Formation of a Fully Anionic Supported Lipid Bilayer to Model Bacterial Inner Membrane for QCM-D Studies
Abstract
:1. Introduction
1.1. Quartz Crystal Microbalance with Dissipation Monitoring (QCM-D)
1.2. Selecting Lipids for Model Membranes
1.3. Past Attempts Incorporating Anionic Lipids in SLBs
1.4. Approach of This Study
2. Materials and Methods
2.1. Lipids
2.2. Lipid Vesicle Formation
2.3. Surface Treatment of Quartz Crystal
2.4. QCM-D Experiments of SLB Formation
3. Results and Discussion
3.1. Vesicle Size and Stability
3.2. Anionic SLB Formation
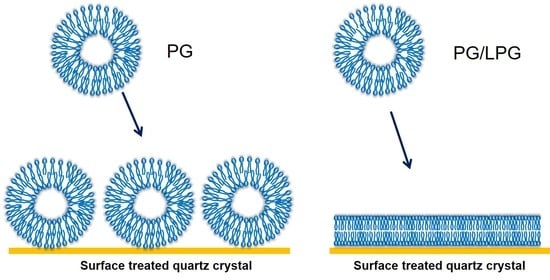
3.2.1. PG Vesicles form a Vesicle Layer and Not a Bilayer on APTMS-Coated Silica
3.2.2. LPG Addition Promotes Bilayer Formation
3.2.3. Increasing LPG to 40% Promotes Lipid Removal from the Membrane
3.3. Dynamics of Vesicle Adsorption and Bilayer Formation
3.4. Estimation of SLB Molecular Packing Characteristics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Analysis of QCM-D Data
References
- Clifton, L.A.; Campbell, R.A.; Sebastiani, F.; Campos-Terán, J.; Gonzalez-Martinez, J.F.; Björklund, S.; Sotres, J.; Cárdenas, M. Design and use of model membranes to study biomolecular interactions using complementary surface-sensitive techniques. Adv. Colloid Interface Sci. 2020, 27, 102118. [Google Scholar] [CrossRef] [PubMed]
- Watts, T.H.; Brian, A.A.; Kappler, J.W.; Marrack, P.; McConnell, H.M. Antigen presentation by supported planar membranes containing affinity-purified I-Ad. Proc. Natl. Acad. Sci. USA 1984, 81, 7564–7568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McConnell, H.M.; Watts, T.H.; Weis, R.M.; Brian, A.A. Supported planar membranes in studies of cell-cell recognition in the immune system. Biochim. Biophys. Acta 1986, 864, 95–106. [Google Scholar] [CrossRef]
- Richter, R.P.; Berat, R.; Brisson, A.R. Formation of solid-supported lipid bilayers: An integrated view. Langmuir 2006, 22, 3497–3505. [Google Scholar] [CrossRef]
- Keller, C.A.; Kasemo, B. Surface specific kinetics of lipid vesicle adsorption measured with a quartz crystal microbalance. Biophys. J. 1998, 75, 1397–1402. [Google Scholar] [CrossRef] [Green Version]
- Richter, R.; Mukhopadhyay, A.; Brisson, A. Pathways of lipid vesicle deposition on solid surfaces: A combined QCM-D and AFM study. Biophys. J. 2003, 85, 3035–3047. [Google Scholar] [CrossRef] [Green Version]
- Seantier, B.; Breffa, C.; Félix, O.; Decher, G. Dissipation-enhanced quartz crystal microbalance studies on the experimental parameters controlling the formation of supported lipid bilayers. J. Phys. Chem. B 2005, 109, 21755–21765. [Google Scholar] [CrossRef]
- Hardy, G.J.; Nayak, R.; Zauscher, S. Model cell membranes: Techniques to form complex biomimetic supported lipid bilayers via vesicle fusion. Curr. Opin. Colloid Interface Sci. 2013, 18, 448–458. [Google Scholar] [CrossRef] [Green Version]
- Mechler, A.; Praporski, S.; Atmuri, K.; Boland, M.; Separovic, F.; Martin, L.L. Specific and selective peptide-membrane interactions revealed using quartz crystal microbalance. Biophys. J. 2007, 93, 3907–3916. [Google Scholar] [CrossRef] [Green Version]
- Glasmästar, K.; Larsson, C.; Höök, F.; Kasemo, B. Protein adsorption on supported phospholipid bilayers. J. Colloid Interface Sci. 2002, 246, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Rydell, G.E.; Dahlin, A.B.; Höök, F.; Larson, G. QCM-D studies of human norovirus VLPs binding to glycosphingolipids in supported lipid bilayers reveal strain-specific characteristics. Glycobiology 2009, 19, 1176–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kotarek, J.A.; Moss, M.A. Impact of phospholipid bilayer saturation on amyloid-β protein aggregation intermediate growth: A quartz crystal microbalance analysis. Anal. Biochem. 2010, 399, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.F.; Nagarajan, R.; Mello, C.M.; Camesano, T.A. Characterization of supported lipid bilayer disruption by chrysophsin-3 using QCM-D. J. Phys. Chem. 2011, 115, 15228–15235. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.F.; Nagarajan, R.; Camesano, T.A. Antimicrobial peptide alamethicin insertion into lipid bilayer: A QCM-D exploration. Colloids Surf. B Biointerfaces 2014, 116, 472–481. [Google Scholar] [CrossRef]
- Wang, K.F.; Nagarajan, R.; Camesano, T.A. Differentiating antimicrobial peptides interacting with lipid bilayer: Molecular signatures derived from quartz crystal microbalance with dissipation monitoring. Biophys. Chem. 2015, 196, 53–67. [Google Scholar] [CrossRef]
- Bailey, C.M.; Kamaloo, E.; Waterman, K.L.; Wang, K.F.; Nagarajan, R.; Camesano, T.A. Size dependence of gold nanoparticle interactions with a supported lipid bilayer: A QCM-D study. Biophys. Chem. 2015, 203–204, 51–61. [Google Scholar] [CrossRef] [Green Version]
- Keller, C.A.; Glasmastar, K.; Zhdanov, V.P.; Kasemo, B. Formation of supported membranes from vesicles. Phys. Rev. Lett. 2000, 84, 5443–5446. [Google Scholar] [CrossRef]
- Richter, R.P.; Brisson, A.R. Following the formation of supported lipid bilayers on mica: A study combining AFM, QCM-D, and Ellipsometry. Biophys. J. 2005, 88, 3422–3433. [Google Scholar] [CrossRef] [Green Version]
- Cho, N.-J.; Frank, C.W.; Kasemo, B.; Höök, F. Quartz crystal microbalance with dissipation monitoring of supported lipid bilayers on various substrates. Nat. Protoc. 2010, 5, 1096–1106. [Google Scholar] [CrossRef]
- Jackman, J.A.; Choi, J.H.; Zhdanov, V.P.; Cho, N.J. Influence of osmotic pressure on adhesion of lipid vesicles to solid supports. Langmuir 2013, 29, 11375–11384. [Google Scholar] [CrossRef]
- Anderson, T.H.; Min, Y.; Weirich, K.L.; Zeng, H.; Fygenson, D.; Israelachvili, J.N. Formation of supported bilayers on silica substrates. Langmuir 2009, 25, 6997–7005. [Google Scholar] [CrossRef] [PubMed]
- Basit, H.; Lopez, S.G.; Keyes, T.E. Fluorescence correlation and lifetime correlation spectroscopy applied to the study of supported lipid bilayer models of the cell membrane. Methods 2014, 68, 286–299. [Google Scholar] [CrossRef] [PubMed]
- Lind, T.K.; Cárdenas, M. Understanding the formation of supported lipid bilayers via vesicle fusion—A case that exemplifies the need for the complementary method approach. Biointerphases 2016, 11, 020801. [Google Scholar] [CrossRef] [Green Version]
- Koutsioubas, A.; Appavou, M.S.; Lairez, D. Time-resolved neutron reflectivity during supported membrane formation by vesicle fusion. Langmuir 2017, 33, 10598–10605. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Hofferek, V.; Separovic, F.; Reid, G.E.; Aguilar, M.-I. The role of bacterial lipid diversity and membrane properties in modulating antimicrobial peptide activity and drug resistance. Curr. Opin. Chem. Biol. 2019, 52, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- López-Lara, I.M.; Geiger, O. Bacterial lipid diversity. Biochim. Biophys. Acta 2017, 1862, 1287–1299. [Google Scholar] [CrossRef]
- Luchini, A.; Vitiello, G. Mimicking the Mammalian Plasma Membrane: An Overview of Lipid Membrane Models for Biophysical Studies. Biomimetics 2021, 6, 3. [Google Scholar] [CrossRef]
- Epand, R.F.; Pollard, J.E.; Wright, J.O.; Savage, P.B.; Epand, R.M. Depolarization, Bacterial Membrane Composition, and the Antimicrobial Action of Ceragenins. Antimicrob. Agents Chemother. 2010, 54, 3708–3713. [Google Scholar] [CrossRef] [Green Version]
- Travaglia, A.; Satriano, C.; Giuffrida, M.L.; La Mendola, D.; Rampazzo, E.; Prodi, L.; Rizzarelli, E. Electrostatically driven interaction of silica-supported lipid bilayer nanoplatforms and a nerve growth factor-mimicking peptide. Soft Matter 2013, 9, 4648–4654. [Google Scholar] [CrossRef]
- Weng, K.C.; Kanter, J.L.; Robinson, W.H.; Frank, C.W. Fluid supported lipid bilayers containing monosialoganglioside GM1: A QCM-D and FRAP study. Colloids Surf. B Biointerfaces 2006, 50, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Viitala, T.; Hautala, J.T.; Vuorinen, J.; Wiedmer, S.K. Structure of anionic phospholipid coatings on silica by dissipative quartz crystal microbalance. Langmuir 2007, 23, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Mechler, A.; Praporski, S.; Piantavigna, S.; Heaton, S.M.; Hall, K.N.; Aguilar, M.I.; Martin, L.L. Structure and homogeneity of pseudo-physiological phospholipid bilayers and their deposition characteristics on carboxylic acid terminated self-assembled monolayers. Biomaterials 2009, 30, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Dodd, C.E.; Johnson, B.R.G.; Jeuken, L.J.C.; Bugg, T.D.H.; Bushby, R.J.; Evans, S.D. Native E. coli inner membrane incorporation in solid-supported lipid bilayer membranes. Biointerphases 2008, 3, FA59–FA67. [Google Scholar] [CrossRef] [Green Version]
- Hasan, I.Y.; Mechler, A. Formation of planar unilamellar phospholipid membranes on oxidized gold substrate. Biointerphases 2016, 11, 031017. [Google Scholar] [CrossRef]
- Luchini, A.; Nzulumike, A.N.O.; Lind, T.K.; Nylander, T.; Barker, R.; Arleth, L.; Mortensen, K.; Cárdenas, M. Towards biomimics of cell membranes: Structural effect of phosphatidylinositol triphosphate (PIP3) on a lipid bilayer. Colloids Surf. B Biointerfaces 2019, 173, 202–209. [Google Scholar] [CrossRef]
- Wolanin, J.; Barré, L.; Dalmazzone, C.; Frot, D.; Jestin, J.; Perrot, H.; Bauer, D. Insight into kinetics and mechanisms of AOT vesicle adsorption on silica in unfavorable conditions. Langmuir 2020, 36, 1937–1949. [Google Scholar] [CrossRef]
- Feng, Y.; Zhang, Y.; Liu, G.; Liu, X.; Gao, S. Interaction of graphene oxide with artificial cell membranes: Role of anionic phospholipid and cholesterol in nanoparticle attachment and membrane disruption. Colloids Surf. B Biointerfaces 2021, 202, 111685. [Google Scholar] [CrossRef]
- John, L.H.; Preston, G.M.; Sansom, M.S.P.; Clifton, L.A. Large scale model lipid membrane movement induced by a cation switch. J. Colloid Interface Sci. 2021, 596, 297–311. [Google Scholar] [CrossRef]
- Arouri, A.; Kiessling, V.; Tamm, L.; Dathe, M.; Blume, A. Morphological changes induced by the action of antimicrobial peptides on supported lipid bilayers. J. Phys. Chem. 2011, 115, 158–167. [Google Scholar] [CrossRef] [Green Version]
- Choi, E.J.; Dimitriadis, E.K. Cytochrome c adsorption to supported, anionic lipid bilayers studied via atomic force microscopy. Biophys. J. 2004, 87, 3234–3241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagarajan, R. Molecular packing parameter and surfactant self-assembly: The neglected role of the surfactant tail. Langmuir 2002, 18, 31–38. [Google Scholar] [CrossRef]
- Oliver, R.C.; Lipfert, J.; Fox, D.A.; Lo, R.H.; Doniach, S.; Columbus, J. Dependence of micelle size and shape on detergent alkyl chain length and head group. PLoS ONE 2013, 8, e62488. [Google Scholar] [CrossRef] [PubMed]
- Voinova, M.V.; Jonson, M.; Kasemo, B. ‘Missing mass’ effect in biosensor’s QCM applications. Biosens. Bioelectron. 2002, 17, 835–841. [Google Scholar] [CrossRef]
- Zwang, T.J.; Fletcher, W.R.; Lane, T.J.; Johal, M.S. Quantification of the layer of hydration of a supported lipid bilayer. Langmuir 2010, 26, 4598–4601. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Heberle, F.A.; Tristram-Nagle, S.; Szymanski, M.; Koepfinger, M.; Katsaras, J.; Kučerka, N. Molecular structures of fluid phase phosphatidylglycerol bilayers as determined by small angle neutron and X-ray scattering. Biochim. Biophys. Acta 2012, 1818, 2135–2148. [Google Scholar] [CrossRef]






| Bacteria | Lipid Composition (Mole %) | ||
|---|---|---|---|
| PE | PG | CL | |
| Gram-Positive Bacteria | |||
| B. polymyxa | 60 | 3 | 8 |
| B. cereus | 43 | 40 | 17 |
| E. faecalis | 0 | 27 | 19 |
| S. epidermis | 0 | 90 | 1 |
| S. aureus | 0 | 57 | 19 |
| Gram-Negative Bacteria | |||
| E. coli | 85 | 15 | 5 |
| K. pneumoniae | 82 | 5 | 6 |
| P. aeruginosa | 60 | 21 | 11 |
| Lipid Composition | Comments | Ref. |
|---|---|---|
| POPC/POPS (75:25) | SLB on silicon oxide-coated quartz crystal; 10 mM MgCl2 was added to the buffer for SLB formation. Use of a divalent cation was found to facilitate the SLB incorporating the anionic lipid. | [30] |
| PE/Egg PC/PS (50:45:5) | SLB on silicon oxide-coated quartz crystal. Acyl chain information for PE and PC not specified in the paper. | [10] |
| DOPC/DOPS (80:20) | SLB on mica sheet glued to quartz crystal, in the presence of 2 mM CaCl2. | [18] |
| Egg PC/GM1 (98:2, 95:5) | SLB on silicon oxide-coated quartz crystal. | [31] |
| POPC/Egg PA (80:20) POPC/Egg PG (80:20) POPC/Brain PS (80:20) | SLB on silicon oxide-coated crystal. SLB formation was monitored with or without 3 mM CaCl2. POPC/PG with or without Ca2+ and POPC/PA without Ca2+ form an SLB, while POPC/PS with or without Ca2+ and POPC/PA with Ca2+ form a layer of unruptured vesicles. | [32] |
| DMPC/DMPG (80:20) | SLB on gold-coated quartz crystal functionalized with 3-mercaptopropionic acid (MPA) with the carboxyl group of MPA exposed to the vesicles. | [9,33] |
| Egg PC/E. coli IM | SLB on silicon oxide-coated quartz crystal. | [34] |
| DOPC/DOPS (70:30, 80:20, 90:10) DOPC/DOEPC (70:30, 80:20, 90:10) | SLB on silicon oxide-coated quartz crystal. For the lipid mixture with DOEPC (which is a cationic lipid), vesicle adsorption followed by spontaneous vesicle rupture occurs because of the attractive electrostatic interactions between the positively charged vesicles and the negatively charged crystal surface. It was found that a critical coverage of adsorbed vesicles on the substrate is not necessary to induce spontaneous vesicle rupture. | [19] |
| DMPC/DMPG (70:30) | SLB on gold-coated quartz crystal functionalized with MPA. | [35] |
| POPC/DOPIP3 (90:10) | SLB on silicon oxide-coated quartz crystal. | [36] |
| AOT | SLB on silicon oxide-coated quartz crystal at pH of 1.5 (when the crystal surface maintains a positive surface charge). | [37] |
| DOPC/DOPS (95:5, 90:10, 80:20) | SLB on silicon oxide-coated quartz crystal. | [38] |
| POPC/POPS (80:20) POPC/POPS/DOPIP3 (70:20:10) | Quartz crystal was functionalized with self-assembled monolayers of oligoethyleneglycol with partial ionized carboxyl terminal groups. SLB was formed on this surface in the presence of 2 mM CaCl2. | [39] |
| Vesicle Composition | Average Slope (Hz/min) |
|---|---|
| PC | −41.4 ± 3.9 |
| PG | −1.4 ± 0.0 |
| 9:1 PG/LPG | −13.6 ± 4.2 |
| 8:2 PG/LPG | −11.8 ± 1.1 |
| 7:3 PG/LPG | −8.8 ± 1.0 |
| 6:4 PG/LPG | −12.0 ± 2.5 |
| Lipid | Avg MW g/mol | Final Δf (Hz) | ML ng/Lipid | NL (Lipids/nm2) | aL (nm2/Lipid) | vL (nm3/Lipid) | hL (nm) |
|---|---|---|---|---|---|---|---|
| PC | 770 | −26 | 1.28 × 10−12 | 1.41 | 0.71 | 0.96 | 2.71 |
| 9:1 PG/LPG | 754 | −25 | 1.25 × 10−12 | 1.37 | 0.73 | 0.91 | 2.49 |
| 8:2 PG/LPG | 727 | −24 | 1.21 × 10−12 | 1.35 | 0.74 | 0.86 | 2.32 |
| 7:3 PG/LPG | 699 | −24 | 1.16 × 10−12 | 1.40 | 0.71 | 0.81 | 2.27 |
| 6:4 PG/LPG | 672 | −18 | 1.12 × 10−12 | 0.98 | 1.02 | 0.76 | 1.49 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Swana, K.W.; Camesano, T.A.; Nagarajan, R. Formation of a Fully Anionic Supported Lipid Bilayer to Model Bacterial Inner Membrane for QCM-D Studies. Membranes 2022, 12, 558. https://doi.org/10.3390/membranes12060558
Swana KW, Camesano TA, Nagarajan R. Formation of a Fully Anionic Supported Lipid Bilayer to Model Bacterial Inner Membrane for QCM-D Studies. Membranes. 2022; 12(6):558. https://doi.org/10.3390/membranes12060558
Chicago/Turabian StyleSwana, Kathleen W., Terri A. Camesano, and Ramanathan Nagarajan. 2022. "Formation of a Fully Anionic Supported Lipid Bilayer to Model Bacterial Inner Membrane for QCM-D Studies" Membranes 12, no. 6: 558. https://doi.org/10.3390/membranes12060558