Targets for Automotive Fuel Cells by FCCJ Membrane, Catalyst, MEA WG. ECS Trans. 2011, 41, 775–784. [Google Scholar] [CrossRef]
- Gardner, C.; Anantaraman, A. Studies on ion-exchange membranes. II. Measurement of the anisotropic conductance of Nafion®. J. Electroanal. Chem. 1998, 449, 209–214. [Google Scholar] [CrossRef]
- Gadim, T.D.O.; Loureiro, F.J.A.; Vilela, C.; Rosero-Navarro, N.; Silvestre, A.J.D.; Freire, C.S.R.; Figueiredo, F.M.L. Protonic Conductivity and Fuel Cell Tests of Nanocomposite Membranes Based on Bacterial Cellulose. Electrochim. Acta 2017, 233, 52–61. [Google Scholar] [CrossRef]











| Membrane Description in Original Work | Type of Cellulose, Fabrication Procedure | Young’s Modulus (MPa) | Tensile Strength at Break 1 (MPa) | Elongation at Break (%) | Reference |
|---|---|---|---|---|---|
| Cellulose/SSA 5% | Microcrystalline powder (Sigma Aldrich), dissolved in DMSO (stirring), mixed with SSA (stirring 4 h), cast on Teflon-coated glass, dried 80 °C/48 h, annealed 120 °C/3 h Washing: not reported | 2.075 | 2.568 | 0.808 | [24] |
| Cellulose/SSA 10% | 4.457 | 4.772 | 0.955 | ||
| Cellulose/SSA 15% | 5.649 | 5.915 | 0.955 | ||
| Cellulose/SSA 20% | 5.55 | 2.471 | 2.246 | ||
| Cellulose/SSA 25% | 0.606 | 0.298 | 2.034 | ||
| Cellulose/SSA 30% | 1.057 | 0.498 | 2.121 | ||
| NC | Cellulose nanofibers (prepared from Para-rubber wood sawdust). Membranes fabricated by 0.82% H2O suspension pressure filtration, following by hot pressing at 120 °C/1 h. Membranes were soaked in aq. SSA solutions (0.1–10wt.%)/3 days, followed by hot pressing 120 °C/1 h | 1.36 | 0.29 | 4.66 | [27] |
| NC-0.1SSA | 0.31 | 0.19 | 1.59 | ||
| NC-1SSA | 0.15 | 0.36 | 0.46 | ||
| NC-3SSA | 0.09 | 0.86 | 0.11 | ||
| NC-5SSA | 0.08 | 0.91 | 0.09 | ||
| CNC | Cellulose nanocrystals (CNC, Celluforce Inc. Montreal, QC, Canada), dissolved in H2O (mechanical blender), mixed with SSA (ultrasonication 5 min), cast on PTFE Petri-dish, dried 35 °C/48 h, annealed 120 °C/10 min Washing: thorough in DI water, three cycles | 5850 | 42.7 | 1.27 | This work |
| CNC-10%-SSA | 5249 | 48.5 | 1.6 | ||
| CNC-15%-SSA | 2233 | 33.6 | 1.67 | ||
| CNC-20%-SSA | 2252 | 25.9 | 1.9 | ||
| CNC-25%-SSA | 1965 | 26.1 | 3.1 | ||
| CNC-30%-SSA | 1950 | 33.3 | 4.3 | ||
| CNC-35%-SSA | 1752 | 33.7 | 6.6 | ||
| Nafion-212 | Commercially available | 245 | 14 | 50 | This work |
| Membrane Material | Ion Exchange Capacity, mmol [H+]/g | Measurement Approach | Proton Conductivity, mS/cm | Measurement Condition | Reference |
|---|---|---|---|---|---|
| CNC | n/a | Through-plane | 4.6 | 120 °C, 100% RH | [16] |
| CNF | n/a | 0.05 | 100 °C, 100% RH | ||
| S-CNF | 2 | 120 °C, 100% RH | [18] | ||
| Cellulose/SA 5% | 0.12 | In-plane | 0.9 | 20 °C (in DI water) | [26] |
| Cellulose/SA 10% | 0.09 | 2.4 | |||
| Cellulose/SA 15% | 0.27 | 8.0 | |||
| Cellulose/SA 20% | 0.52 | ||||
| Cellulose/SA 25% | 0.42 | 15.0 | |||
| Cellulose/SA 30% | 0.53 | 23 | |||
| 40 | 85 °C (in DI water) | ||||
| CNF | 0.005 | Through-plane | 0.48 | r.t. (in DI water) | [27] |
| CNF—0.1SSA | 0.006 | 0.37 | |||
| CNF—1SSA | 0.010 | 0.16 | |||
| CNF—3SSA | 0.033 | 0.12 | |||
| CNF—5SSA | 0.043 | 0.73 | |||
| CNF—10SSA | 0.069 | 3.17 | |||
| H-CNF-600 (14 um) | n/a | In fuel cell | 1.4 | 30 °C, 95% RH | [38] |
| H-CNF-1550 (14 um) | n/a | 1.5 | |||
| H-CNF-600 (24 um) | n/a | 1.2 | |||
| H-CNF-1550 (24 um) | n/a | 1.4 | |||
| BC | n/a | Through-plane | 0.063 | 94 °C, 98% RH | [39] |
| BC/Fuc_50 | 0.76 | 80 °C, 98% RH | |||
| BC/Fuc_75 | 0.78 | 80 °C, 98% RH | |||
| BC/Fuc_75 | 1.6 | 94 °C, 98% RH | |||
| CNC | n/a | Through-plane | 0.4 | 120 °C, ~96% RH | This work |
| CNC-10%-SSA | 0.399 | 4.8 | 120 °C, ~96% RH | ||
| CNC-15%-SSA | 0.705 | 7.5 | 120 °C, ~96% RH | ||
| CNC-20%-SSA | 0.964 | 11.6 | 120 °C, ~96% RH | ||
| CNC-25%-SSA | 1.214 | 12.7 | 120 °C, ~96% RH | ||
| CNC-30%-SSA | 1.423 | 14.0 | 120 °C, ~96% RH | ||
| CNC-35%-SSA | 1.464 | 10.1 * | 100 °C, ~96% RH |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Selyanchyn, O.; Bayer, T.; Klotz, D.; Selyanchyn, R.; Sasaki, K.; Lyth, S.M. Cellulose Nanocrystals Crosslinked with Sulfosuccinic Acid as Sustainable Proton Exchange Membranes for Electrochemical Energy Applications. Membranes 2022, 12, 658. https://doi.org/10.3390/membranes12070658
Selyanchyn O, Bayer T, Klotz D, Selyanchyn R, Sasaki K, Lyth SM. Cellulose Nanocrystals Crosslinked with Sulfosuccinic Acid as Sustainable Proton Exchange Membranes for Electrochemical Energy Applications. Membranes. 2022; 12(7):658. https://doi.org/10.3390/membranes12070658
Chicago/Turabian StyleSelyanchyn, Olena, Thomas Bayer, Dino Klotz, Roman Selyanchyn, Kazunari Sasaki, and Stephen Matthew Lyth. 2022. "Cellulose Nanocrystals Crosslinked with Sulfosuccinic Acid as Sustainable Proton Exchange Membranes for Electrochemical Energy Applications" Membranes 12, no. 7: 658. https://doi.org/10.3390/membranes12070658