State-of-the-Art Organic- and Inorganic-Based Hollow Fiber Membranes in Liquid and Gas Applications: Looking Back and Beyond
Abstract
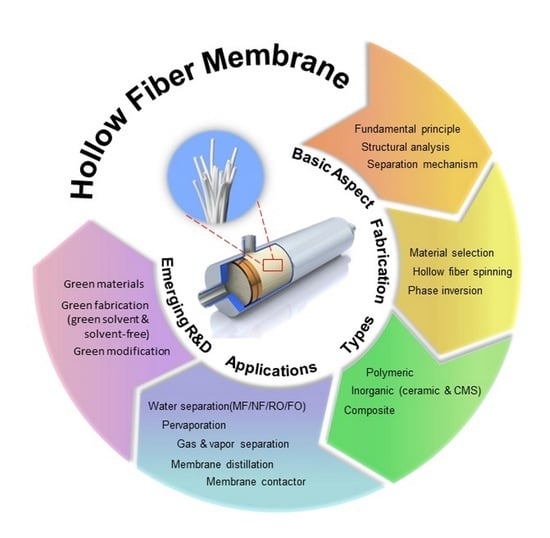
:1. Introduction
2. Fundamental Aspects of Hollow Fiber Membranes
2.1. Structural Analyses on Hollow Fiber Membrane
2.2. Phase Inversion Mechanism during Hollow Fiber Formation
3. Types of Hollow Fiber and Their Preparation Route
3.1. Organic and Inorganic Materials-Derived Hollow Fiber Membranes
3.2. Composite Hollow Fiber Membranes
4. Applications of Hollow Fiber Membranes
4.1. Microfiltration (MF)
4.2. Nanofiltration (NF)/Organic Solvent Nanofiltration (OSN)
4.3. Reverse Osmosis (RO)/Organic Solvent Reverse Osmosis (OSRO)
4.4. Forward Osmosis (FO)/Organic Solvent Forward Osmosis (OSFO)
4.5. Pervaporation
| Membrane a | Water-Organic Mixture | Feed Composition (wt%) | Operating Temperature (°C) | Water Flux (kg/m2 h) | Separation Factor | Reference |
|---|---|---|---|---|---|---|
| CS-PSS/ceramic b | water-ethanol | 10/90 | 70 | 0.495 | 904 | [355] |
| NaA/alumina b | water-ethanol | 10/90 | 75 | 12.8 | 10,000 | [356] |
| NaA/alumina b | water-ethanol | 10/90 | 75 | 19.7 | >80,000 | [357] |
| T-type zeolite/YSZ b | water-ethanol | 10/90 | 70 | 0.78 | >90 | [358] |
| CTA/UiO-66-NH2/Ultem c | water-ethanol | 15/85 | 50 | 2.667 | 152 | [359] |
| PA/PES/silicon rubber d | water-ethanol | 15/85 | 50 | 7.5 | 60 | [360] |
| PA/TEPA/PAN d | water-ethanol | 10/90 | 25 | 0.342 | 366 | [361] |
| CS-PVA/PVDF b | water-isopropanol | 10/90 | 60 | 0.306 | 2140 | [362] |
| CS-TMC/alumina b | water-isopropanol | 10/90 | 70 | 0.908 | 8993 | [363] |
| CTA/Ultem b | water-isopropanol | 13.3/86.7 | 125 | 13.41 | 1332 | [364] |
| MoS2-PEI/TiO2/ceramic b | water-isopropanol | 10/90 | 70 | 5.697 | 320 | [365] |
| PA/Ultem/PDMS b | water-isopropanol | 15/85 | 50 | 2.65 | 246 | [366] |
| Teflon/Ultem b | water-isopropanol | 5/95 | 125 | 4.625 | 383 | [367] |
| T-type zeolite/YSZ b | water-isopropanol | 10/90 | 75 | 7.36 | >10,000 | [368] |
| PA/TiO2/alumina d | water-isopropanol | 10/90 | 60 | 6.44 | >12,000 | [188] |
| UiO-66/YSZ b | water-isobutanol | 5/95 | 50 | 4.81 | >45,000 | [369] |
| SA/TDI-GA/CTA-PAN d | water-isobutanol | 2/98 | 25 | 0.021 | 3229 | [370] |
| CHA zeolite/YSZ b | water-acetic acid | 50/50 | 75 | 12 | >10,000 | [372] |
| DD3R/ceramic b | water-acetic acid | 70/30 | 95 | 0.58 | 800 | [191] |
| DD3R-APTES/ceramic b | water-acetic acid | 10/90 | 75 | 0.23 | 1700 | [373] |
| SA-TDI-GA/CTA-PAN b | water-acetic acid | 5/95 | 25 | 0.012 | 708 | [370] |
| P84/EDA b | water-acetone | 15/85 | 50 | 1.8 | 53 | [374] |
| PBI/PEI’ b | water-ethyl acetate | 2/98 | 60 | 0.82 | 2478 | [375] |
| UiO-66/YSZ b | water-furfural | 5/95 | 50 | 5.95 | >45,000 | [369] |
| water-tetrahydrofuran | 5/95 | 70 | 4.06 | >45,000 | [369] | |
| T-type zeolite/alumina b | water-ethanol-acetic acid | 9.3/83.8/6.9 | 75 | 2.25 | 1348 | [395] |
| GO-PVA-TEOS/alumina b | water-isopropanol-epichlorohydrin | 20/30/50 | 30 | 0.09 | 4844 | [396] |
4.6. Gas and Vapor Separation
4.7. Membrane Distillation (MD)
4.8. Membrane Contactor
4.9. Other

5. Emerging R&D on Hollow Fiber Membranes
5.1. Green Fabrication Technique
5.1.1. Green Solvent
5.1.2. Solvent-Free Method
5.2. Modification of Hollow Fiber Membranes via Greener Approach

5.3. Green Materials for Hollow Fiber Membranes Fabrication
6. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 2D | Two-dimensional |
| 6FDA | 4,4′-(hexafluoroisopropylidene) diphthalic anhydride |
| Abn-NH | Amino functionalized acid activated bentonite clay |
| AF | Acid Fuchsin |
| Ag | Silver |
| Al2O3 | Aluminium oxide |
| APTES | 3-aminopropyltriethoxysilane |
| AR88 | Acid Red 88 |
| B2RL | Direct Fast Blue B2RL |
| BBR | Brilliant Blue R |
| BCBZ | BaCo0.85Bi0.05Zr0.1O3−δ |
| BPDA | 3,3′-4,4′-biphenyl tetracarboxylic acid dianhydride |
| BSA | Bovine serum albumin |
| BSCF | Ba0.5Sr0.5Co0.8Fe0.2O3−δ |
| BTB | Bromothymol Blue |
| C2H4 | Ethane or ethylene |
| C3H6 | Propylene or propene or paraffin |
| C3H8 | Propane or olefin |
| CECs | Contaminants of emerging concern |
| CFB | Chromatrope FB |
| CHA | Chabazite |
| CL | Chitosan lactate |
| Cl− | Chloride ions |
| CMPs | Conjugated microporous polymers |
| CMS | Carbon molecular sieve |
| CO2 | Carbon dioxide |
| COF(s) | Covalent organic framework(s) |
| CQDs | Carbon quantum dots |
| CR | Congo Red |
| CTA | Cellulose triacetate |
| c-TiO2 | Carboxylated TiO2 |
| DABA | Diaminobenzoic acid |
| DAM | 2,4,6-trimethyl-1,3-phenylene diamine |
| DAPE | 1,3-diaminopropane |
| DCMD | Direct contact membrane distillation |
| DD3R | Decadodecasil 3R |
| DES(s) | Deep eutectic solvent(s) |
| DETDA | Diethyltoluenediamine |
| DIPS | Diffusion-induced phase separation |
| DMAc | Dimethylacetamide |
| DMF | Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| DR23 | Direct Red 23 |
| EBT | Eriochrome Black T |
| ECHA | European Chemical Agency |
| EDA | Ethylenediamine |
| ETS-4 | Engelhard titanosilicate-4 |
| EVAL | Poly(ethylene vinyl) alcohol |
| Fe | Iron |
| FESEM | Field emission scanning electron microscope |
| FO | Forward osmosis |
| FRR | flux recovery ratio |
| GA | Glutaraldehyde |
| GBL | γ-butyrolactone |
| GO | Graphene oxide |
| GRL | Cationic Red X-GRL |
| GTL | Cationic red GTL |
| H2 | Hydrogen |
| H2S | Hydrogen sulfide |
| IL(s) | Ionic liquid(s) |
| K2S2O8 | Potassium persulphate |
| KNO3/KNO2-BSCF | potassium nitrate/potassium nitrite-BSCF |
| LCCF | (La0.6Ca0.4)(Co0.8Fe0.2)O3−δ |
| LCF | La0.8Ca0.2Fe0.94O3−a |
| LSC | La0.6Sr0.4CoO3−δ |
| LSCF | La0.6Sr0.4Co0.2Fe0.8O3−δ |
| MB | Methylene Blue |
| MD | Membrane distillation |
| MF | Microfiltration |
| MgCl2 | Magnesium chloride |
| MIL | Materials of Institute Lavoisier |
| MO | Methyl Orange |
| MOF(s) | Metal organic framework(s) |
| MoS2 | Molybdenum disulfide |
| MPD | M-phenylenediamine |
| MR | Methyl Red |
| MS-S | Melt-spinning and stretching |
| MWCNT | Multi-walled carbon nanotubes |
| MWCO | Molecular weight cut off |
| N2 | Nitrogen |
| Na+ | Sodium ions |
| Na+-CQDs | Sodium-functionalized CQDs |
| Na2SO4 | Sodium sulfate |
| NaCl | Sodium chloride |
| NADES | Natural deep eutectic solvent |
| NaOH | Sodium hydroxide |
| NF | Nanofiltration |
| NH2-MWCNT | Amine-functionalized MWCNT |
| NIPS | Non-solvent induced phase separation |
| NMP | N-methyl-2-pyrrolidone |
| NO | Nitric oxide |
| O2 | Oxygen |
| OARO | Osmotically assisted reverse osmosis |
| OSFO | Organic solvent forward osmosis |
| OSN | Organic solvent nanofiltration |
| OSRO | Organic solvent reverse osmosis |
| P(VTES-AA-SSNa) | Poly(triethoxyvinylsilane-acrylic acid-sodium 4-vinylbenzenssulfonate) |
| PA | Polyamide |
| PAA | Polyamic acid |
| PAN | Polyacrylonitrile |
| PANI | Polyaniline |
| PBI | Polybenzimidazole |
| PC | Polycarbonate |
| PCL | Polycaprolactone |
| Pd | Palladium |
| PDA | Polydopamine |
| PDMS | Polydimethylsiloxane |
| PEG | Polyethylene glycol |
| PEI | Polyethyleneimine |
| PEO | Polyethylene oxide |
| PES | Polyethersulfone |
| PET | Polyethylene terephthalate |
| PHAs | Polyhydroxyalkanoates |
| PI/LPSQ | Polyimide/ladder-structured polysilsesquioxane |
| PIMs | Polymers of intrinsic microporosity |
| PIP | Piperazine |
| PLA | Polylactic acid |
| PMDA-MDA | Pyromellitic dianhydride-4,4′-diaminodiphenylmethane |
| PMDA-ODA | Poly(4,4′-oxydiphenylene pyromellitimide) |
| PMIA | Poly(m-phenylene isophthalamide) |
| PMMOF | Polymer-modification-enabled in-site metal-organic framework |
| PMP | Poly(4-methyl-1-pentene) |
| PPA | Poly(piperazine-amide) |
| PPTA | Poly(p-phenylene terephthalamide) |
| PPTA | Poly(p-phenylene terephthalamide) |
| PSF | Polysulfone |
| PSS | Poly(4-styrenesulfonic acid) |
| PTFE | Polytetrafluoroethylene |
| PVA | Poly(vinyl alcohol) |
| PVDF | Polyvinylidene fluoride |
| PWP | Pure water permeability |
| RB | Rose Bengal |
| RB5 | Reactive Black 5 |
| RBB | Remazol Brilliant Blue |
| RDB | Rhodamine B |
| rGO | Reduced GO |
| RhB | Rhodamine B |
| RO | Reverse osmosis |
| RR195 | Reactive Red 195 |
| RY3 | Reactive Yellow 3 |
| SAPO-34 | Silicoaluminophosphate chabazite |
| SEM | Scanning electron microscope |
| SILMs | Supported ionic liquid membrane(s) |
| SiO2 | Silicon dioxide |
| SMA | Styrene maleic anhydride |
| SO2 | Sulfur dioxide |
| ST | Safranine T |
| TDI | 2,4-toluene diisocyanate |
| TEOS | Tetraethyl orthosilicate |
| TEP | Triethyl phosphate |
| TEPA | Tetraethylenepentamine |
| TFC | Thin film composite |
| TFN | Thin film nanocomposite |
| THF | Tetrahydrofuran |
| TiO2 | Titanium dioxide |
| TIPS | Thermally induced phase separation |
| TMC | Trimesoyl chloride |
| TR | Thermally rearraged |
| UF | Ultrafiltration |
| UiO | Universitetet i Oslo |
| VTMS | Vinyltrimethoxysilane |
| XDLVO | Extended Derjaguin-Landau-Verwey-Overbeek |
| YSZ | Yttria-stabilized zirconia |
| ZIF(s) | Zeolitic imidazolate framework(s) |
| ZnCl2 | Zinc chloride |
| ZrO2 | Zirconium dioxide |
| β-CD | β-cyclodextrin |
References
- Guo, Y.; Tong, L.; Mei, L. Evaluation and influencing factors of industrial pollution in Jilin restricted development zone: A spatial econometric analysis. Sustainability 2021, 13, 4194. [Google Scholar] [CrossRef]
- Kim, S. Membranes for water, gas and ion separation. Membranes 2021, 11, 325. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, A.; Macedonio, F.; Barbieri, G.; Drioli, E. Membrane engineering for environmental protection and sustainable industrial growth: Options for water and gas treatment. Environ. Eng. Res. 2015, 20, 307–328. [Google Scholar] [CrossRef]
- Font-Palma, C.; Cann, D.; Udemu, C. Review of cryogenic carbon capture innovations and their potential applications. C 2021, 7, 58. [Google Scholar] [CrossRef]
- Sircar, S.; Golden, T. Purification of hydrogen by pressure swing adsorption. Sep. Sci. Technol. 2000, 35, 667–687. [Google Scholar] [CrossRef]
- Sholl, D.S.; Lively, R.P. Seven chemical separations to change the world. Nature 2016, 532, 435–437. [Google Scholar] [CrossRef]
- Liang, C.Z.; Chung, T.-S.; Lai, J.-Y. A review of polymeric composite membranes for gas separation and energy production. Prog. Polym. Sci. 2019, 97, 101141. [Google Scholar] [CrossRef]
- Pagliero, M.; Khayet, M.; Garcia-Payo, C.; García-Fernández, L. Hollow fibre polymeric membranes for desalination by membrane distillation technology: A review of different morphological structures and key strategic improvements. Desalination 2021, 516, 115235. [Google Scholar] [CrossRef]
- Chung, T.-S.; Feng, Y. Hollow Fiber Membranes: Fabrication and Applications; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Pagliero, M.; Comite, A.; Soda, O.; Costa, C. Effect of support on PVDF membranes for distillation process. J. Membr. Sci. 2021, 635, 119528. [Google Scholar] [CrossRef]
- Peng, N.; Widjojo, N.; Sukitpaneenit, P.; Teoh, M.M.; Lipscomb, G.G.; Chung, T.-S.; Lai, J.-Y. Evolution of polymeric hollow fibers as sustainable technologies: Past, present, and future. Prog. Polym. Sci. 2012, 37, 1401–1424. [Google Scholar] [CrossRef]
- Lau, H.S.; Yong, W.F. Recent progress and prospects of polymeric hollow fiber membranes for gas application, water vapor separation and particulate matter removal. J. Mater. Chem. A 2021, 9, 26454–26497. [Google Scholar] [CrossRef]
- Moch, I., Jr. Membranes, Hollow-Fiber; John Wiley & Sons Inc.: New York, NY, USA, 2000. [Google Scholar]
- Mahon, H.I. Permeability Separatory Apparatus, Permeability Separatory Membrane Element, Method of Making the Same and Process Utilizing the Same. U.S. Patent 3,228,876A, 11 January 1966. [Google Scholar]
- Lonsdale, H. The growth of membrane technology. J. Membr. Sci. 1982, 10, 31–181. [Google Scholar] [CrossRef]
- Mclain, E.A. Wound Hollow Fiber Permeability Apparatus and Process of Making the Same. U.S. Patent 3,422,008, 14 January 1969. [Google Scholar]
- Li, N.N.; Fane, A.G.; Ho, W.W.; Matsuura, T. Advanced Membrane Technology and Applications; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011. [Google Scholar]
- Henis, J.M.; Tripodi, M.K. A novel approach to gas separations using composite hollow fiber membranes. Sep. Sci. Technol. 1980, 15, 1059–1068. [Google Scholar] [CrossRef]
- Henis, J.M.; Tripodi, M.K. Composite hollow fiber membranes for gas separation: The resistance model approach. J. Membr. Sci. 1981, 8, 233–246. [Google Scholar] [CrossRef]
- Henis, J.M.; Tripodi, M.K. Multicomponent Membranes for Gas Separations. U.S. Patent 4,230,463, 28 October 1980. [Google Scholar]
- Waterland, L.R.; Robertson, C.R.; Michaels, A.S. Enzymatic catalysis using asymmetric hollow fiber membranes. Chem. Eng. Commun. 1975, 2, 37–47. [Google Scholar] [CrossRef]
- Bollinger, W.; Chen, G.; Metzer, T. Commerical Hydrogen Purification and Recovery Using PRISM Separators; Monsanto Co.: St. Louis, MO, USA, 1981. [Google Scholar]
- Moattari, R.M.; Mohammadi, T.; Rajabzadeh, S.; Dabiryan, H.; Matsuyama, H. Reinforced hollow fiber membranes: A comprehensive review. J. Taiwan Inst. Chem. Eng. 2021, 122, 284–310. [Google Scholar] [CrossRef]
- Jung, J.T.; Wang, H.H.; Kim, J.F.; Lee, J.; Kim, J.S.; Drioli, E.; Lee, Y.M. Tailoring nonsolvent-thermally induced phase separation (N-TIPS) effect using triple spinneret to fabricate high performance PVDF hollow fiber membranes. J. Membr. Sci. 2018, 559, 117–126. [Google Scholar] [CrossRef]
- Hassankiadeh, N.T.; Cui, Z.; Kim, J.H.; Shin, D.W.; Sanguineti, A.; Arcella, V.; Lee, Y.M.; Drioli, E. PVDF hollow fiber membranes prepared from green diluent via thermally induced phase separation: Effect of PVDF molecular weight. J. Membr. Sci. 2014, 471, 237–246. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Lu, X.; Xiao, C. Morphology changes of polyvinylidene fluoride membrane under different phase separation mechanisms. J. Membr. Sci. 2008, 320, 277–482. [Google Scholar] [CrossRef]
- Li, G.; Kujawski, W.; Válek, R.; Koter, S. A review-The development of hollow fibre membranes for gas separation processes. Int. J. Greenh. Gas Control 2021, 104, 103195. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Peng, N.; Chung, T.-S. Gas separation membranes developed through integration of polymer blending and dual-layer hollow fiber spinning process for hydrogen and natural gas enrichments. J. Membr. Sci. 2010, 349, 156–166. [Google Scholar] [CrossRef]
- Li, D.F.; Chung, T.-S.; Wang, R.; Liu, Y. Fabrication of fluoropolyimide/polyethersulfone (PES) dual-layer asymmetric hollow fiber membranes for gas separation. J. Membr. Sci. 2002, 198, 211–223. [Google Scholar] [CrossRef]
- Jo, E.-S.; An, X.; Ingole, P.G.; Choi, W.-K.; Park, Y.-S.; Lee, H.-K. CO2/CH4 separation using inside coated thin film composite hollow fiber membranes prepared by interfacial polymerization. Chin. J. Chem. Eng. 2017, 25, 278–287. [Google Scholar] [CrossRef]
- Wenten, I.; Khoiruddin, K.; Wardani, A.; Aryanti, P.; Astuti, D.; Komaladewi, A. Preparation of antifouling polypropylene/ZnO composite hollow fiber membrane by dip-coating method for peat water treatment. J. Water Process. Eng. 2020, 34, 101158. [Google Scholar] [CrossRef]
- Sharma, A.K.; Juelfs, A.; Colling, C.; Sharma, S.; Conover, S.P.; Puranik, A.A.; Chau, J.; Rodrigues, L.; Sirkar, K.K. Porous hydrophobic–hydrophilic composite hollow fiber and flat membranes prepared by plasma polymerization for direct contact membrane distillation. Membranes 2021, 11, 120. [Google Scholar] [CrossRef]
- Park, H.J.; Bhatti, U.H.; Nam, S.C.; Park, S.Y.; Lee, K.B.; Baek, I.H. Nafion/TiO2 nanoparticle decorated thin film composite hollow fiber membrane for efficient removal of SO2 gas. Sep. Purif. Technol. 2019, 211, 377–390. [Google Scholar] [CrossRef]
- Liang, C.Z.; Yong, W.F.; Chung, T.-S. High-performance composite hollow fiber membrane for flue gas and air separations. J. Membr. Sci. 2017, 541, 367–377. [Google Scholar] [CrossRef]
- Li, M.; Zhu, Z.; Zhou, M.; Jie, X.; Wang, L.; Kang, G.; Cao, Y. Removal of CO2 from biogas by membrane contactor using PTFE hollow fibers with smaller diameter. J. Membr. Sci. 2021, 627, 119232. [Google Scholar] [CrossRef]
- Naderi, A.; Chung, T.-S.; Weber, M.; Maletzko, C. High performance dual-layer hollow fiber membrane of sulfonated polyphenylsulfone/polybenzimidazole for hydrogen purification. J. Membr. Sci. 2019, 591, 117292. [Google Scholar] [CrossRef]
- Yang, D.; Barbero, R.S.; Devlin, D.J.; Cussler, E.; Colling, C.W.; Carrera, M.E. Hollow fibers as structured packing for olefin/paraffin separations. J. Membr. Sci. 2006, 279, 21–69. [Google Scholar] [CrossRef]
- Qi, Z.; Cussler, E. Microporous hollow fibers for gas absorption: II. Mass transfer across the membrane. J. Membr. Sci. 1985, 23, 333–345. [Google Scholar] [CrossRef]
- Huang, Y.; Xiao, C.; Huang, Q.; Liu, H.; Zhao, J. Progress on polymeric hollow fiber membrane preparation technique from the perspective of green and sustainable development. Chem. Eng. J. 2021, 403, 126295. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, C.; Hu, X.; Bai, Q. Preparation and properties of homogeneous-reinforced polyvinylidene fluoride hollow fiber membrane. Appl. Surf. Sci. 2013, 264, 801–810. [Google Scholar] [CrossRef]
- Hu, X.; Chen, Y.; Liang, H.; Xiao, C. Preparation of polyurethane/poly (vinylidene fluoride) blend hollow fibre membrane using melt spinning and stretching. Mater. Sci. Technol. 2011, 27, 661–665. [Google Scholar] [CrossRef]
- Aslan, T.; Arslan, S.; Eyvaz, M.; Güçlü, S.; Yüksel, E.; Koyuncu, I. A novel nanofiber microfiltration membrane: Fabrication and characterization of tubular electrospun nanofiber (TuEN) membrane. J. Membr. Sci. 2016, 520, 616–629. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, eaab0530. [Google Scholar] [CrossRef] [Green Version]
- Hays, S.S.; Sanyal, O.; León, N.E.; Arab, P.; Koros, W.J. Envisioned role of slit bypass pores in physical aging of carbon molecular sieve membranes. Carbon 2020, 157, 385–394. [Google Scholar] [CrossRef]
- Chen, C.-C.; Qiu, W.; Miller, S.J.; Koros, W.J. Plasticization-resistant hollow fiber membranes for CO2/CH4 separation based on a thermally crosslinkable polyimide. J. Membr. Sci. 2011, 382, 212–221. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, X.; Paul, D.R. Physical aging of thin glassy polymer films: Free volume interpretation. J. Membr. Sci. 2006, 277, 219–229. [Google Scholar] [CrossRef]
- Dong, G.; Li, H.; Chen, V. Plasticization mechanisms and effects of thermal annealing of Matrimid hollow fiber membranes for CO2 removal. J. Membr. Sci. 2011, 369, 206–220. [Google Scholar] [CrossRef]
- Alklaibi, A.M.; Lior, N. Membrane-distillation desalination: Status and potential. Desalination 2005, 171, 111–131. [Google Scholar] [CrossRef]
- Lau, S.K.; Yong, W.F. Recent progress of zwitterionic materials as antifouling membranes for ultrafiltration, nanofiltration, and reverse osmosis. ACS Appl. Polym. Mater. 2021, 3, 4390–4412. [Google Scholar] [CrossRef]
- Zhang, R.; Liu, Y.; He, M.; Su, Y.; Zhao, X.; Elimelech, M.; Jiang, Z. Antifouling membranes for sustainable water purification: Strategies and mechanisms. Chem. Soc. Rev. 2016, 45, 5888–5924. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.U.; Othman, M.H.D.; Ismail, A.; Matsuura, T.; Hashim, H.; Nordin, N.A.H.M.; Rahman, M.A.; Jaafar, J.; Jilani, A. Status and improvement of dual-layer hollow fiber membranes via co-extrusion process for gas separation: A review. J. Nat. Gas Sci. Eng. 2018, 52, 215–234. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Galiano, F.; Figoli, A. Recent advances in pervaporation hollow fiber membranes for dehydration of organics. Chem. Eng. Res. Des. 2020, 164, 68–85. [Google Scholar] [CrossRef]
- Sirkar, K.K. Membranes, phase interfaces, and separations: Novel techniques and membranes—An overview. Ind. Eng. Chem. Res. 2008, 47, 5250–5266. [Google Scholar] [CrossRef]
- Sewerin, T.; Elshof, M.G.; Matencio, S.; Boerrigter, M.; Yu, J.; de Grooth, J. Advances and applications of hollow fiber nanofiltration membranes: A review. Membranes 2021, 11, 890. [Google Scholar] [CrossRef]
- Yang, Z.; Zhou, Y.; Feng, Z.; Rui, X.; Zhang, T.; Zhang, Z. A review on reverse osmosis and nanofiltration membranes for water purification. Polymers 2019, 11, 1252. [Google Scholar] [CrossRef] [Green Version]
- Turken, T.; Sengur-Tasdemir, R.; Ates-Genceli, E.; Tarabara, V.V.; Koyuncu, I. Progress on reinforced braided hollow fiber membranes in separation technologies: A review. J. Water Process. Eng. 2019, 32, 100938. [Google Scholar] [CrossRef]
- Ahmad, A.L.; Otitoju, T.A.; Ooi, B.S. Hollow fiber (HF) membrane fabrication: A review on the effects of solution spinning conditions on morphology and performance. J. Ind. Eng. Chem. 2019, 70, 35–50. [Google Scholar] [CrossRef]
- Dashti, A.; Asghari, M. Recent progresses in ceramic hollow-fiber membranes. ChemBioEng Rev. 2015, 2, 54–70. [Google Scholar] [CrossRef]
- Feng, C.; Khulbe, K.; Matsuura, T.; Ismail, A. Recent progresses in polymeric hollow fiber membrane preparation, characterization and applications. Sep. Purif. Technol. 2013, 111, 43–71. [Google Scholar] [CrossRef]
- Feng, Y.; Chung, T.-S. Chapter 1—Introduction. In Hollow Fiber Membranes; Chung, T.-S., Feng, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 1–11. [Google Scholar]
- Baker, R.W.; Low, B.T. Gas separation membrane materials: A perspective. Macromolecules 2014, 47, 6999–7013. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Deng, L. Recent advances in multi-layer composite polymeric membranes for CO2 separation: A review. Green Energy Environ. 2016, 1, 102–128. [Google Scholar] [CrossRef] [Green Version]
- Baker, R.W. Membrane Technology and Applications, 3rd ed.; John Wiley & Sons, Ltd.: San Francisco, CA, USA, 2012. [Google Scholar]
- Matsuyama, H.; Karkhanechi, H.; Rajabzadeh, S. Chapter 3—Polymeric membrane fabrication via thermally induced phase separation (TIPS) method. In Hollow Fiber Membranes; Chung, T.-S., Feng, Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 57–83. [Google Scholar]
- Mulder, M.; Mulder, J. Basic Principles of Membrane Technology, 2nd ed.; Springer: Dordrecht, The Netherlands, 1996. [Google Scholar]
- Yong, W.F.; Chung, T.-S.; Weber, M.; Maletzko, C. New polyethersulfone (PESU) hollow fiber membranes for CO2 capture. J. Membr. Sci. 2018, 552, 305–314. [Google Scholar] [CrossRef]
- Li, M.; Feng, Y.; Wang, K.; Yong, W.F.; Yu, L.; Chung, T.-S. Novel hollow fiber air filters for the removal of ultrafine particles in PM2.5 with repetitive usage capability. Environ. Sci. Technol. 2017, 51, 10041–10049. [Google Scholar] [CrossRef]
- Li, Y.; Cao, B.; Li, P. Fabrication of PMDA-ODA hollow fibers with regular cross-section morphologies and study on the formation mechanism. J. Membr. Sci. 2017, 544, 1–11. [Google Scholar] [CrossRef]
- Tham, H.M.; Wang, K.Y.; Hua, D.; Japip, S.; Chung, T.-S. From ultrafiltration to nanofiltration: Hydrazine cross-linked polyacrylonitrile hollow fiber membranes for organic solvent nanofiltration. J. Membr. Sci. 2017, 542, 289–299. [Google Scholar] [CrossRef]
- Liang, C.Z.; Liu, J.T.; Lai, J.-Y.; Chung, T.-S. High-performance multiple-layer PIM composite hollow fiber membranes for gas separation. J. Membr. Sci. 2018, 563, 93–106. [Google Scholar] [CrossRef]
- Cheng, D.; Zhao, L.; Li, N.; Smith, S.J.; Wu, D.; Zhang, J.; Ng, D.; Wu, C.; Martinez, M.R.; Batten, M.P. Aluminum fumarate MOF/PVDF hollow fiber membrane for enhancement of water flux and thermal efficiency in direct contact membrane distillation. J. Membr. Sci. 2019, 588, 117204. [Google Scholar] [CrossRef]
- Ren, J.; Wang, R. Preparation of polymeric membranes. In Membrane and Desalination Technologies; Wang, L.K., Chen, J.P., Hung, Y.-T., Shammas, N.K., Eds.; Springer: New York, NY, USA, 2011; pp. 47–100. [Google Scholar]
- Tompa, H. Polymer Solutions; Butterworths Scientific Publications: London, UK, 1956. [Google Scholar]
- Strathmann, H.; Scheible, P.; Baker, R. A rationale for the preparation of Loeb-Sourirajan-type cellulose acetate membranes. J. Appl. Polym. Sci. 1971, 15, 811–828. [Google Scholar] [CrossRef]
- Michaels, A.S. High Flow Membrane. U.S. Patent 3,615,024, 26 October 1971. [Google Scholar]
- Figoli, A.; Cassano, A.; Basile, A. Membrane Technologies for Biorefining; Woodhead Publishing: Duxford, UK, 2016. [Google Scholar]
- Purkait, M.K.; Sinha, M.K.; Mondal, P.; Singh, R. Introduction to membranes. In Interface Science and Technology; Elsevier: Amsterdam, The Netherlands, 2018; Volume 25, pp. 1–37. [Google Scholar]
- Kapantaidakis, G.; Koops, G.; Wessling, M. Effect of spinning conditions on the structure and the gas permeation properties of high flux polyethersulfone-polyimide blend hollow fibers. Desalination 2002, 144, 121–125. [Google Scholar] [CrossRef]
- Tasselli, F.; Jansen, J.; Sidari, F.; Drioli, E. Morphology and transport property control of modified poly(ether ether ketone)(PEEKWC) hollow fiber membranes prepared from PEEKWC/PVP blends: Influence of the relative humidity in the air gap. J. Membr. Sci. 2005, 255, 13–22. [Google Scholar] [CrossRef]
- Da Silva Barbosa Ferreira, R.; Dias, R.A.; Araújo, E.M.; Oliveira, S.S.L.; da Nóbrega Medeiros, V.; de Lucena Lira, H. Hollow fiber membranes of polysulfone/attapulgite for oil removal in wastewater. Polym. Bull. 2022, 1–21. [Google Scholar] [CrossRef]
- Mondal, R.; De, S. Removal of copper (II) from aqueous solution using zinc oxide nanoparticle impregnated mixed matrix hollow fiber membrane. Environ. Technol. Innov. 2022, 26, 102300. [Google Scholar] [CrossRef]
- Lim, Y.-G.; Bak, C.-u.; Kim, Y.-D. Comprehensive experimental and theoretical insights into the performance of polysulfone hollow-fiber membrane modules in biogas purification process. Chem. Eng. J. 2022, 433, 134616. [Google Scholar] [CrossRef]
- Zou, D.; Kim, H.W.; Jeon, S.M.; Lee, Y.M. Fabrication and modification of PVDF/PSF hollow-fiber membranes for ginseng extract and saline water separations via direct contact membrane distillation. J. Membr. Sci. 2022, 644, 120101. [Google Scholar] [CrossRef]
- Dibrov, G.; Kagramanov, G.; Sudin, V.; Molchanov, S.; Grushevenko, E.; Yushkin, A.; Volkov, V. Influence of draw ratio and take-up velocity on properties of ultrafiltration hollow fiber membranes from polyethersulfone. Fibers 2022, 10, 29. [Google Scholar] [CrossRef]
- Liang, C.Z.; Yong, W.F.; Wu, J.; Weber, M.; Maletzko, C.; Lai, J.-Y.; Chung, T.-S. Plasticization-enhanced trimethylbenzene functionalized polyethersulfone hollow fiber membranes for propylene and propane separation. J. Membr. Sci. 2022, 647, 120293. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Hu, X.; Guo, P. Engineering novel high-flux thin-film composite (TFC) hollow fiber nanofiltration membranes via a facile and scalable coating procedure. Desalination 2022, 526, 115531. [Google Scholar] [CrossRef]
- Czieborowski, M.; Kemperman, A.J.; Rolevink, E.; Blom, J.; Visser, T.; Philipp, B. A two-step bioluminescence assay for optimizing antibacterial coating of hollow-fiber membranes with polydopamine in an integrative approach. J. Microbiol. Methods 2022, 196, 106452. [Google Scholar] [CrossRef] [PubMed]
- Vatanpour, V.; Pasaoglu, M.E.; Barzegar, H.; Teber, O.O.; Kaya, R.; Bastug, M.; Khataee, A.; Koyuncu, I. Cellulose acetate in fabrication of polymeric membranes: A review. Chemosphere 2022, 295, 133914. [Google Scholar] [CrossRef] [PubMed]
- Sunder, N.; Fong, Y.Y.; Bustam, M.A. Investigation on the effects of air gap distance on the formation of cellulose triacetate hollow fiber membrane for CO2 and CH4 gases permeation. Mater. Today Proc. 2022, in press. [Google Scholar] [CrossRef]
- Nakao, T.; Goda, S.; Miura, Y.; Yasukawa, M.; Ishibashi, M.; Nakagawa, K.; Shintani, T.; Matsuyama, H.; Yoshioka, T. Development of cellulose triacetate asymmetric hollow fiber membranes with highly enhanced compaction resistance for osmotically assisted reverse osmosis operation applicable to brine concentration. J. Membr. Sci. 2022, 653, 120508. [Google Scholar] [CrossRef]
- Theodorakopoulos, G.V.; Karousos, D.S.; Mansouris, K.G.; Sapalidis, A.A.; Kouvelos, E.P.; Favvas, E.P. Graphene nanoplatelets based polyimide/Pebax dual-layer mixed matrix hollow fiber membranes for CO2/CH4 and He/N2 separations. Int. J. Greenh. Gas Control 2022, 114, 103588. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Li, S.; Xu, S.; Tian, L.; Su, B.; Han, L.; Mandal, B. Fundamental understanding on the preparation conditions of high-performance polyimide-based hollow fiber membranes for organic solvent nanofiltration (OSN). Sep. Purif. Technol. 2021, 254, 117600. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, X.; Wang, G.-R.; Lu, T.-D.; Shi, Q.; Sun, S.-P. Inner-selective coordination nanofiltration hollow fiber membranes from assist-pressure modified substrate. J. Membr. Sci. 2021, 626, 119186. [Google Scholar] [CrossRef]
- Chen, M.; Yang, R.; Li, P. Preparation of defect-free hollow fiber membranes derived from PMDA-ODA polyimide for gas separation. Chem. Eng. Res. Des. 2022, 179, 154–161. [Google Scholar] [CrossRef]
- Mousavi, S.A.; Aboosadi, Z.A.; Mansourizadeh, A.; Honarvar, B. Surface modified porous polyetherimide hollow fiber membrane for sweeping gas membrane distillation of dyeing wastewater. Colloids. Surf. A Physicochem. Eng. Asp. 2021, 610, 125439. [Google Scholar] [CrossRef]
- Mousavi, S.; Arab Aboosadi, Z.; Mansourizadeh, A.; Honarvar, B. Modification of porous polyetherimide hollow fiber membrane by dip-coating of Zonyl® BA for membrane distillation of dyeing wastewater. Water Sci. Technol. 2021, 83, 3092–3109. [Google Scholar] [CrossRef]
- Li, G.; Knozowska, K.; Kujawa, J.; Tonkonogovas, A.; Stankevičius, A.; Kujawski, W. Fabrication of polydimethysiloxane (PDMS) dense layer on polyetherimide (PEI) hollow fiber support for the efficient CO2/N2 separation membranes. Polymers 2021, 13, 756. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Kujawski, W.; Knozowska, K.; Kujawa, J. The effects of PEI hollow fiber substrate characteristics on PDMS/PEI hollow fiber membranes for CO2/N2 separation. Membranes 2021, 11, 56. [Google Scholar] [CrossRef] [PubMed]
- Alihemati, Z.; Hashemifard, S.; Matsuura, T.; Ismail, A. On performance and fouling of thin film composite hollow Fiber membranes using polycarbonate/polyvinylchloride as porous substrates for forward osmosis applications. J. Environ. Chem. Eng. 2022, 10, 106828. [Google Scholar] [CrossRef]
- Zheng, M.; Yang, Y.; Qiao, S.; Zhou, J.; Quan, X. A porous carbon-based electro-Fenton hollow fiber membrane with good antifouling property for microalgae harvesting. J. Membr. Sci. 2021, 626, 119189. [Google Scholar] [CrossRef]
- Zou, L.; Gusnawan, P.; Jiang, Y.-B.; Zhang, G.; Yu, J. Macrovoid-inhibited PVDF hollow fiber membranes via spinning process delay for direct contact membrane distillation. ACS Appl. Mater. Interfaces 2020, 12, 28655–28668. [Google Scholar] [CrossRef]
- El-badawy, T.; Othman, M.H.D.; Adam, M.R.; Kamaludin, R.; Ismail, A.; Rahman, M.A.; Jaafar, J.; Rajabzadeh, S.; Matsuyama, H.; Usman, J. Braid-reinforced PVDF hollow fiber membranes for high-efficiency separation of oily wastewater. J. Environ. Chem. Eng. 2022, 10, 107258. [Google Scholar] [CrossRef]
- Madupathi, M.; Nandala, S.; Sundergopal, S. Experimental and modeling investigation of ultrafine polyvinylidene fluoride hollow fiber membrane module for recovery of lactic acid from aqueous solutions. Polym. Eng. Sci. 2022, 62, 758–771. [Google Scholar] [CrossRef]
- Chen, Y.; Lu, K.-J.; Liang, C.Z.; Chung, T.-S. Mechanically strong Janus tri-bore hollow fiber membranes with asymmetric pores for anti-wetting and anti-fouling membrane distillation. Chem. Eng. J. 2022, 429, 132455. [Google Scholar] [CrossRef]
- Wang, C.; Chen, Y.; Yang, K.; Hu, X.; Zhang, Y. Fabrication of tight GO/PVDF hollow fiber membranes with improved permeability for efficient fractionation of dyes and salts in textile wastewater. Polym. Bull. 2022, 79, 443–462. [Google Scholar] [CrossRef]
- Zakaria, N.; Zaliman, S.; Leo, C.; Ahmad, A.; Ooi, B.; Poh, P.E. 3D imprinted superhydrophobic polyvinylidene fluoride/carbon black membrane for membrane distillation with electrochemical cleaning evaluation. J. Environ. Chem. Eng. 2022, 10, 107346. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, G.; Yu, J. Desirable PVDF hollow fiber membrane engineered with synergism between small molecular weight additives for DCMD treating of a hypersaline brine. J. Water Process. Eng. 2022, 45, 102528. [Google Scholar] [CrossRef]
- Zhao, B.; Shi, G.M.; Wang, K.Y.; Lai, J.-Y.; Chung, T.-S. Employing a green cross-linking method to fabricate polybenzimidazole (PBI) hollow fiber membranes for organic solvent nanofiltration (OSN). Sep. Purif. Technol. 2021, 255, 117702. [Google Scholar] [CrossRef]
- Dahe, G.J.; Singh, R.P.; Dudeck, K.W.; Yang, D.; Berchtold, K.A. Influence of non-solvent chemistry on polybenzimidazole hollow fiber membrane preparation. J. Membr. Sci. 2019, 577, 91–103. [Google Scholar] [CrossRef]
- Yong, W.F.; Li, F.Y.; Xiao, Y.C.; Chung, T.-S.; Tong, Y.W. High performance PIM-1/Matrimid hollow fiber membranes for CO2/CH4, O2/N2 and CO2/N2 separation. J. Membr. Sci. 2013, 443, 156–169. [Google Scholar] [CrossRef]
- Anderson, M.R.; Mattes, B.R.; Reiss, H.; Kaner, R.B. Conjugated polymer films for gas separations. Science 1991, 252, 1412–1415. [Google Scholar] [CrossRef]
- Pellegrino, J. The use of conducting polymers in membrane-based separations. Ann. N. Y. Acad. Sci. 2003, 984, 289–305. [Google Scholar] [CrossRef]
- Chen, D.; Miao, Y.-E.; Liu, T. Electrically conductive polyaniline/polyimide nanofiber membranes prepared via a combination of electrospinning and subsequent in situ polymerization growth. ACS Appl. Mater. Interfaces 2013, 5, 1206–1212. [Google Scholar] [CrossRef]
- Hardison, L.M.; Zhao, X.; Jiang, H.; Schanze, K.S.; Kleiman, V.D. Energy transfer dynamics in a series of conjugated polyelectrolytes with varying chain length. J. Phys. Chem. C 2008, 112, 16140–16147. [Google Scholar] [CrossRef]
- Zhao, X.; Pinto, M.R.; Hardison, L.M.; Mwaura, J.; Müller, J.; Jiang, H.; Witker, D.; Kleiman, V.D.; Reynolds, J.R.; Schanze, K.S. Variable band gap poly(arylene ethynylene) conjugated polyelectrolytes. Macromolecules 2006, 39, 6355–6366. [Google Scholar] [CrossRef]
- Masuda, T. Substituted polyacetylenes. J. Polym. Sci. Part A Polym. Chem. 2007, 45, 165–180. [Google Scholar] [CrossRef]
- Saini, N.; Pandey, K.; Awasthi, K. Conjugate polymer-based membranes for gas separation applications: Current status and future prospects. Mater. Today Chem. 2021, 22, 100558. [Google Scholar] [CrossRef]
- Bini, K.; Xu, X.; Andersson, M.R.; Wang, E. Alcohol-soluble conjugated polymers as cathode interlayers for all-polymer solar cells. ACS Appl. Energy Mater. 2018, 1, 2176–2182. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, L.; Ju, X.; He, T.; Chen, P. Molten salt assisted synthesis of microporous polyaniline nanosheets with superior gas sorption properties. Microporous Mesoporous Mater. 2018, 267, 100–106. [Google Scholar] [CrossRef]
- Majumdar, S.; Sarmah, K.; Mahanta, D. A simple route to prepare polypyrrole-coated filter papers via vapor phase polymerization and their gas sensing application. ACS Appl. Polym. Mater. 2020, 2, 1933–1942. [Google Scholar] [CrossRef]
- Huang, Y.; Zang, Y.; Xu, L.; Lei, T.; Cui, J.; Xie, Y.; Wang, J.; Jia, H.; Miao, F. Synthesis of chiral conjugated microporous polymer composite membrane and improvements in permeability and selectivity during enantioselective permeation. Sep. Purif. Technol. 2021, 266, 118529. [Google Scholar] [CrossRef]
- Monga Mulunda, M. Development of Conjugated Polymer Membranes for Gas Separation; KU Leuven: Leuven, Belgium, 2018. [Google Scholar]
- García-Fernández, L.; Wang, B.; García-Payo, M.; Li, K.; Khayet, M. Morphological design of alumina hollow fiber membranes for desalination by air gap membrane distillation. Desalination 2017, 420, 226–240. [Google Scholar] [CrossRef]
- Ko, C.C.; Chen, C.H.; Chen, Y.R.; Wu, Y.H.; Lu, S.C.; Hu, F.C.; Li, C.L.; Tung, K.L. Increasing the performance of vacuum membrane distillation using micro-structured hydrophobic aluminum hollow fiber membranes. Appl. Sci. 2017, 7, 357. [Google Scholar] [CrossRef] [Green Version]
- Magnone, E.; Lee, H.J.; Che, J.W.; Park, J.H. High-performance of modified Al2O3 hollow fiber membranes for CO2 absorption at room temperature. J. Ind. Eng. Chem. 2016, 42, 19–22. [Google Scholar] [CrossRef]
- Han, N.; Zhang, C.; Tan, X.; Wang, Z.; Kawi, S.; Liu, S. Re-evaluation of La0.6Sr0.4Co0.2Fe0.8O3−δ hollow fiber membranes for oxygen separation after long-term storage of five and ten years. J. Membr. Sci. 2019, 587, 117180. [Google Scholar] [CrossRef]
- Han, N.; Zhang, W.; Guo, W.; Xie, S.; Zhang, C.; Zhang, X.; Fransaer, J.; Liu, S. Novel oxygen permeable hollow fiber perovskite membrane with surface wrinkles. Sep. Purif. Technol. 2021, 261, 118295. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Liu, G.; Liu, Z.; Jin, W.; Xu, N. Perovskite hollow fibers with precisely controlled cation stoichiometry via one-step thermal processing. Adv. Mater. 2017, 29, 1606377. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zheng, Q.; Wang, S.; Zhang, Z.; Liu, Z.; Zhang, G.; Jin, W. Fabrication of molten nitrate/nitrite dual-phase four-channel hollow fiber membranes for nitrogen oxides separation. J. Membr. Sci. 2021, 635, 119506. [Google Scholar] [CrossRef]
- Araki, S.; Li, T.; Li, K.; Yamamoto, H. Preparation of zeolite hollow fibers for high-efficiency cadmium removal from waste water. Sep. Purif. Technol. 2019, 221, 393–398. [Google Scholar] [CrossRef]
- Makhtar, S.N.N.M.; Rahman, M.A.; Ismail, A.F.; Othman, M.H.D.; Jaafar, J. Preparation and characterization of glass hollow fiber membrane for water purification applications. Environ. Sci. Pollut. Res. 2017, 24, 15918–15928. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Ismail, A.; Rahman, M.A.; Jaafar, J. A low cost hydrophobic kaolin hollow fiber membrane (h-KHFM) for arsenic removal from aqueous solution via direct contact membrane distillation. Sep. Purif. Technol. 2019, 214, 31–39. [Google Scholar] [CrossRef]
- Lohaus, T.; De Wit, P.; Kather, M.; Menne, D.; Benes, N.; Pich, A.; Wessling, M. Tunable permeability and selectivity: Heatable inorganic porous hollow fiber membrane with a thermo-responsive microgel coating. J. Membr. Sci. 2017, 539, 451–457. [Google Scholar] [CrossRef]
- Wang, M.; Huang, M.L.; Cao, Y.; Ma, X.H.; Xu, Z.L. Fabrication, characterization and separation properties of three-channel stainless steel hollow fiber membrane. J. Membr. Sci. 2016, 515, 144–153. [Google Scholar] [CrossRef]
- Wang, M.; Song, J.; Wu, X.; Tan, X.; Meng, B.; Liu, S. Metallic nickel hollow fiber membranes for hydrogen separation at high temperatures. J. Membr. Sci. 2016, 509, 156–163. [Google Scholar] [CrossRef]
- Allioux, F.-M.; David, O.; Merenda, A.; Maina, J.W.; Benavides, M.E.; Tanaka, A.P.; Dumée, L.F. Catalytic nickel and nickel–copper alloy hollow-fiber membranes for the remediation of organic pollutants by electrocatalysis. J. Mater. Chem. A 2018, 6, 6904–6915. [Google Scholar] [CrossRef]
- Lei, L.; Pan, F.; Lindbråthen, A.; Zhang, X.; Hillestad, M.; Nie, Y.; Bai, L.; He, X.; Guiver, M.D. Carbon hollow fiber membranes for a molecular sieve with precise-cutoff ultramicropores for superior hydrogen separation. Nat. Commun. 2021, 12, 268. [Google Scholar] [CrossRef]
- Liu, L.; Liu, D.; Zhang, C. High-temperature hydrogen/propane separations in asymmetric carbon molecular sieve hollow fiber membranes. J. Membr. Sci. 2022, 642, 119978. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, K.; Sanyal, O.; Koros, W.J. Carbon molecular sieve membrane preparation by economical coating and pyrolysis of porous polymer hollow fibers. Angew. Chem. Int. Ed. 2019, 58, 12149–12153. [Google Scholar] [CrossRef]
- Qiu, W.; Xu, L.; Liu, Z.; Liu, Y.; Arab, P.; Brayden, M.; Martinez, M.; Liu, J.; Roy, A.; Koros, W.J. Surprising olefin/paraffin separation performance recovery of highly aged carbon molecular sieve hollow fiber membranes by a super-hyperaging treatment. J. Membr. Sci. 2021, 620, 118701. [Google Scholar] [CrossRef]
- Qiu, W.; Vaughn, J.; Liu, G.; Xu, L.; Brayden, M.; Martinez, M.; Fitzgibbons, T.; Wenz, G.; Koros, W.J. Hyperaging tuning of a carbon molecular-sieve hollow fiber membrane with extraordinary gas-separation performance and stability. Angew. Chem. Int. Ed. 2019, 58, 11700–11703. [Google Scholar] [CrossRef] [PubMed]
- Kamath, M.G.; Fu, S.; Itta, A.K.; Qiu, W.; Liu, G.; Swaidan, R.; Koros, W.J. 6FDA-DETDA: DABE polyimide-derived carbon molecular sieve hollow fiber membranes: Circumventing unusual aging phenomena. J. Membr. Sci. 2018, 546, 197–205. [Google Scholar] [CrossRef]
- Zhang, C.; Wenz, G.B.; Williams, P.J.; Mayne, J.M.; Liu, G.; Koros, W.J. Purification of aggressive supercritical natural gas using carbon molecular sieve hollow fiber membranes. Ind. Eng. Chem. Res. 2017, 56, 10482–10490. [Google Scholar] [CrossRef]
- Seong, J.G.; Lewis, J.C.; Matteson, J.A.; Craddock, E.; Martinez, U.; Thakkar, H.; Benavidez, A.D.; Berchtold, K.A.; Singh, R.P. Polybenzimidazole-derived carbon molecular sieve hollow fiber membranes with tailored oxygen selective transport. Carbon 2022, 192, 71–83. [Google Scholar] [CrossRef]
- Shin, J.H.; Yu, H.J.; An, H.; Lee, A.S.; Hwang, S.S.; Lee, S.Y.; Lee, J.S. Rigid double-stranded siloxane-induced high-flux carbon molecular sieve hollow fiber membranes for CO2/CH4 separation. J. Membr. Sci. 2019, 570–571, 504–512. [Google Scholar] [CrossRef]
- Ismail, A.F.; Li, K. From polymeric precursors to hollow fiber carbon and ceramic membranes. Membr. Sci. Technol. 2008, 13, 81–119. [Google Scholar]
- Reed, J.S. Principles of Ceramics Processing, 2nd ed.; Wiley: New York, NY, USA, 1995. [Google Scholar]
- Jue, M.L.; Ma, Y.; Lively, R.P. Streamlined fabrication of asymmetric carbon molecular sieve hollow fiber membranes. ACS Appl. Polym. Mater. 2019, 1, 1960–1964. [Google Scholar] [CrossRef]
- Ma, Y.; Jue, M.L.; Zhang, F.; Mathias, R.; Jang, H.Y.; Lively, R.P. Creation of well-defined “mid-sized” micropores in carbon molecular sieve membranes. Angew. Chem. Int. Ed. 2019, 58, 13259–13265. [Google Scholar] [CrossRef] [PubMed]
- Salinas, O.; Ma, X.; Litwiller, E.; Pinnau, I. Ethylene/ethane permeation, diffusion and gas sorption properties of carbon molecular sieve membranes derived from the prototype ladder polymer of intrinsic microporosity (PIM-1). J. Membr. Sci. 2016, 504, 133–140. [Google Scholar] [CrossRef] [Green Version]
- Seong, K.H.; Song, J.S.; Koh, H.C.; Ha, S.Y.; Han, M.H.; Cho, C.H. Effect of carbonization conditions on gas permeation of methyl imide based carbon molecular sieve hollow fiber membranes. Membr. J. 2013, 23, 332–342. [Google Scholar]
- Haider, S.; Lindbråthen, A.; Lie, J.A.; Andersen, I.C.T.; Hägg, M.-B. CO2 separation with carbon membranes in high pressure and elevated temperature applications. Sep. Purif. Technol. 2018, 190, 177–189. [Google Scholar] [CrossRef] [Green Version]
- Richter, H.; Voss, H.; Kaltenborn, N.; Kämnitz, S.; Wollbrink, A.; Feldhoff, A.; Caro, J.; Roitsch, S.; Voigt, I. High-flux carbon molecular sieve membranes for gas separation. Angew. Chem. Int. Ed. 2017, 56, 7760–7763. [Google Scholar] [CrossRef]
- Wijmans, J.; Hao, P. Influence of the porous support on diffusion in composite membranes. J. Membr. Sci. 2015, 494, 78–85. [Google Scholar] [CrossRef]
- Xia, Q.-C.; Liu, M.-L.; Cao, X.-L.; Wang, Y.; Xing, W.; Sun, S.-P. Structure design and applications of dual-layer polymeric membranes. J. Membr. Sci. 2018, 562, 85–111. [Google Scholar] [CrossRef]
- Xia, Q.-C.; Wang, J.; Wang, X.; Chen, B.-Z.; Guo, J.-L.; Jia, T.-Z.; Sun, S.-P. A hydrophilicity gradient control mechanism for fabricating delamination-free dual-layer membranes. J. Membr. Sci. 2017, 539, 392–402. [Google Scholar] [CrossRef]
- Hermans, S.; Bernstein, R.; Volodin, A.; Vankelecom, I.F. Study of synthesis parameters and active layer morphology of interfacially polymerized polyamide-polysulfone membranes. React. Funct. Polym. 2015, 86, 199–208. [Google Scholar] [CrossRef]
- Chen, H.Z.; Xiao, Y.C.; Chung, T.-S. Multi-layer composite hollow fiber membranes derived from poly(ethylene glycol) (PEG) containing hybrid materials for CO2/N2 separation. J. Membr. Sci. 2011, 381, 211–220. [Google Scholar] [CrossRef]
- Li, P.; Chen, H.Z.; Chung, T.-S. The effects of substrate characteristics and pre-wetting agents on PAN–PDMS composite hollow fiber membranes for CO2/N2 and O2/N2 separation. J. Membr. Sci. 2013, 434, 18–25. [Google Scholar] [CrossRef]
- Liang, C.Z.; Chung, T.-S. Robust thin film composite PDMS/PAN hollow fiber membranes for water vapor removal from humid air and gases. Sep. Purif. Technol. 2018, 202, 345–356. [Google Scholar] [CrossRef]
- Liang, C.Z.; Chung, T.S. Ultrahigh flux composite hollow fiber membrane via highly crosslinked PDMS for recovery of hydrocarbons: Propane and propene. Macromol. Rapid Commun. 2018, 39, 1700535. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Z.; Thong, Z.; Li, P.; Chung, T.-S. High performance composite hollow fiber membranes for CO2/H2 and CO2/N2 separation. Int. J. Hydrog. Energy 2014, 39, 5043–5053. [Google Scholar] [CrossRef]
- Veríssimo, S.; Peinemann, K.V.; Bordado, J. Thin-film composite hollow fiber membranes: An optimized manufacturing method. J. Membr. Sci. 2005, 264, 48–55. [Google Scholar] [CrossRef]
- Park, C.H.; Kwak, S.J.; Choi, J.; Lee, K.; Lee, J.H. Fabrication of a pilot scale module of thin film composite hollow fiber membrane for osmotic pressure-driven processes. J. Appl. Polym. Sci. 2018, 135, 46110. [Google Scholar] [CrossRef]
- Ren, J.; McCutcheon, J.R. Making thin film composite hollow fiber forward osmosis membranes at the module scale using commercial ultrafiltration membranes. Ind. Eng. Chem. Res. 2017, 56, 4074–4082. [Google Scholar] [CrossRef]
- Sun, S.-P.; Chung, T.-S. Outer-selective pressure-retarded osmosis hollow fiber membranes from vacuum-assisted interfacial polymerization for osmotic power generation. Environ. Sci. Technol. 2013, 47, 13167–13174. [Google Scholar] [CrossRef]
- Choi, O.; Peck, D.-H.; Park, C.H. High-performance nanofiltration of outer-selective thin-film composite hollow-fiber membranes via continuous interfacial polymerization. J. Ind. Eng. Chem. 2021, 103, 373–380. [Google Scholar] [CrossRef]
- Khulbe, K.C.; Matsuura, T. Thin film composite and/or thin film nanocomposite hollow fiber membrane for water treatment, pervaporation, and gas/vapor separation. Polymers 2018, 10, 1051. [Google Scholar] [CrossRef] [Green Version]
- Wang, M.; Wang, Z.; Li, S.; Zhang, C.; Wang, J.; Wang, S. A high performance antioxidative and acid resistant membrane prepared by interfacial polymerization for CO2 separation from flue gas. Energy Environ. Sci. 2013, 6, 539–551. [Google Scholar] [CrossRef]
- Hu, L.; Cheng, J.; Li, Y.; Liu, J.; Zhou, J.; Cen, K. Amino-functionalized surface modification of polyacrylonitrile hollow fiber-supported polydimethylsiloxane membranes. Appl. Surf. Sci. 2017, 413, 27–34. [Google Scholar] [CrossRef]
- Zhou, F.; Tien, H.N.; Xu, W.L.; Chen, J.T.; Liu, Q.; Hicks, E.; Fathizadeh, M.; Li, S.; Yu, M. Ultrathin graphene oxide-based hollow fiber membranes with brush-like CO2-philic agent for highly efficient CO2 capture. Nat. Commun. 2017, 8, 2107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, F.; Tien, H.N.; Dong, Q.; Xu, W.L.; Li, H.; Li, S.; Yu, M. Ultrathin, ethylenediamine-functionalized graphene oxide membranes on hollow fibers for CO2 capture. J. Membr. Sci. 2019, 573, 184–191. [Google Scholar] [CrossRef]
- Yong, W.F.; Zhang, H. Recent advances in polymer blend membranes for gas separation and pervaporation. Prog. Mater. Sci. 2021, 116, 100713. [Google Scholar] [CrossRef]
- Govardhan, B.; Chandrasekhar, S.; Sridhar, S. Purification of surface water using novel hollow fiber membranes prepared from polyetherimide/polyethersulfone blends. J. Environ. Chem. Eng. 2017, 5, 1068–1078. [Google Scholar] [CrossRef]
- Ter Beek, O.; Pavlenko, D.; Stamatialis, D. Hollow fiber membranes for long-term hemodialysis based on polyethersulfone-SlipSkin™ polymer blends. J. Membr. Sci. 2020, 604, 118068. [Google Scholar] [CrossRef]
- Wu, P.; Jiang, L.Y.; Hu, B. Fabrication of novel PVDF/(PVDF-co-HFP) blend hollow fiber membranes for DCMD. J. Membr. Sci. 2018, 566, 442–454. [Google Scholar] [CrossRef]
- Noor, N.; Koll, J.; Scharnagl, N.; Abetz, C.; Abetz, V. Hollow fiber membranes of blends of polyethersulfone and sulfonated polymers. Membranes 2018, 8, 54. [Google Scholar] [CrossRef] [Green Version]
- Sikorska, W.; Wasyłeczko, M.; Przytulska, M.; Wojciechowski, C.; Rokicki, G.; Chwojnowski, A. Chemical degradation of PSF-PUR blend hollow fiber membranes-assessment of changes in properties and morphology after hydrolysis. Membranes 2021, 11, 51. [Google Scholar] [CrossRef]
- Fosi-Kofal, M.; Mustafa, A.; Ismail, A.; Rezaei-DashtArzhandi, M.; Matsuura, T. PVDF/CaCO3 composite hollow fiber membrane for CO2 absorption in gas-liquid membrane contactor. J. Nat. Gas Sci. Eng. 2016, 31, 428–436. [Google Scholar] [CrossRef]
- Villalobos, L.F.; Hilke, R.; Akhtar, F.H.; Peinemann, K.V. Fabrication of polybenzimidazole/palladium nanoparticles hollow fiber membranes for hydrogen purification. Adv. Energy Mater. 2018, 8, 1701567. [Google Scholar] [CrossRef]
- Jamil, A.; Oh, P.C.; Shariff, A.M. Polyetherimide-montmorillonite mixed matrix hollow fibre membranes: Effect of inorganic/organic montmorillonite on CO2/CH4 separation. Sep. Purif. Technol. 2018, 206, 256–267. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, K.; Zhang, C.; Koros, W.J. Carbon molecular sieve hollow fiber membranes derived from dip-coated precursor hollow fibers comprising nanoparticles. J. Membr. Sci. 2022, 649, 120279. [Google Scholar] [CrossRef]
- Choi, O.; Kim, Y.; Jeon, J.-D.; Kim, T.-H. Preparation of thin film nanocomposite hollow fiber membranes with polydopamine-encapsulated Engelhard titanosilicate-4 for gas separation applications. J. Membr. Sci. 2021, 620, 118946. [Google Scholar] [CrossRef]
- Dong, Z.; Zhu, H.; Hang, Y.; Liu, G.; Jin, W. Polydimethylsiloxane (PDMS) composite membrane fabricated on the inner surface of a ceramic hollow fiber: From single-channel to multi-channel. Engineering 2020, 6, 89–99. [Google Scholar] [CrossRef]
- Wan, C.F.; Yang, T.; Gai, W.; De Lee, Y.; Chung, T.-S. Thin-film composite hollow fiber membrane with inorganic salt additives for high mechanical strength and high power density for pressure-retarded osmosis. J. Membr. Sci. 2018, 555, 388–397. [Google Scholar] [CrossRef]
- Abdullah, N.; Rahman, M.A.; Othman, M.H.D.; Ismail, A.; Jaafar, J.; Abd Aziz, A. Preparation and characterization of self-cleaning alumina hollow fiber membrane using the phase inversion and sintering technique. Ceram. Int. 2016, 42, 12312–12322. [Google Scholar] [CrossRef]
- Lin, Y.; Xu, Y.; Loh, C.H.; Wang, R. Development of robust fluorinated TiO2/PVDF composite hollow fiber membrane for CO2 capture in gas-liquid membrane contactor. Appl. Surf. Sci. 2018, 436, 670–681. [Google Scholar] [CrossRef]
- Liu, X.-W.; Cao, Y.; Li, Y.-X.; Xu, Z.-L.; Li, Z.; Wang, M.; Ma, X.-H. High-performance polyamide/ceramic hollow fiber TFC membranes with TiO2 interlayer for pervaporation dehydration of isopropanol solution. J. Membr. Sci. 2019, 576, 26–35. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Zhang, X.; Hu, X.; Wang, C.; Yang, Y. Preparation and characterization of PSF-TiO2 hybrid hollow fiber UF membrane by sol-gel method. J. Polym. Res. 2020, 27, 1–9. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, J.; Pati, S.; Chen, T.; Deng, Y.; Dewangan, N.; Meng, L.; Lin, J.Y.; Kawi, S. High H2 permeable SAPO-34 hollow fiber membrane for high temperature propane dehydrogenation application. AIChE J. 2020, 66, e16278. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.; Shi, R.; Du, P.; Qiu, X.; Gu, X. Pervaporation dehydration of acetic acid through hollow fiber supported DD3R zeolite membrane. Sep. Purif. Technol. 2018, 204, 234–242. [Google Scholar] [CrossRef]
- Muhamad, N.; Makhtar, S.N.N.M.; Abdullah, N.; Pauzi, M.Z.M.; Mahpoz, N.M.A.; Othman, M.H.D.; Jaafar, J.; Abas, K.H.; Fadil, N.A.; Rahman, M.A. Composite zeolite hollow fiber membrane for the removal of nickel using forward osmosis. J. Water Process. Eng. 2021, 40, 101806. [Google Scholar] [CrossRef]
- Amirabedi, P.; Akbari, A.; Yegani, R. Fabrication of hydrophobic PP/CH3SiO2 composite hollow fiber membrane for membrane contactor application. Sep. Purif. Technol. 2019, 228, 115689. [Google Scholar] [CrossRef]
- Gong, H.; Pang, H.; Du, M.; Chen, Z. Fabrication of a superhydrophobic mixed matrix PVDF-SiO2-HDTMS hollow fiber membrane for membrane contact carbon dioxide absorption. Clean. Eng. Technol. 2021, 5, 100278. [Google Scholar] [CrossRef]
- Zhang, H.; Li, B.; Sun, D.; Miao, X.; Gu, Y. SiO2-PDMS-PVDF hollow fiber membrane with high flux for vacuum membrane distillation. Desalination 2018, 429, 33–43. [Google Scholar] [CrossRef]
- Marti, A.M.; Wickramanayake, W.; Dahe, G.; Sekizkardes, A.; Bank, T.L.; Hopkinson, D.P.; Venna, S.R. Continuous flow processing of ZIF-8 membranes on polymeric porous hollow fiber supports for CO2 capture. ACS Appl. Mater. Interfaces 2017, 9, 5678–5682. [Google Scholar] [CrossRef]
- Etxeberria-Benavides, M.; Johnson, T.; Cao, S.; Zornoza, B.; Coronas, J.; Sanchez-Lainez, J.; Sabetghadam, A.; Liu, X.; Andres-Garcia, E.; Kapteijn, F.; et al. PBI mixed matrix hollow fiber membrane: Influence of ZIF-8 filler over H2/CO2 separation performance at high temperature and pressure. Sep. Purif. Technol. 2020, 237, 116347. [Google Scholar] [CrossRef]
- Lin, Y.; Chen, Y.; Wang, R. Thin film nanocomposite hollow fiber membranes incorporated with surface functionalized HKUST-1 for highly-efficient reverses osmosis desalination process. J. Membr. Sci. 2019, 589, 117249. [Google Scholar] [CrossRef]
- Liu, Q.; Li, Y.; Li, Q.; Liu, G.; Liu, G.; Jin, W. Mixed-matrix hollow fiber composite membranes comprising of PEBA and MOF for pervaporation separation of ethanol/water mixtures. Sep. Purif. Technol. 2019, 214, 2–10. [Google Scholar] [CrossRef]
- Zhu, J.; Meng, X.; Zhao, J.; Jin, Y.; Yang, N.; Zhang, S.; Sunarso, J.; Liu, S. Facile hydrogen/nitrogen separation through graphene oxide membranes supported on YSZ ceramic hollow fibers. J. Membr. Sci. 2017, 535, 143–150. [Google Scholar] [CrossRef]
- Qu, K.; Dai, L.; Xia, Y.; Wang, Y.; Zhang, D.; Wu, Y.; Yao, Z.; Huang, K.; Guo, X.; Xu, Z. Self-crosslinked MXene hollow fiber membranes for H2/CO2 separation. J. Membr. Sci. 2021, 638, 119669. [Google Scholar] [CrossRef]
- Janakiram, S.; Espejo, J.L.M.; Høisæter, K.K.; Lindbråthen, A.; Ansaloni, L.; Deng, L. Three-phase hybrid facilitated transport hollow fiber membranes for enhanced CO2 separation. Appl. Mater. Today 2020, 21, 100801. [Google Scholar] [CrossRef]
- Syed Ibrahim, G.; Isloor, A.M.; Ismail, A.; Farnood, R. One-step synthesis of zwitterionic graphene oxide nanohybrid: Application to polysulfone tight ultrafiltration hollow fiber membrane. Sci. Rep. 2020, 10, 6880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, W.; Zhang, X.; Qin, L.; Li, X.; Meng, Q.; Shen, C.; Zhang, G. Enhanced MPBR with polyvinylpyrrolidone-graphene oxide/PVDF hollow fiber membrane for efficient ammonia nitrogen wastewater treatment and high-density Chlorella cultivation. Chem. Eng. J. 2020, 379, 122368. [Google Scholar] [CrossRef]
- Xu, Y.; Lin, Y.; Lee, M.; Malde, C.; Wang, R. Development of low mass-transfer-resistance fluorinated TiO2-SiO2/PVDF composite hollow fiber membrane used for biogas upgrading in gas-liquid membrane contactor. J. Membr. Sci. 2018, 552, 253–264. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Wang, S.; Xu, C.; Yang, K.; Xu, M. Removal of Pb (II) from wastewater using Al2O3-NaA zeolite composite hollow fiber membranes synthesized from solid waste coal fly ash. Chemosphere 2018, 206, 278–284. [Google Scholar] [CrossRef]
- Wang, S.; Tian, J.; Wang, Q.; Zhao, Z.; Cui, F.; Li, G. Low-temperature sintered high-strength CuO doped ceramic hollow fiber membrane: Preparation, characterization and catalytic activity. J. Membr. Sci. 2019, 570, 333–342. [Google Scholar] [CrossRef]
- Charcosset, C. Membrane Processes in Biotechnology and Pharmaceutics; Elsevier: Oxford, UK, 2012. [Google Scholar]
- Manni, A.; Achiou, B.; Karim, A.; Harrati, A.; Sadik, C.; Ouammou, M.; Alami Younssi, S.; El Bouari, A. New low-cost ceramic microfiltration membrane made from natural magnesite for industrial wastewater treatment. J. Environ. Chem. Eng. 2020, 8, 103906. [Google Scholar] [CrossRef]
- Goswami, L.; Kumar, R.V.; Pakshirajan, K.; Pugazhenthi, G. A novel integrated biodegradation-microfiltration system for sustainable wastewater treatment and energy recovery. J. Hazard. Mater. 2019, 365, 707–715. [Google Scholar] [CrossRef] [PubMed]
- Carter, B.; DiMarzo, L.; Pranata, J.; Barbano, D.M.; Drake, M. Efficiency of removal of whey protein from sweet whey using polymeric microfiltration membranes. J. Dairy Sci. 2021, 104, 8630–8643. [Google Scholar] [CrossRef] [PubMed]
- Doulia, D.S.; Anagnos, E.K.; Liapis, K.S.; Klimentzos, D.A. Removal of pesticides from white and red wines by microfiltration. J. Hazard. Mater. 2016, 317, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Rouquié, C.; Dahdouh, L.; Delalonde, M.; Wisniewski, C. New prospects for immersed hollow-fiber membranes in fruit juices microfiltration: Case of grapefruit juice. J. Food Eng. 2019, 246, 75–85. [Google Scholar] [CrossRef]
- Liu, D.; Zhu, J.; Qiu, M.; He, C. Antifouling performance of poly (lysine methacrylamide)-grafted PVDF microfiltration membrane for solute separation. Sep. Purif. Technol. 2016, 171, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Hernández, S.; Lei, S.; Rong, W.; Ormsbee, L.; Bhattacharyya, D. Functionalization of flat sheet and hollow fiber microfiltration membranes for water applications. ACS Sustain. Chem. Eng. 2016, 4, 907–918. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Chen, M.; Dong, Y.; Tang, C.Y.; Huang, A.; Li, L. A low-cost mullite-titania composite ceramic hollow fiber microfiltration membrane for highly efficient separation of oil-in-water emulsion. Water Res. 2016, 90, 277–285. [Google Scholar] [CrossRef]
- Schopf, R.; Schmidt, F.; Linner, J.; Kulozik, U. Comparative assessment of tubular ceramic, spiral wound, and hollow fiber membrane microfiltration module systems for milk protein fractionation. Foods 2021, 10, 692. [Google Scholar] [CrossRef]
- Schopf, R.; Schmidt, F.; Kulozik, U. Impact of hollow fiber membrane length on the milk protein fractionation. J. Membr. Sci. 2021, 620, 118834. [Google Scholar] [CrossRef]
- Giacobbo, A.; Meneguzzi, A.; Bernardes, A.M.; de Pinho, M.N. Pressure-driven membrane processes for the recovery of antioxidant compounds from winery effluents. J. Clean. Prod. 2017, 155, 172–178. [Google Scholar] [CrossRef]
- Mondal, M.; De, S. Enrichment of (−) epigallocatechin gallate (EGCG) from aqueous extract of green tea leaves by hollow fiber microfiltration: Modeling of flux decline and identification of optimum operating conditions. Sep. Purif. Technol. 2018, 206, 107–117. [Google Scholar] [CrossRef]
- Behroozi, A.H.; Ataabadi, M.R. Improvement in microfiltration process of oily wastewater: A comprehensive review over two decades. J. Environ. Chem. Eng. 2021, 9, 104981. [Google Scholar] [CrossRef]
- Abd Aziz, M.H.; Othman, M.H.D.; Hashim, N.A.; Adam, M.R.; Mustafa, A. Fabrication and characterization of mullite ceramic hollow fiber membrane from natural occurring ball clay. Appl. Clay Sci. 2019, 177, 51–62. [Google Scholar] [CrossRef]
- Bai, Z.; Zhang, R.; Wang, S.; Gao, S.; Tian, J. Membrane fouling behaviors of ceramic hollow fiber microfiltration (MF) membranes by typical organic matters. Sep. Purif. Technol. 2021, 274, 118951. [Google Scholar] [CrossRef]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based membranes for water and wastewater treatment. Colloids. Surf. A Physicochem. Eng. Asp. 2019, 578, 123513. [Google Scholar] [CrossRef]
- Abadikhah, H.; Wang, J.-W.; Xu, X.; Agathopoulos, S. SiO2 nanoparticles modified Si3N4 hollow fiber membrane for efficient oily wastewater microfiltration. J. Water Process. Eng. 2019, 29, 100799. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Rahman, M.A.; Ismail, A.; Jaafar, J. Preparation and characterization of inexpensive kaolin hollow fibre membrane (KHFM) prepared using phase inversion/sintering technique for the efficient separation of real oily wastewater. Arab. J. Chem. 2020, 13, 2349–2367. [Google Scholar] [CrossRef]
- Paul, M.; Jons, S.D. Chemistry and fabrication of polymeric nanofiltration membranes: A review. Polymer 2016, 103, 417–456. [Google Scholar] [CrossRef]
- Labban, O.; Liu, C.; Chong, T.H. Fundamentals of low-pressure nanofiltration: Membrane characterization, modeling, and understanding the multi-ionic interactions in water softening. J. Membr. Sci. 2017, 521, 18–32. [Google Scholar] [CrossRef]
- Setiawan, L.; Shi, L.; Wang, R. Dual layer composite nanofiltration hollow fiber membranes for low-pressure water softening. Polymer 2014, 55, 1367–1374. [Google Scholar] [CrossRef]
- Lidén, A.; Lavonen, E.; Persson, K.M.; Larson, M. Integrity breaches in a hollow fiber nanofilter–Effects on natural organic matter and virus-like particle removal. Water Res. 2016, 105, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Dorji, P.; Shon, H.K.; Hong, S. Applications of capacitive deionization: Desalination, softening, selective removal, and energy efficiency. Desalination 2019, 449, 118–130. [Google Scholar] [CrossRef]
- Sengur-Tasdemir, R.; Urper-Bayram, G.M.; Turken, T.; Ates-Genceli, E.; Tarabara, V.V.; Koyuncu, I. Hollow fiber nanofiltration membranes for surface water treatment: Performance evaluation at the pilot scale. J. Water Process. Eng. 2021, 42, 102100. [Google Scholar] [CrossRef]
- Conidi, C.; Castro-Muñoz, R.; Cassano, A. Membrane-based operations in the fruit juice processing industry: A review. Beverages 2020, 6, 18. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Zeng, X.; Shi, W.; Zhang, H.; Huang, S.; Zhou, R.; Qin, X. Recovery and purification of potato proteins from potato starch wastewater by hollow fiber separation membrane integrated process. Innov. Food Sci. Emerg. Technol. 2020, 63, 102380. [Google Scholar] [CrossRef]
- Paltrinieri, L.; Remmen, K.; Müller, B.; Chu, L.; Köser, J.; Wintgens, T.; Wessling, M.; de Smet, L.C.; Sudhölter, E.J. Improved phosphoric acid recovery from sewage sludge ash using layer-by-layer modified membranes. J. Membr. Sci. 2019, 587, 117162. [Google Scholar] [CrossRef]
- Remmen, K.; Müller, B.; Köser, J.; Wessling, M.; Wintgens, T. Assessment of layer-by-layer modified nanofiltration membrane stability in phosphoric acid. Membranes 2020, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Remmen, K.; Schäfer, R.; Hedwig, S.; Wintgens, T.; Wessling, M.; Lenz, M. Layer-by-layer membrane modification allows scandium recovery by nanofiltration. Environ. Sci. Water Res. Technol. 2019, 5, 1683–1688. [Google Scholar] [CrossRef]
- Kazemabad, M.; Verliefde, A.; Cornelissen, E.R.; D’Haese, A. Crown ether containing polyelectrolyte multilayer membranes for lithium recovery. J. Membr. Sci. 2020, 595, 117432. [Google Scholar] [CrossRef]
- Bóna, Á.; Bakonyi, P.; Galambos, I.; Bélafi-Bakó, K.; Nemestóthy, N. Separation of volatile fatty acids from model anaerobic effluents using various membrane technologies. Membranes 2020, 10, 252. [Google Scholar] [CrossRef]
- Monte, J.; Sá, M.; Galinha, C.F.; Costa, L.; Hoekstra, H.; Brazinha, C.; Crespo, J.G. Harvesting of Dunaliella salina by membrane filtration at pilot scale. Sep. Purif. Technol. 2018, 190, 252–260. [Google Scholar] [CrossRef]
- Avram, A.M.; Ahmadiannamini, P.; Vu, A.; Qian, X.; Sengupta, A.; Wickramasinghe, S.R. Polyelectrolyte multilayer modified nanofiltration membranes for the recovery of ionic liquid from dilute aqueous solutions. J. Appl. Polym. Sci. 2017, 134, 45349. [Google Scholar] [CrossRef]
- Wang, Q.; Lu, T.-D.; Yan, X.-Y.; Zhao, L.-L.; Yin, H.; Xiong, X.-X.; Zhou, R.; Sun, S.-P. Designing nanofiltration hollow fiber membranes based on dynamic deposition technology. J. Membr. Sci. 2020, 610, 118336. [Google Scholar] [CrossRef]
- He, Y.; Miao, J.; Chen, S.; Zhang, R.; Zhang, L.; Tang, H.; Yang, H. Preparation and characterization of a novel positively charged composite hollow fiber nanofiltration membrane based on chitosan lactate. RSC Adv. 2019, 9, 4361–4369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haghighat, N.; Vatanpour, V. Fouling decline and retention increase of polyvinyl chloride nanofiltration membranes blended by polypyrrole functionalized multiwalled carbon nanotubes. Mater. Today Commun. 2020, 23, 100851. [Google Scholar] [CrossRef]
- Rohani, R.; Yusoff, I.I. Towards electrically tunable nanofiltration membranes: Polyaniline-coated polyvinylidene fluoride membranes with tunable permeation properties. Iran. Polym. J. 2019, 28, 789–800. [Google Scholar] [CrossRef]
- Ma, G.; Zhao, S.; Wang, Y.; Wang, Z.; Wang, J. Conjugated polyaniline derivative membranes enable ultrafast nanofiltration and organic-solvent nanofiltration. J. Membr. Sci. 2022, 645, 120241. [Google Scholar] [CrossRef]
- Xia, L.; Ren, J.; McCutcheon, J.R. Braid-reinforced thin film composite hollow fiber nanofiltration membranes. J. Membr. Sci. 2019, 585, 109–114. [Google Scholar] [CrossRef]
- Wang, T.; He, X.; Li, Y.; Li, J. Novel poly (piperazine-amide)(PA) nanofiltration membrane based poly (m-phenylene isophthalamide)(PMIA) hollow fiber substrate for treatment of dye solutions. Chem. Eng. J. 2018, 351, 1013–1026. [Google Scholar] [CrossRef]
- Tian, L.; Jiang, Y.; Li, S.; Han, L.; Su, B. Graphene oxide interlayered thin-film nanocomposite hollow fiber nanofiltration membranes with enhanced aqueous electrolyte separation performance. Sep. Purif. Technol. 2020, 248, 117153. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W.; Xu, N. Graphene-based membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Guo, Y.; Meng, B.; Wang, Z.; Liu, G.; Jin, W. Two-dimensional MXene hollow fiber membrane for divalent ions exclusion from water. Chin. J. Chem. Eng. 2022, 41, 260–266. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Daems, B.; Wilms, D.; Vandecasteele, C. Mechanisms of retention and flux decline for the nanofiltration of dye baths from the textile industry. Sep. Purif. Technol. 2001, 22, 519–528. [Google Scholar] [CrossRef]
- Koyuncu, I.; Topacik, D.; Yuksel, E. Reuse of reactive dyehouse wastewater by nanofiltration: Process water quality and economical implications. Sep. Purif. Technol. 2004, 36, 77–85. [Google Scholar] [CrossRef]
- Chu, Z.; Chen, K.; Xiao, C.; Ji, D.; Ling, H.; Li, M.; Liu, H. Improving pressure durability and fractionation property via reinforced PES loose nanofiltration hollow fiber membranes for textile wastewater treatment. J. Taiwan Inst. Chem. Eng. 2020, 108, 71–81. [Google Scholar] [CrossRef]
- Song, C.; Tang, S.; Yue, S.; Cui, Z.; Du, X.; Jiang, T.; He, B.; Li, J. Design of microstructure for hollow fiber loose nanofiltration separation layer and its compactness-tailoring mechanism. J. Hazard. Mater. 2022, 421, 126800. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Li, T.; Li, H.; Du, Q.; Zhang, H.; Qin, X. An attempt to enhance water flux of hollow fiber polyamide composite nanofiltration membrane by the incorporation of hydrophilic and compatible PPTA/PSF microparticles. Sep. Purif. Technol. 2022, 280, 119821. [Google Scholar] [CrossRef]
- Tan, Z.; Chen, S.; Peng, X.; Zhang, L.; Gao, C. Polyamide membranes with nanoscale Turing structures for water purification. Science 2018, 360, 518–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchetti, P.; Jimenez Solomon, M.F.; Szekely, G.; Livingston, A.G. Molecular separation with organic solvent nanofiltration: A critical review. Chem. Rev. 2014, 114, 10735–10806. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.K.; Goh, K.; Bae, T.-H.; Wang, R. Polymer-based membranes for solvent-resistant nanofiltration: A review. Chin. J. Chem. Eng. 2017, 25, 1653–1675. [Google Scholar] [CrossRef]
- Shi, G.M.; Feng, Y.; Li, B.; Tham, H.M.; Lai, J.-Y.; Chung, T.-S. Recent progress of organic solvent nanofiltration membranes. Prog. Polym. Sci. 2021, 123, 101470. [Google Scholar] [CrossRef]
- Li, Y.; Cao, B.; Li, P. Effects of dope compositions on morphologies and separation performances of PMDA-ODA polyimide hollow fiber membranes in aqueous and organic solvent systems. Appl. Surf. Sci. 2019, 473, 1038–1048. [Google Scholar] [CrossRef]
- Li, Y.; Xue, J.; Zhang, X.; Cao, B.; Li, P. Formation of macrovoid-free PMDA-MDA polyimide membranes using a gelation/non-solvent-induced phase separation method for organic solvent nanofiltration. Ind. Eng. Chem. Res. 2019, 58, 6712–6720. [Google Scholar] [CrossRef]
- Goh, K.S.; Chong, J.Y.; Chen, Y.; Fang, W.; Bae, T.-H.; Wang, R. Thin-film composite hollow fibre membrane for low pressure organic solvent nanofiltration. J. Membr. Sci. 2020, 597, 117760. [Google Scholar] [CrossRef]
- Goh, K.S.; Chen, Y.; Chong, J.Y.; Bae, T.H.; Wang, R. Thin film composite hollow fibre membrane for pharmaceutical concentration and solvent recovery. J. Membr. Sci. 2021, 621, 119008. [Google Scholar] [CrossRef]
- Tashvigh, A.A.; Chung, T.-S. Robust polybenzimidazole (PBI) hollow fiber membranes for organic solvent nanofiltration. J. Membr. Sci. 2019, 572, 580–587. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Chung, T.-S. A novel crosslinking technique towards the fabrication of high-flux polybenzimidazole (PBI) membranes for organic solvent nanofiltration (OSN). Sep. Purif. Technol. 2019, 209, 182–192. [Google Scholar] [CrossRef]
- Chen, D.; Yan, C.; Li, X.; Liu, L.; Wu, D.; Li, X. A highly stable PBI solvent resistant nanofiltration membrane prepared via versatile and simple crosslinking process. Sep. Purif. Technol. 2019, 224, 15–22. [Google Scholar] [CrossRef]
- Jiang, J.X.; Su, F.; Trewin, A.; Wood, C.D.; Campbell, N.L.; Niu, H.; Dickinson, C.; Ganin, A.Y.; Rosseinsky, M.J.; Khimyak, Y.Z. Conjugated microporous poly (aryleneethynylene) networks. Angew. Chem. Int. Ed. 2007, 119, 8728–8732. [Google Scholar] [CrossRef]
- Liang, B.; Wang, H.; Shi, X.; Shen, B.; He, X.; Ghazi, Z.A.; Khan, N.A.; Sin, H.; Khattak, A.M.; Li, L. Microporous membranes comprising conjugated polymers with rigid backbones enable ultrafast organic-solvent nanofiltration. Nat. Chem. 2018, 10, 961–967. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Sin, H.; Liang, B.; Ghazi, Z.A.; Khattak, A.M.; Khan, N.A.; Alanagh, H.R.; Li, L.; Lu, X.; Tang, Z. Controlling the selectivity of conjugated microporous polymer membrane for efficient organic solvent nanofiltration. Adv. Funct. Mater. 2019, 29, 1900134. [Google Scholar] [CrossRef]
- Gong, J.; Lin, R.-B.; Chen, B. Conjugated microporous polymers with rigid backbones for organic solvent nanofiltration. Chem 2018, 4, 2269–2271. [Google Scholar] [CrossRef] [Green Version]
- Farahani, M.H.D.A.; Hua, D.; Chung, T.-S. Cross-linked mixed matrix membranes (MMMs) consisting of amine-functionalized multi-walled carbon nanotubes and P84 polyimide for organic solvent nanofiltration (OSN) with enhanced flux. J. Membr. Sci. 2018, 548, 319–331. [Google Scholar] [CrossRef]
- Ran, J.; Zhang, P.; Chu, C.; Cui, P.; Ai, X.; Pan, T.; Wu, Y.; Xu, T. Ultrathin lamellar MoS2 membranes for organic solvent nanofiltration. J. Membr. Sci. 2020, 602, 117963. [Google Scholar] [CrossRef]
- Guo, B.-Y.; Jiang, S.-D.; Tang, M.-J.; Li, K.; Sun, S.; Chen, P.-Y.; Zhang, S. MoS2 membranes for organic solvent nanofiltration: Stability and structural control. J. Phys. Chem. Lett. 2019, 10, 4609–4617. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Song, X.; Su, B.; Mandal, B.; Prasad, B.; Gao, X.; Gao, C. Graphene quantum dots-doped thin film nanocomposite polyimide membranes with enhanced solvent resistance for solvent-resistant nanofiltration. ACS Appl. Mater. Interfaces 2019, 11, 6527–6540. [Google Scholar] [CrossRef]
- Li, S.; Li, C.; Su, B.; Hu, M.Z.; Gao, X.; Gao, C. Amino-functionalized graphene quantum dots (aGQDs)-embedded thin film nanocomposites for solvent resistant nanofiltration (SRNF) membranes based on covalence interactions. J. Membr. Sci. 2019, 588, 117212. [Google Scholar] [CrossRef]
- Li, Y.; Li, J.; Soria, R.B.; Volodine, A.; Van der Bruggen, B. Aramid nanofiber and modified ZIF-8 constructed porous nanocomposite membrane for organic solvent nanofiltration. J. Membr. Sci. 2020, 603, 118002. [Google Scholar] [CrossRef]
- Karimi, A.; Khataee, A.; Safarpour, M.; Vatanpour, V. Development of mixed matrix ZIF-8/polyvinylidene fluoride membrane with improved performance in solvent resistant nanofiltration. Sep. Purif. Technol. 2020, 237, 116358. [Google Scholar] [CrossRef]
- Dai, J.; Li, S.; Liu, J.; He, J.; Li, J.; Wang, L.; Lei, J. Fabrication and characterization of a defect-free mixed matrix membrane by facile mixing PPSU with ZIF-8 core–shell microspheres for solvent-resistant nanofiltration. J. Membr. Sci. 2019, 589, 117261. [Google Scholar] [CrossRef]
- Wang, S.; Mahalingam, D.; Sutisna, B.; Nunes, S.P. 2D-dual-spacing channel membranes for high performance organic solvent nanofiltration. J. Mater. Chem. A 2019, 7, 11673–11682. [Google Scholar] [CrossRef] [Green Version]
- Fei, F.; Cseri, L.; Szekely, G.; Blanford, C.F. Robust covalently cross-linked polybenzimidazole/graphene oxide membranes for high-flux organic solvent nanofiltration. ACS Appl. Mater. Interfaces 2018, 10, 16140–16147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.-L.; Wang, J.; Guo, J.-L.; Lu, T.-D.; Cao, X.-L.; Sun, S.-P. Graphene oxide/cross-linked polyimide (GO/CLPI) composite membranes for organic solvent nanofiltration. Chem. Eng. Res. Des. 2019, 146, 182–189. [Google Scholar] [CrossRef]
- Su, J.; Lv, X.; Li, S.; Jiang, Y.; Liu, S.; Zhang, X.; Li, H.; Su, B. High separation performance thin film composite and thin film nanocomposite hollow fiber membranes via interfacial polymerization for organic solvent nanofiltration. Sep. Purif. Technol. 2021, 278, 119567. [Google Scholar] [CrossRef]
- Abadikhah, H.; Kalali, E.N.; Behzadi, S.; Khan, S.A.; Xu, X.; Shabestari, M.E.; Agathopoulos, S. High flux thin film nanocomposite membrane incorporated with functionalized TiO2@ reduced graphene oxide nanohybrids for organic solvent nanofiltration. Chem. Eng. Sci. 2019, 204, 99–109. [Google Scholar] [CrossRef]
- Farahani, M.H.D.A.; Chung, T.-S. Solvent resistant hollow fiber membranes comprising P84 polyimide and amine-functionalized carbon nanotubes with potential applications in pharmaceutical, food, and petrochemical industries. Chem. Eng. J. 2018, 345, 174–185. [Google Scholar] [CrossRef]
- Elimelech, M.; Phillip, W.A. The future of seawater desalination: Energy, technology, and the environment. Science 2011, 333, 712–717. [Google Scholar] [CrossRef]
- Xu, J.; Feng, X.; Gao, C. Surface modification of thin-film-composite polyamide membranes for improved reverse osmosis performance. J. Membr. Sci. 2011, 370, 116–123. [Google Scholar] [CrossRef]
- Yang, T.; Wan, C.F.; Zhang, J.; Gudipati, C.; Chung, T.-S. Optimization of interfacial polymerization to fabricate thin-film composite hollow fiber membranes in modules for brackish water reverse osmosis. J. Membr. Sci. 2021, 626, 119187. [Google Scholar] [CrossRef]
- Zhao, D.L.; Japip, S.; Zhang, Y.; Weber, M.; Maletzko, C.; Chung, T.-S. Emerging thin-film nanocomposite (TFN) membranes for reverse osmosis: A review. Water Res. 2020, 173, 115557. [Google Scholar] [CrossRef]
- Teow, Y.H.; Mohammad, A.W. New generation nanomaterials for water desalination: A review. Desalination 2019, 451, 2–17. [Google Scholar] [CrossRef]
- Gai, W.; Zhang, Y.; Zhao, Q.; Chung, T.-S. Highly permeable thin film composite hollow fiber membranes for brackish water desalination by incorporating amino functionalized carbon quantum dots and hypochlorite treatment. J. Membr. Sci. 2021, 620, 118952. [Google Scholar] [CrossRef]
- Teramura, K.; Iguchi, S.; Mizuno, Y.; Shishido, T.; Tanaka, T. Photocatalytic conversion of CO2 in water over layered double hydroxides. Angew. Chem. Int. Ed. 2012, 51, 8008–8011. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Zhao, D.L.; Feng, F.; Chung, T.-S.; Chen, S.B. Thin-film nanocomposite reverse osmosis membranes incorporated with citrate-modified layered double hydroxides (LDHs) for brackish water desalination and boron removal. Desalination 2022, 527, 115583. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Y.; Zhou, Y.; Yang, B.; Xiao, K.; Zhao, H.-Z. High-hydrophilic and antifouling reverse osmosis membrane prepared based an unconventional radiation method for pharmaceutical plant effluent treatment. Sep. Purif. Technol. 2022, 280, 119838. [Google Scholar] [CrossRef]
- Chen, X.; Yip, N.Y. Unlocking high-salinity desalination with cascading osmotically mediated reverse osmosis: Energy and operating pressure analysis. Environ. Sci. Technol. 2018, 52, 2242–2250. [Google Scholar] [CrossRef]
- Peters, C.D.; Hankins, N.P. Osmotically assisted reverse osmosis (OARO): Five approaches to dewatering saline brines using pressure-driven membrane processes. Desalination 2019, 458, 1–13. [Google Scholar] [CrossRef]
- Al-Najar, B.; Peters, C.D.; Albuflasa, H.; Hankins, N.P. Pressure and osmotically driven membrane processes: A review of the benefits and production of nano-enhanced membranes for desalination. Desalination 2020, 479, 114323. [Google Scholar] [CrossRef]
- Askari, M.; Liang, C.Z.; Choong, L.T.S.; Chung, T.-S. Optimization of TFC-PES hollow fiber membranes for reverse osmosis (RO) and osmotically assisted reverse osmosis (OARO) applications. J. Membr. Sci. 2021, 625, 119156. [Google Scholar] [CrossRef]
- Liu, C.; Takagi, R.; Shintani, T.; Cheng, L.; Tung, K.L.; Matsuyama, H. Organic liquid mixture separation using an aliphatic polyketone-supported polyamide organic solvent reverse osmosis (OSRO) membrane. ACS Appl. Mater. Interfaces 2020, 12, 7586–7594. [Google Scholar] [CrossRef]
- Liu, C.; Takagi, R.; Saeki, D.; Cheng, L.; Shintani, T.; Yasui, T.; Matsuyama, H. Highly improved organic solvent reverse osmosis (OSRO) membrane for organic liquid mixture separation by simple heat treatment. J. Membr. Sci. 2021, 618, 118710. [Google Scholar] [CrossRef]
- Chau, J.; Basak, P.; Sirkar, K.K. Reverse osmosis separation of particular organic solvent mixtures by a perfluorodioxole copolymer membrane. J. Membr. Sci. 2018, 563, 541–551. [Google Scholar] [CrossRef]
- Jang, H.Y.; Johnson, J.; Ma, Y.; Mathias, R.; Bhandari, D.A.; Lively, R.P. Torlon® hollow fiber membranes for organic solvent reverse osmosis separation of complex aromatic hydrocarbon mixtures. AIChE J. 2019, 65, 16757. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, F.; Yang, S.; Lively, R.P. Evidence for entropic diffusion selection of xylene isomers in carbon molecular sieve membranes. J. Membr. Sci. 2018, 564, 404–414. [Google Scholar] [CrossRef]
- Koh, D.-Y.; McCool, B.A.; Deckman, H.W.; Lively, R.P. Reverse osmosis molecular differentiation of organic liquids using carbon molecular sieve membranes. Science 2016, 353, 804–807. [Google Scholar] [CrossRef]
- Liu, C.; Dong, G.; Tsuru, T.; Matsuyama, H. Organic solvent reverse osmosis membranes for organic liquid mixture separation: A review. J. Membr. Sci. 2021, 620, 118882. [Google Scholar] [CrossRef]
- Liu, C.; Cheng, L.; Shintani, T.; Matsuyama, H. AF2400/polyketone composite organic solvent reverse osmosis membrane for organic liquid separation. J. Membr. Sci. 2021, 628, 119270. [Google Scholar] [CrossRef]
- Gonzales, R.R.; Kato, N.; Awaji, H.; Matsuyama, H. Development of polydimethylsiloxane composite membrane for organic solvent separation. Sep. Purif. Technol. 2022, 285, 120369. [Google Scholar] [CrossRef]
- Rivera, M.P.; Bruno, N.C.; Finn, M.G.; Lively, R.P. Organic solvent reverse osmosis using CuAAC-crosslinked molecularly-mixed composite membranes. J. Membr. Sci. 2021, 638, 119700. [Google Scholar] [CrossRef]
- Koros, W.J.; Zhang, C. Materials for next-generation molecularly selective synthetic membranes. Nat. Mater. 2017, 16, 289–297. [Google Scholar] [CrossRef]
- Jafarinejad, S. Forward osmosis membrane technology for nutrient removal/recovery from wastewater: Recent advances, proposed designs, and future directions. Chemosphere 2021, 263, 128116. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Chen, Q.; Ge, Q. Recent advances in forward osmosis (FO) membrane: Chemical modifications on membranes for FO processes. Desalination 2017, 419, 101–116. [Google Scholar] [CrossRef]
- Zheng, L.; Price, W.E.; McDonald, J.; Khan, S.J.; Fujioka, T.; Nghiem, L.D. New insights into the relationship between draw solution chemistry and trace organic rejection by forward osmosis. J. Membr. Sci. 2019, 587, 117184. [Google Scholar] [CrossRef] [Green Version]
- Lim, S.; Park, K.H.; Akther, N.; Phuntsho, S.; Choi, J.Y.; Shon, H.K. Size-controlled graphene oxide for highly permeable and fouling-resistant outer-selective hollow fiber thin-film composite membranes for forward osmosis. J. Membr. Sci. 2020, 609, 118171. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Quan, X. A novel reduced graphene oxide/carbon nanotube hollow fiber membrane with high forward osmosis performance. Desalination 2019, 451, 117–124. [Google Scholar] [CrossRef]
- Choi, P.J.; Lim, S.; Shon, H.; An, A.K. Incorporation of negatively charged silver nanoparticles in outer-selective hollow fiber forward osmosis (OSHF-FO) membrane for wastewater dewatering. Desalination 2022, 522, 115402. [Google Scholar] [CrossRef]
- Makhtar, S.N.N.M.; Muhammad, N.; Rahman, M.A.; Abas, K.H.; Abd Aziz, A.; Sokri, M.N.M.; Othman, M.H.D.; Jaafar, J. Zeolite-A deposited on glass hollow fiber for forward osmosis applications. J. Water Process. Eng. 2020, 33, 100991. [Google Scholar] [CrossRef]
- Fan, X.; Liu, Y.; Quan, X.; Chen, S. Highly permeable thin-film composite forward osmosis membrane based on carbon nanotube hollow fiber scaffold with electrically enhanced fouling resistance. Environ. Sci. Technol. 2018, 52, 1444–1452. [Google Scholar] [CrossRef]
- Ren, J.; McCutcheon, J.R. A new commercial biomimetic hollow fiber membrane for forward osmosis. Desalination 2018, 442, 44–50. [Google Scholar] [CrossRef]
- Engelhardt, S.; Sadek, A.; Duirk, S. Rejection of trace organic water contaminants by an Aquaporin-based biomimetic hollow fiber membrane. Sep. Purif. Technol. 2018, 197, 170–177. [Google Scholar] [CrossRef]
- Mahpoz, N.M.; Makhtar, S.N.N.M.; Pauzi, M.Z.M.; Abdullah, N.; Rahman, M.A.; Abas, K.H.; Ismail, A.F.; Othman, M.H.D.; Jaafar, J. ZIF-8 membrane supported on alumina hollow fiber with enhanced salt removal by forward osmosis. Desalination 2020, 496, 114697. [Google Scholar] [CrossRef]
- Lim, S.; Akther, N.; Bae, T.H.; Phuntsho, S.; Merenda, A.; Dumée, L.F.; Shon, H.K. Covalent organic framework incorporated outer-selective hollow fiber thin-film nanocomposite membranes for osmotically driven desalination. Desalination 2020, 485, 114461. [Google Scholar] [CrossRef]
- Engelhardt, S.; Vogel, J.; Duirk, S.E.; Moore, F.B.; Barton, H.A. Urea and ammonium rejection by an aquaporin-based hollow fiber membrane. J. Water Process. Eng. 2019, 32, 100903. [Google Scholar] [CrossRef]
- Sanahuja-Embuena, V.; Lim, S.; Górecki, R.; Trzaskus, K.; Hélix-Nielsen, C.; Shon, H.K. Enhancing selectivity of novel outer-selective hollow fiber forward osmosis membrane by polymer nanostructures. Chem. Eng. J. 2022, 433, 133634. [Google Scholar] [CrossRef]
- Salamanca, M.; López-Serna, R.; Palacio, L.; Hernández, A.; Prádanos, P.; Peña, M. Study of the rejection of contaminants of emerging concern by a biomimetic aquaporin hollow fiber forward osmosis membrane. J. Water Process. Eng. 2021, 40, 101914. [Google Scholar] [CrossRef]
- Firouzjaei, M.D.; Seyedpour, S.F.; Aktij, S.A.; Giagnorio, M.; Bazrafshan, N.; Mollahosseini, A.; Samadi, F.; Ahmadalipour, S.; Firouzjaei, F.D.; Esfahani, M.R. Recent advances in functionalized polymer membranes for biofouling control and mitigation in forward osmosis. J. Membr. Sci. 2020, 596, 117604. [Google Scholar] [CrossRef]
- Rastgar, M.; Bozorg, A.; Shakeri, A.; Sadrzadeh, M. Substantially improved antifouling properties in electro-oxidative graphene laminate forward osmosis membrane. Chem. Eng. Res. Des. 2019, 141, 413–424. [Google Scholar] [CrossRef]
- Xu, M.; Zhao, P.; Tang, C.Y.; Yi, X.; Wang, X. Preparation of electrically enhanced forward osmosis (FO) membrane by two-dimensional MXenes for organic fouling mitigation. Chin. Chem. Lett. 2021, in press. [Google Scholar] [CrossRef]
- Cruz-Tato, P.; Rivera-Fuentes, N.; Flynn, M.; Nicolau, E. Anti-fouling electroconductive forward osmosis membranes: Electrochemical and chemical properties. ACS Appl. Polym. Mater. 2019, 1, 1061–1070. [Google Scholar] [CrossRef]
- Shakeri, A.; Salehi, H.; Rastgar, M. Antifouling electrically conductive membrane for forward osmosis prepared by polyaniline/graphene nanocomposite. J. Water Process. Eng. 2019, 32, 100932. [Google Scholar] [CrossRef]
- Abu-Thabit, N.Y. Chemical oxidative polymerization of polyaniline: A practical approach for preparation of smart conductive textiles. J. Chem. Educ. 2016, 93, 1606–1611. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, H.; Yu, M.; Wang, Y.; Gao, T.; Yang, F. Conductive thin film nanocomposite forward osmosis membrane (TFN-FO) blended with carbon nanoparticles for membrane fouling control. Sci. Total Environ. 2019, 697, 134050. [Google Scholar] [CrossRef] [PubMed]
- Law, J.Y.; Mohammad, A.W. Multiple-solute salts as draw solution for osmotic concentration of succinate feed by forward osmosis. J. Ind. Eng. Chem. 2017, 51, 264–270. [Google Scholar] [CrossRef]
- Johnson, D.J.; Suwaileh, W.A.; Mohammed, A.W.; Hilal, N. Osmotic’s potential: An overview of draw solutes for forward osmosis. Desalination 2018, 434, 100–120. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhang, X.; Yao, J.; Simon, G.P.; Wang, H. Stimuli-responsive polymer hydrogels as a new class of draw agent for forward osmosis desalination. Chem. Commun. 2011, 47, 1710–1712. [Google Scholar] [CrossRef] [PubMed]
- Mohammadifakhr, M.; De Grooth, J.; Trzaskus, K.; Roesink, H.; Kemperman, A. Single-step synthesis of a polyelectrolyte complex hollow-fiber membrane for forward osmosis. Sep. Purif. Technol. 2021, 264, 118430. [Google Scholar] [CrossRef]
- Huang, J.J.; Chen, S.; Liao, Y.; Chen, Y.; You, X.; Wang, R. Performance, fouling and cleaning of a thin film composite hollow fiber membrane during fertiliser-drawn forward osmosis process for micro-polluted water. Environ. Sci. Water Res. Technol. 2021, 7, 1279–1291. [Google Scholar] [CrossRef]
- Almoalimi, K.; Liu, Y.Q. Enhancing ammonium rejection in forward osmosis for wastewater treatment by minimizing cation exchange. J. Membr. Sci. 2022, 648, 120365. [Google Scholar] [CrossRef]
- Akhtar, A.; Singh, M.; Subbiah, S.; Mohanty, K. Sugarcane juice concentration using a novel aquaporin hollow fiber forward osmosis membrane. Food Bioprod. Process. 2021, 126, 195–206. [Google Scholar] [CrossRef]
- Teklu, H.; Gautam, D.K.; Subbiah, S. Axial flow hollow fiber forward osmosis module analysis for optimum design and operating conditions in desalination applications. Chem. Eng. Sci. 2020, 216, 115494. [Google Scholar] [CrossRef]
- Altaee, A.; Braytee, A.; Millar, G.J.; Naji, O. Energy efficiency of hollow fibre membrane module in the forward osmosis seawater desalination process. J. Membr. Sci. 2019, 587, 117165. [Google Scholar] [CrossRef]
- Im, S.J.; Jeong, S.; Jang, A. Forward osmosis (FO)-reverse osmosis (RO) hybrid process incorporated with hollow fiber FO. npj Clean Water 2021, 4, 51. [Google Scholar] [CrossRef]
- Low, K.S.; Wang, Y.; Ng, D.Y.F.; Goh, K.; Li, Y.; Wang, R. Understanding the effect of transverse vibration on hollow fiber membranes for submerged forward osmosis processes. J. Membr. Sci. 2020, 610, 118211. [Google Scholar] [CrossRef]
- Li, T.; Law, A.W.-K.; Jiang, Y.; Harijanto, A.K.; Fane, A.G. Fouling control of submerged hollow fibre membrane bioreactor with transverse vibration. J. Membr. Sci. 2016, 505, 216–224. [Google Scholar] [CrossRef]
- Cui, Y.; Chung, T.-S. Pharmaceutical concentration using organic solvent forward osmosis for solvent recovery. Nat. Commun. 2018, 9, 1426. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Chung, T.-S. Solvent recovery via organic solvent pressure assisted osmosis. Ind. Eng. Chem. Res. 2019, 58, 4970–4978. [Google Scholar] [CrossRef]
- Liang, B.; He, X.; Hou, J.; Li, L.; Tang, Z. Membrane separation in organic liquid: Technologies, achievements, and opportunities. Adv. Mater. 2019, 31, 1806090. [Google Scholar] [CrossRef]
- Goh, K.S.; Chen, Y.; Ng, D.Y.F.; Chew, J.W.; Wang, R. Organic solvent forward osmosis membranes for pharmaceutical concentration. J. Membr. Sci. 2022, 642, 119965. [Google Scholar] [CrossRef]
- Seo, H.; Yoon, S.; Oh, B.; Chung, Y.G.; Koh, D.Y. Shape-selective ultramicroporous carbon membranes for sub-0.1 nm organic liquid separation. Adv. Sci. 2021, 8, 2004999. [Google Scholar] [CrossRef]
- Wu, X.; Ding, M.; Xu, H.; Yang, W.; Zhang, K.; Tian, H.; Wang, H.; Xie, Z. Scalable Ti3C2Tx Mxene interlayered forward osmosis membranes for enhanced water purification and organic solvent recovery. ACS Nano 2020, 14, 9125–9135. [Google Scholar] [CrossRef]
- Tong, Z.; Guo, H.; Liu, X.; Zhang, B. Organic solvent forward osmosis of graphene oxide-based membranes for enrichment of target products. Ind. Eng. Chem. Res. 2020, 59, 19012–19019. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y. Liquid separation by membrane pervaporation: A review. Ind. Eng. Chem. Res. 1997, 36, 1048–1066. [Google Scholar] [CrossRef]
- Liu, G.; Jin, W. Pervaporation membrane materials: Recent trends and perspectives. J. Membr. Sci. 2021, 636, 119557. [Google Scholar] [CrossRef]
- Ong, Y.K.; Shi, G.M.; Le, N.L.; Tang, Y.P.; Zuo, J.; Nunes, S.P.; Chung, T.-S. Recent membrane development for pervaporation processes. Prog. Polym. Sci. 2016, 57, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Castro-Muñoz, R.; Galiano, F.; Fíla, V.; Drioli, E.; Figoli, A. Mixed matrix membranes (MMMs) for ethanol purification through pervaporation: Current state of the art. Rev. Chem. Eng. 2019, 35, 565–590. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, M.; Ji, C.-H.; Xu, X.-R.; Ma, X.-H.; Xu, Z.-L. Multilayer assembled CS-PSS/ceramic hollow fiber membranes for pervaporation dehydration. Sep. Purif. Technol. 2018, 203, 84–92. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, Y.; Jiang, J.; Wang, X.; Zhang, C.; Gu, X. High-performance NaA zeolite membranes supported on four-channel ceramic hollow fibers for ethanol dehydration. RSC Adv. 2015, 5, 95866–95871. [Google Scholar] [CrossRef]
- Cao, Y.; Li, Y.-X.; Wang, M.; Xu, Z.-L.; Wei, Y.-M.; Shen, B.-J.; Zhu, K.-K. High-flux NaA zeolite pervaporation membranes dynamically synthesized on the alumina hollow fiber inner-surface in a continuous flow system. J. Membr. Sci. 2019, 570, 445–454. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, J.; Liu, D.; Xue, Y.; Zhang, C.; Gu, X. Evaluation of hollow fiber T-type zeolite membrane modules for ethanol dehydration. Chin. J. Chem. Eng. 2017, 25, 581–586. [Google Scholar] [CrossRef]
- Xu, Y.M.; Japip, S.; Chung, T.-S. UiO-66-NH2 incorporated dual-layer hollow fibers made by immiscibility induced phase separation (I2PS) process for ethanol dehydration via pervaporation. J. Membr. Sci. 2020, 595, 117571. [Google Scholar] [CrossRef]
- Sukitpaneenit, P.; Chung, T.-S. Fabrication and use of hollow fiber thin film composite membranes for ethanol dehydration. J. Membr. Sci. 2014, 450, 124–137. [Google Scholar] [CrossRef]
- Tsai, H.-A.; Wang, T.-Y.; Huang, S.-H.; Hu, C.-C.; Hung, W.-S.; Lee, K.-R.; Lai, J.-Y. The preparation of polyamide/polyacrylonitrile thin film composite hollow fiber membranes for dehydration of ethanol mixtures. Sep. Purif. Technol. 2017, 187, 221–232. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, W.; Li, W.; Xing, W. Preparation and characterization of chitosan-poly (vinyl alcohol)/polyvinylidene fluoride hollow fiber composite membranes for pervaporation dehydration of isopropanol. Korean J. Chem. Eng. 2015, 32, 1369–1376. [Google Scholar] [CrossRef]
- Zhang, X.; Li, M.-P.; Huang, Z.-H.; Zhang, H.; Liu, W.-L.; Xu, X.-R.; Ma, X.-H.; Xu, Z.-L. Fast surface crosslinking ceramic hollow fiber pervaporation composite membrane with outstanding separation performance for isopropanol dehydration. Sep. Purif. Technol. 2020, 234, 116116. [Google Scholar] [CrossRef]
- Zuo, J.; Hua, D.; Maricar, V.; Ong, Y.K.; Chung, T.S. Dehydration of industrial isopropanol (IPA) waste by pervaporation and vapor permeation membranes. J. Appl. Polym. Sci. 2018, 135, 45086. [Google Scholar] [CrossRef]
- Taymazov, D.; Zhang, H.; Li, W.-X.; Li, P.-P.; Xie, F.; Gong, X.-Y.; Zhang, S.-N.; Ma, X.-H.; Xu, Z.-L. Construction of MoS2 hybrid membranes on ceramic hollow fibers for efficient dehydration of isopropanol solution via pervaporation. Sep. Purif. Technol. 2021, 277, 119452. [Google Scholar] [CrossRef]
- Hua, D.; Ong, Y.K.; Wang, P.; Chung, T.-S. Thin-film composite tri-bore hollow fiber (TFC TbHF) membranes for isopropanol dehydration by pervaporation. J. Membr. Sci. 2014, 471, 155–167. [Google Scholar] [CrossRef]
- Hua, D.; Chung, T.S.; Shi, G.M.; Fang, C. Teflon AF2400/Ultem composite hollow fiber membranes for alcohol dehydration by high-temperature vapor permeation. AIChE J. 2016, 62, 1747–1757. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Zhang, C.; Gu, X.; Xu, N. Preparation and characterization of high-flux T-type zeolite membranes supported on YSZ hollow fibers. J. Membr. Sci. 2014, 455, 294–304. [Google Scholar] [CrossRef]
- Liu, X.; Wang, C.; Wang, B.; Li, K. Novel organic-dehydration membranes prepared from zirconium metal-organic frameworks. Adv. Funct. Mater. 2017, 27, 1604311. [Google Scholar] [CrossRef]
- Badiger, H.; Shukla, S.; Kalyani, S.; Sridhar, S. Thin film composite sodium alginate membranes for dehydration of acetic acid and isobutanol. J. Appl. Polym. Sci. 2014, 131, 1–9. [Google Scholar] [CrossRef]
- Raza, W.; Wang, J.; Yang, J.; Tsuru, T. Progress in pervaporation membranes for dehydration of acetic acid. Sep. Purif. Technol. 2021, 262, 118338. [Google Scholar] [CrossRef]
- Jiang, J.; Peng, L.; Wang, X.; Qiu, H.; Ji, M.; Gu, X. Effect of Si/Al ratio in the framework on the pervaporation properties of hollow fiber CHA zeolite membranes. Microporous Mesoporous Mater. 2019, 273, 196–202. [Google Scholar] [CrossRef]
- Zhang, Y.; Qiu, X.; Hong, Z.; Du, P.; Song, Q.; Gu, X. All-silica DD3R zeolite membrane with hydrophilic-functionalized surface for efficient and highly-stable pervaporation dehydration of acetic acid. J. Membr. Sci. 2019, 581, 236–242. [Google Scholar] [CrossRef]
- Mangindaan, D.W.; Shi, G.M.; Chung, T.-S. Pervaporation dehydration of acetone using P84 co-polyimide flat sheet membranes modified by vapor phase crosslinking. J. Membr. Sci. 2014, 458, 76–85. [Google Scholar] [CrossRef]
- Wang, Y. Pervaporation dehydration of ethyl acetate via PBI/PEI hollow fiber membranes. Ind. Eng. Chem. Res. 2015, 54, 3082–3089. [Google Scholar] [CrossRef]
- Prasad, N.S.; Moulik, S.; Bohra, S.; Rani, K.Y.; Sridhar, S. Solvent resistant chitosan/poly (ether-block-amide) composite membranes for pervaporation of n-methyl-2-pyrrolidone/water mixtures. Carbohydr. Polym. 2016, 136, 1170–1181. [Google Scholar] [CrossRef]
- Han, Y.-J.; Su, W.-C.; Lai, J.-Y.; Liu, Y.-L. Hydrophilically surface-modified and crosslinked polybenzimidazole membranes for pervaporation dehydration on tetrahydrofuran aqueous solutions. J. Membr. Sci. 2015, 475, 496–503. [Google Scholar] [CrossRef]
- Zhang, W.; Ying, Y.; Ma, J.; Guo, X.; Huang, H.; Liu, D.; Zhong, C. Mixed matrix membranes incorporated with polydopamine-coated metal-organic framework for dehydration of ethylene glycol by pervaporation. J. Membr. Sci. 2017, 527, 8–17. [Google Scholar] [CrossRef]
- Castro-Muñoz, R.; Ahmad, M.Z.; Fíla, V. Tuning of nano-based materials for embedding into low-permeability polyimides for a featured gas separation. Front. Chem. 2020, 7, 897. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.-X.; Wang, N.; Zhao, C.; Ji, S.; Li, J.-R. Membrane materials in the pervaporation separation of aromatic/aliphatic hydrocarbon mixtures—A review. Chin. J. Chem. Eng. 2018, 26, 1–16. [Google Scholar] [CrossRef]
- Wu, T.; Wang, N.; Li, J.; Wang, L.; Zhang, W.; Zhang, G.; Ji, S. Tubular thermal crosslinked-PEBA/ceramic membrane for aromatic/aliphatic pervaporation. J. Membr. Sci. 2015, 486, 1–9. [Google Scholar] [CrossRef]
- Arregoitia-Sarabia, C.; González-Revuelta, D.; Fallanza, M.; Ortiz, A.; Gorri, D. Polyether-block-amide thin-film composite hollow fiber membranes for the recovery of butanol from ABE process by pervaporation. Sep. Purif. Technol. 2021, 279, 119758. [Google Scholar] [CrossRef]
- Mirfendereski, S.M.; Lin, J.Y. High-performance MFI zeolite hollow fiber membranes synthesized by double-layer seeding with variable temperature secondary growth. J. Membr. Sci. 2021, 618, 118573. [Google Scholar] [CrossRef]
- Xia, S.; Peng, Y.; Wang, Z. Microstructure manipulation of MFI-type zeolite membranes on hollow fibers for ethanol-water separation. J. Membr. Sci. 2016, 498, 324–335. [Google Scholar] [CrossRef]
- Huang, K.; Li, Q.; Liu, G.; Shen, J.; Guan, K.; Jin, W. A ZIF-71 hollow fiber membrane fabricated by contra-diffusion. ACS Appl. Mater. Interfaces 2015, 7, 16157–16160. [Google Scholar] [CrossRef]
- Li, D.; Yao, J.; Sun, H.; Liu, B.; van Agtmaal, S.; Feng, C. Recycling of phenol from aqueous solutions by pervaporation with ZSM-5/PDMS/PVDF hollow fiber composite membrane. Appl. Surf. Sci. 2018, 427, 288–297. [Google Scholar] [CrossRef]
- Li, J.; Labreche, Y.; Wang, N.; Ji, S.; An, Q. PDMS/ZIF-8 coating polymeric hollow fiber substrate for alcohol permselective pervaporation membranes. Chin. J. Chem. Eng. 2019, 27, 2376–2382. [Google Scholar] [CrossRef]
- Li, Y.; Shen, J.; Guan, K.; Liu, G.; Zhou, H.; Jin, W. PEBA/ceramic hollow fiber composite membrane for high-efficiency recovery of bio-butanol via pervaporation. J. Membr. Sci. 2016, 510, 338–347. [Google Scholar] [CrossRef]
- Dong, Z.; Liu, G.; Liu, S.; Liu, Z.; Jin, W. High performance ceramic hollow fiber supported PDMS composite pervaporation membrane for bio-butanol recovery. J. Membr. Sci. 2014, 450, 38–47. [Google Scholar] [CrossRef]
- Saw, E.T.; Ang, K.L.; He, W.; Dong, X.; Ramakrishna, S. Molecular sieve ceramic pervaporation membranes in solvent recovery: A comprehensive review. J. Environ. Chem. Eng. 2019, 7, 103367. [Google Scholar] [CrossRef]
- Yang, D.; Majumdar, S.; Kovenklioglu, S.; Sirkar, K.K. Hollow fiber contained liquid membrane pervaporation system for the removal of toxic volatile organics from wastewater. J. Membr. Sci. 1995, 103, 195–210. [Google Scholar] [CrossRef]
- Uragami, T.; Matsuoka, Y.; Miyata, T. Permeation and separation characteristics in removal of dilute volatile organic compounds from aqueous solutions through copolymer membranes consisted of poly(styrene) and poly(dimethylsiloxane) containing a hydrophobic ionic liquid by pervaporation. J. Membr. Sci. 2016, 506, 109–118. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Removal of hazardous volatile organic compounds from water by vacuum pervaporation with hydrophobic ceramic membranes. J. Membr. Sci. 2015, 474, 11–19. [Google Scholar] [CrossRef]
- Kujawa, J.; Cerneaux, S.; Kujawski, W. Highly hydrophobic ceramic membranes applied to the removal of volatile organic compounds in pervaporation. Chem. Eng. J. 2015, 260, 43–54. [Google Scholar] [CrossRef]
- Ji, M.; Gao, X.; Wang, X.; Zhang, Y.; Jiang, J.; Gu, X. An ensemble synthesis strategy for fabrication of hollow fiber T-type zeolite membrane modules. J. Membr. Sci. 2018, 563, 460–469. [Google Scholar] [CrossRef]
- Chaudhari, S.; Chang, D.W.; Cho, K.Y.; Shon, M.Y.; Kwon, Y.S.; Nam, S.E.; Park, Y.I. Polyvinyl alcohol and graphene oxide blending surface coated alumina hollow fiber (AHF) membrane for pervaporation dehydration of epichlorohydrin (ECH)/isopropanol (IPA)/water ternary feed mixture. J. Taiwan Inst. Chem. Eng. 2020, 114, 103–114. [Google Scholar] [CrossRef]
- Goh, S.H.; Lau, H.S.; Yong, W.F. Metal-organic frameworks (MOFs)-based mixed matrix membranes (MMMs) for gas separation: A review on advanced materials in harsh environmental applications. Small 2022, 18, 2107536. [Google Scholar] [CrossRef]
- Li, Y.; Cao, C.; Chung, T.-S.; Pramoda, K.P. Fabrication of dual-layer polyethersulfone (PES) hollow fiber membranes with an ultrathin dense-selective layer for gas separation. J. Membr. Sci. 2004, 245, 23–60. [Google Scholar] [CrossRef]
- Jiang, L.; Chung, T.-S.; Li, D.F.; Cao, C.; Kulprathipanja, S. Fabrication of Matrimid/polyethersulfone dual-layer hollow fiber membranes for gas separation. J. Membr. Sci. 2004, 240, 21–103. [Google Scholar] [CrossRef]
- Liu, Y.; Chung, T.-S.; Wang, R.; Li, D.F.; Chng, M.L. Chemical cross-linking modification of polyimide/poly(ether sulfone) dual-layer hollow-fiber membranes for gas separation. Ind. Eng. Chem. Res. 2003, 42, 1190–1195. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.-S.; Xiao, Y. Superior gas separation performance of dual-layer hollow fiber membranes with an ultrathin dense-selective layer. J. Membr. Sci. 2008, 325, 23–27. [Google Scholar] [CrossRef]
- Li, Y.; Chung, T.-S. Silver ionic modification in dual-layer hollow fiber membranes with significant enhancement in CO2/CH4 and O2/N2 separation. J. Membr. Sci. 2010, 350, 226–231. [Google Scholar] [CrossRef]
- Li, H.; Choi, W.; Ingole, P.G.; Lee, H.K.; Baek, I.H. Oxygen separation membrane based on facilitated transport using cobalt tetraphenylporphyrin-coated hollow fiber composites. Fuel 2016, 185, 133–141. [Google Scholar] [CrossRef]
- Lau, C.H.; Low, B.T.; Shao, L.; Chung, T.-S. A vapor-phase surface modification method to enhance different types of hollow fiber membranes for industrial scale hydrogen separation. Int. J. Hydrog. Energy 2010, 35, 8970–8982. [Google Scholar] [CrossRef]
- Ingole, P.G.; Choi, W.K.; Baek, I.-H.; Lee, H.K. Highly selective thin film composite hollow fiber membranes for mixed vapor/gas separation. RSC Adv. 2015, 5, 78950–78957. [Google Scholar] [CrossRef]
- Ingole, P.G.; Choi, W.K.; Lee, G.B.; Lee, H.K. Thin-film-composite hollow-fiber membranes for water vapor separation. Desalination 2017, 403, 12–23. [Google Scholar] [CrossRef]
- Roslan, R.A.; Lau, W.J.; Sakthivel, D.B.; Khademi, S.; Zulhairun, A.K.; Goh, P.S.; Ismail, A.F.; Chong, K.C.; Lai, S.O. Separation of CO2/CH4 and O2/N2 by polysulfone hollow fiber membranes: Effects of membrane support properties and surface coating materials. J. Polym. Eng. 2018, 38, 871–880. [Google Scholar] [CrossRef]
- Qin, J.-J.; Chung, T.-S. Development of high-performance polysulfone/poly(4-vinylpyridine) composite hollow fibers for CO2/CH4 separation. Desalination 2006, 192, 112–116. [Google Scholar] [CrossRef]
- Li, H.B.; Shi, W.Y.; Li, J.C.; Zhang, Y.F. Preparation of hollow fiber composite membrane for carbon dioxide/methane separation via orthogonal experimental design. Fibers Polym. 2014, 15, 2553–2563. [Google Scholar] [CrossRef]
- Chong, K.C.; Lai, S.O.; Lau, W.J.; Thiam, H.S.; Ismail, A.F.; Roslan, R.A. Preparation, characterization, and performance evaluation of polysulfone hollow fiber membrane with PEBAX or PDMS coating for oxygen enhancement process. Polymers 2018, 10, 126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, X.; Cao, Y.; Zhao, H.; Wang, L.; Yuan, Q. Fabrication of high performance Matrimid/polysulfone dual-layer hollow fiber membranes for O2/N2 separation. J. Membr. Sci. 2008, 323, 352–361. [Google Scholar] [CrossRef]
- Ingole, P.G.; Baig, M.I.; Choi, W.K.; Lee, H.K. Synthesis and characterization of polyamide/polyester thin-film nanocomposite membranes achieved by functionalized TiO2 nanoparticles for water vapor separation. J. Mater. Chem. A 2016, 4, 5592–5604. [Google Scholar] [CrossRef]
- Baig, M.I.; Ingole, P.G.; Choi, W.K.; Park, S.R.; Kang, E.C.; Lee, H.K. Development of carboxylated TiO2 incorporated thin film nanocomposite hollow fiber membranes for flue gas dehydration. J. Membr. Sci. 2016, 514, 622–635. [Google Scholar] [CrossRef]
- Ingole, P.G.; Pawar, R.R.; Baig, M.I.; Jeon, J.D.; Lee, H.K. Thin film nanocomposite (TFN) hollow fiber membranes incorporated with functionalized acid-activated bentonite (ABn-NH) clay: Towards enhancement of water vapor permeance and selectivity. J. Mater. Chem. A 2017, 5, 20947–20958. [Google Scholar] [CrossRef]
- Ingole, P.G.; Sohail, M.; Abou-Elanwar, A.M.; Irshad Baig, M.; Jeon, J.-D.; Choi, W.K.; Kim, H.; Lee, H.K. Water vapor separation from flue gas using MOF incorporated thin film nanocomposite hollow fiber membranes. Chem. Eng. J. 2018, 334, 2450–2458. [Google Scholar] [CrossRef]
- Choi, O.; Karki, S.; Pawar, R.R.; Hazarika, S.; Ingole, P.G. A new perspective of functionalized MWCNT incorporated thin film nanocomposite hollow fiber membranes for the separation of various gases. J. Environ. Chem. Eng. 2021, 9, 104774. [Google Scholar] [CrossRef]
- An, X.; Ingole, P.G.; Choi, W.K.; Lee, H.K.; Hong, S.U.; Jeon, J.-D. Enhancement of water vapor separation using ETS-4 incorporated thin film nanocomposite membranes prepared by interfacial polymerization. J. Membr. Sci. 2017, 531, 77–85. [Google Scholar] [CrossRef]
- An, X.; Ingole, P.G.; Choi, W.K.; Lee, H.K.; Hong, S.U.; Jeon, J.-D. Development of thin film nanocomposite membranes incorporated with sulfated β-cyclodextrin for water vapor/N2 mixture gas separation. J. Ind. Eng. Chem. 2018, 59, 259–265. [Google Scholar] [CrossRef]
- Abou-Elanwar, A.M.; Shirke, Y.M.; Ingole, P.G.; Choi, W.K.; Lee, H.; Hong, S.U.; Lee, H.K.; Jeon, J.-D. Nanocomposite hollow fiber membranes with recyclable β-cyclodextrin encapsulated magnetite nanoparticles for water vapor separation. J. Mater. Chem. A 2018, 6, 24569–24579. [Google Scholar] [CrossRef]
- Sridhar, S.; Veerapur, R.S.; Patil, M.B.; Gudasi, K.B.; Aminabhavi, T.M. Matrimid polyimide membranes for the separation of carbon dioxide from methane. J. Appl. Polym. Sci. 2007, 106, 1585–1594. [Google Scholar] [CrossRef]
- Dominic, T.C.; William, J.K. Formation of defect-free polyimide hollow fiber membranes for gas separations. J. Membr. Sci. 2000, 167, 79–89. [Google Scholar]
- Falbo, F.; Tasselli, F.; Brunetti, A.; Drioli, E.; Barbieri, G. Polyimide hollow fiber membranes for CO2 separation from wet gas mixtures. Braz. J. Chem. Eng. 2014, 31, 1023–1034. [Google Scholar] [CrossRef] [Green Version]
- Kosuri, M.R.; Koros, W.J. Defect-free asymmetric hollow fiber membranes from Torlon®, a polyamide-imide polymer, for high-pressure CO2 separations. J. Membr. Sci. 2008, 320, 25–72. [Google Scholar] [CrossRef]
- Jue, M.L.; Breedveld, V.; Lively, R.P. Defect-free PIM-1 hollow fiber membranes. J. Membr. Sci. 2017, 530, 33–41. [Google Scholar] [CrossRef]
- Askari, M.; Yang, T.; Chung, T.-S. Natural gas purification and olefin/paraffin separation using cross-linkable dual-layer hollow fiber membranes comprising β-Cyclodextrin. J. Membr. Sci. 2012, 423–424, 392–403. [Google Scholar] [CrossRef]
- Peng, N.; Chung, T.-S.; Chng, M.L.; Aw, W. Evolution of ultra-thin dense-selective layer from single-layer to dual-layer hollow fibers using novel Extem® polyetherimide for gas separation. J. Membr. Sci. 2010, 360, 28–57. [Google Scholar] [CrossRef]
- Mishra, N.K.; Patil, N.; Long, C.; Yi, S.L.; Hopkinson, D.; Grunlan, J.C.; Wilhite, B.A. Enhancing H2-permselectivity of high-flux hollow fiber membrane via in-situ layer-by-layer surface treatment. J. Membr. Sci. 2020, 615, 118312. [Google Scholar] [CrossRef]
- Zhao, B.; Peng, N.; Liang, C.; Yong, W.F.; Chung, T.-S. Hollow fiber membrane dehumidification device for air conditioning system. Membranes 2015, 5, 722–738. [Google Scholar] [CrossRef] [Green Version]
- Esposito, E.; Clarizia, G.; Bernardo, P.; Jansen, J.C.; Sedláková, Z.; Izák, P.; Curcio, S.; Cindio, B.D.; Tasselli, F. Pebax®/PAN hollow fiber membranes for CO2/CH4 separation. Chem. Eng. Process. Process Intensif. 2015, 94, 53–61. [Google Scholar] [CrossRef]
- Yong, W.F.; Ho, Y.X.; Chung, T.-S. Nanoparticles embedded in amphiphilic membranes for carbon dioxide separation and dehumidification. ChemSusChem 2017, 10, 4046–4055. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Cheng, J.; Li, Y.; Liu, J.; Zhou, J.; Cen, K. In-situ grafting to improve polarity of polyacrylonitrile hollow fiber-supported polydimethylsiloxane membranes for CO2 separation. J. Colloid Interface Sci. 2018, 510, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Dahe, G.J.; Dudeck, K.W.; Berchtold, K.A. Macrovoid-free high performance polybenzimidazole hollow fiber membranes for elevated temperature H2/CO2 separations. Int. J. Hydrog. Energy 2020, 45, 27331–27345. [Google Scholar] [CrossRef]
- Wang, K.Y.; Weber, M.; Chung, T.-S. Polybenzimidazoles (PBIs) and state-of-the-art PBI hollow fiber membranes for water, organic solvent and gas separations: A review. J. Mater. Chem. A 2022, 10, 8687–8718. [Google Scholar] [CrossRef]
- Hao, L.; Zuo, J.; Chung, T.-S. Formation of defect-free polyetherimide/PIM-1 hollow fiber membranes for gas separation. AIChE J. 2014, 60, 3848–3858. [Google Scholar] [CrossRef]
- Salih, A.A.M.; Yi, C.H.; Yang, B.L.; Chen, P. Interfacially polymerized composite hollow fiber membrane for CO2 separation. Adv. Mat. Res. 2013, 781–784, 2040–2046. [Google Scholar]
- Dai, Z.; Deng, J.; Yu, Q.; Helberg, R.M.L.; Janakiram, S.; Ansaloni, L.; Deng, L. Fabrication and evaluation of bio-based nanocomposite TFC hollow fiber membranes for enhanced CO2 capture. ACS Appl. Mater. Interfaces 2019, 11, 10874–10882. [Google Scholar] [CrossRef]
- Dai, Z.; Deng, J.; Ansaloni, L.; Janakiram, S.; Deng, L. Thin-film-composite hollow fiber membranes containing amino acid salts as mobile carriers for CO2 separation. J. Membr. Sci. 2019, 578, 61–68. [Google Scholar] [CrossRef]
- Dai, Y.; Ruan, X.; Bai, F.; Yu, M.; Li, H.; Zhao, Z.; He, G. High solvent resistance PTFPMS/PEI hollow fiber composite membrane for gas separation. Appl. Surf. Sci. 2016, 360, 164–173. [Google Scholar] [CrossRef]
- Kumbharkar, S.C.; Liu, Y.; Li, K. High performance polybenzimidazole based asymmetric hollow fibre membranes for H2/CO2 separation. J. Membr. Sci. 2011, 375, 231–240. [Google Scholar] [CrossRef]
- Kim, D.Y.; Ryu, S.; Kim, H.-J.; Ham, H.C.; Sohn, H.; Yoon, S.P.; Han, J.; Lim, T.-H.; Kim, J.Y.; Lee, S.W.; et al. Highly selective asymmetric polybenzimidazole-4,4′-(hexafluoroisopropylidene) bis(benzoic acid) hollow fiber membranes for hydrogen separation. Sep. Purif. Technol. 2021, 257, 117954. [Google Scholar] [CrossRef]
- Lee, K.R.; Hwang, S.T. Separation of propylene and propane by polyimide hollow-fiber membrane module. J. Membr. Sci. 1992, 73, 37–45. [Google Scholar] [CrossRef]
- Krol, J.J.; Boerrigter, M.; Koops, G.H. Polyimide hollow fiber gas separation membranes: Preparation and the suppression of plasticization in propane/propylene environments. J. Membr. Sci. 2001, 184, 275–286. [Google Scholar] [CrossRef]
- Yoshino, M.; Nakamura, S.; Kita, H.; Okamoto, K.; Tanihara, N.; Kusuki, Y. Olefin/paraffin separation performance of asymmetric hollow fiber membrane of 6FDA/BPDA–DDBT copolyimide. J. Membr. Sci. 2003, 212, 23–27. [Google Scholar] [CrossRef]
- Liu, G.; Labreche, Y.; Li, N.; Liu, Y.; Zhang, C.; Miller, S.J.; Babu, V.P.; Bhuwania, N.; Koros, W.J. Simultaneously tuning denseskin and porous substrate of asymmetric hollow fiber membranes for efficient purification of aggressive natural gas. AIChE J. 2019, 65, 1269–1280. [Google Scholar] [CrossRef]
- Kim, K.; Ingole, P.G.; Kim, J.; Lee, H. Separation performance of PEBAX/PEI hollow fiber composite membrane for SO2/CO2/N2 mixed gas. Chem. Eng. J. 2013, 233, 242–250. [Google Scholar] [CrossRef]
- Kim, K.; Hong, S.; Kim, J.; Lee, H. Preparation and performance evaluation of composite hollow fiber membrane for SO2 separation. AIChE J. 2014, 60, 2298–2306. [Google Scholar] [CrossRef]
- Babu, V.P.; Kraftschik, B.E.; Koros, W.J. Crosslinkable TEGMC asymmetric hollow fiber membranes for aggressive sour gas separations. J. Membr. Sci. 2018, 558, 94–105. [Google Scholar] [CrossRef]
- Wang, L.-Y.; Yong, W.F.; Yu, L.E.; Chung, T.-S. Design of high efficiency PVDF-PEG hollow fibers for air filtration of ultrafine particles. J. Membr. Sci. 2017, 535, 342–349. [Google Scholar] [CrossRef]
- Chung, T.-S.; Teoh, S.K. The ageing phenomenon of polyethersulfone hollow fiber membranes for gas separation and their characteristics. J. Membr. Sci. 1999, 152, 175–188. [Google Scholar] [CrossRef]
- Zhao, C.; Xue, J.; Ran, F.; Sun, S. Modification of polyethersulfone membranes—A review of methods. Prog. Mater. Sci. 2013, 58, 76–150. [Google Scholar] [CrossRef]
- Zhang, M.; Deng, L.; Xiang, D.; Cao, B.; Hosseini, S.; Li, P. Approaches to suppress CO2-induced plasticization of polyimide membranes in gas separation applications. Processes 2019, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Li, K.; Teo, W.K. Highly permeable polyethersulfone hollow fiber gas separation membranes prepared using water as non-solvent additive. J. Membr. Sci. 2000, 176, 147–158. [Google Scholar] [CrossRef]
- Adib, H.; Hassanajili, S.; Mowla, D.; Esmaeilzadeh, F. Fabrication of integrally skinned asymmetric membranes based on nanocomposite polyethersulfone by supercritical CO2 for gas separation. J. Supercrit. Fluids 2015, 97, 6–15. [Google Scholar] [CrossRef]
- Ismail, A.F.; Norida, R.; Rahman, W.A.W.A.; Matsuura, T.; Hashemifard, S.A. Preparation and characterization of hyperthin-skinned and high performances asymmetric polyethersulfone membrane for gas separation. Desalination 2011, 273, 93–104. [Google Scholar] [CrossRef]
- Ma, C.; Koros, W.J. Physical aging of ester-cross-linked hollow fiber membranes for natural gas separations and mitigation thereof. J. Membr. Sci. 2018, 551, 214–221. [Google Scholar] [CrossRef]
- Clarizia, G.; Tasselli, F.; Bernardo, P. Effect of physical aging on gas transport in asymmetric polyimide hollow fibers prepared by triple-orifice spinneret. Polymers 2020, 12, 441. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.-H.; Jansen, J.C.; Tasselli, F.; Barbieri, G.; Drioli, E. In-line formation of chemically cross-linked P84® co-polyimide hollow fibre membranes for H2/CO2 separation. Sep. Purif. Technol. 2010, 76, 132–139. [Google Scholar] [CrossRef]
- Omole, I.C.; Miller, S.J.; Koros, W.J. Increased molecular weight of a crosslinkable polyimide for spinning plasticization resistant hollow fiber membranes. Macromolecules 2008, 41, 6367–6375. [Google Scholar] [CrossRef]
- Chen, C.-C.; Miller, S.J.; Koros, W.J. Characterization of thermally cross-linkable hollow fiber membranes for natural gas separation. Ind. Eng. Chem. Res. 2012, 52, 1015–1022. [Google Scholar] [CrossRef]
- Thür, R.; Lemmens, V.; Van Havere, D.; van Essen, M.; Nijmeijer, K.; Vankelecom, I.F.J. Tuning 6FDA-DABA membrane performance for CO2 removal by physical densification and decarboxylation cross-linking during simple thermal treatment. J. Membr. Sci. 2020, 610, 118195. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Kraftschik, B.E.; Babu, V.P.; Bhuwania, N.; Chinn, D.; Koros, W.J. Natural gas sweetening using TEGMC polyimide hollow fiber membranes. J. Membr. Sci. 2021, 632, 119361. [Google Scholar] [CrossRef]
- Chung, T.-S.; Ren, J.; Wang, R.; Li, D.; Liu, Y.; Pramoda, K.P.; Cao, C.; Loh, W.W. Development of asymmetric 6FDA-2,6DAT hollow fiber membranes for CO2/CH4 separation: Part 2. Suppression of plasticization. J. Membr. Sci. 2003, 214, 57–69. [Google Scholar] [CrossRef]
- Sheng, L.; Ren, J.; Hua, K.; Li, H.; Feng, Y.; Deng, M. The enhancement of mechanical properties of P84 hollow fiber membranes by thermally annealing below and above Tg. J. Membr. Sci. 2020, 595, 117580. [Google Scholar] [CrossRef]
- Shao, L.; Low, B.T.; Chung, T.-S.; Greenberg, A.R. Polymeric membranes for the hydrogen economy: Contemporary approaches and prospects for the future. J. Membr. Sci. 2009, 327, 18–31. [Google Scholar] [CrossRef]
- Masuda, T.; Nagai, K. Synthesis and permeation properties of substituted polyacetylenes for gas separation and pervaporation. In Materials Science of Membranes for Gas and Vapor Separation; John Wiley & Sons, Ltd.: San Francisco, CA, USA, 2006; Volume 8, pp. 231–247. [Google Scholar]
- Inoue, R.; Kanaya, T.; Hu, Y.; Masuda, T.; Nishida, K.; Yamamuro, O. Relationship between the local dynamics and gas permeability of polyacetylenes containing polymethylated indan/tetrahydronaphtalene moieties. Polymer 2014, 55, 182–186. [Google Scholar] [CrossRef]
- Lee, H.J.; Kang, S.W. CO2 separation with polymer/aniline composite membranes. Polymers 2020, 12, 1363. [Google Scholar] [CrossRef]
- Gupta, Y.; Hellgardt, K.; Wakeman, R.J. Enhanced permeability of polyaniline based nano-membranes for gas separation. J. Membr. Sci. 2006, 282, 60–70. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Z.; Wang, J.; Wang, S. High-performance membranes comprising polyaniline nanoparticles incorporated into polyvinylamine matrix for CO2/N2 separation. J. Membr. Sci. 2012, 403–404, 203–215. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, X.; Li, J.; Liu, C.; Gao, Y.; Li, N.; Xie, Z. Enhancing the CO2 separation performance of SPEEK membranes by incorporation of polyaniline-decorated halloysite nanotubes. J. Membr. Sci. 2019, 573, 602–611. [Google Scholar] [CrossRef]
- Illing, G.; Hellgardt, K.; Wakeman, R.J.; Jungbauer, A. Preparation and characterisation of polyaniline based membranes for gas separation. J. Membr. Sci. 2001, 184, 69–78. [Google Scholar] [CrossRef]
- Stolarczyk, A.; Lapkowski, M. Investigation of gas separation on polyaniline laminar composite membranes. Synth. Met. 2001, 121, 1385–1386. [Google Scholar] [CrossRef]
- Orlov, A.V.; Kiseleva, S.G.; Karpacheva, G.P.; Teplyakov, V.V.; Syrtsova, D.A.; Starannikova, L.E.; Lebedeva, T.L. Structure and gas separation properties of composite films based on polyaniline. J. Appl. Polym. Sci. 2003, 89, 1379–1384. [Google Scholar] [CrossRef]
- Ghalei, B.; Kinoshita, Y.; Wakimoto, K.; Sakurai, K.; Mathew, S.; Yue, Y.; Kusuda, H.; Imahori, H.; Sivaniah, E. Surface functionalization of high free-volume polymers as a route to efficient hydrogen separation membranes. J. Mater. Chem. A 2017, 5, 4686–4694. [Google Scholar] [CrossRef]
- Giel, V.; Kredatusová, J.; Trchová, M.; Brus, J.; Žitka, J.; Peter, J. Polyaniline/polybenzimidazole blends: Characterisation of its physico-chemical properties and gas separation behaviour. Eur. Polym. J. 2016, 77, 98–113. [Google Scholar] [CrossRef]
- Giel, V.; Morávková, Z.; Peter, J.; Trchová, M. Thermally treated polyaniline/polybenzimidazole blend membranes: Structural changes and gas transport properties. J. Membr. Sci. 2017, 537, 315–322. [Google Scholar] [CrossRef]
- Huang, N.-Y.; Wang, C.-C.; Chen, C.-Y. Gas-permeation properties of sandwich-like polyaniline/poly(ethylene vinyl acetate) nanocomposite membranes. Chem. Eng. Res. Des. 2021, 170, 239–247. [Google Scholar] [CrossRef]
- Kaner, R.B.; Anderson, M.R.; Reiss, H.; Mattes, B.R. Membranes having selective permeability. U.S. Patent 5,358,556, 25 October 1994. [Google Scholar]
- Rebattet, L.; Escoubes, M.; Genies, E.; Pineri, M. Effect of doping treatment on gas transport properties and on separation factors of polyaniline memebranes. J. Appl. Polym. Sci. 1995, 57, 1595–1604. [Google Scholar] [CrossRef]
- Wang, H.-L.; Mattes, B.R. Gas transport and sorption in polyaniline thin film. Synth. Met. 1999, 102, 1333–1334. [Google Scholar] [CrossRef]
- Wang, H.-L.; Mattes, B.R. Permeable Polyaniline Articles for Gas Separation. U.S. Patent 6,797,325, 28 September 2004. [Google Scholar]
- Parthasarathy, R.V.; Menon, V.P.; Martin, C.R. Unusual gas-transport selectivity in a partially oxidized form of the conductive polymer polypyrrole. Chem. Mater. 1997, 9, 560–566. [Google Scholar] [CrossRef]
- Martin, C.R.; Liang, W.; Menon, V.; Parthasarathy, R.; Parthasarathy, A. Electronically conductive polymers as chemically-selective layers for membrane-based separations. Synth. Met. 1993, 57, 3766–3773. [Google Scholar] [CrossRef]
- Andreeva, D.V.; Pientka, Z.; Brozová, L.; Bleha, M.; Polotskaya, G.A.; Elyashevich, G.K. Effect of polymerization conditions of pyrrole on formation, structure and properties of high gas separation thin polypyrrole films. Thin Solid Films 2002, 406, 54–63. [Google Scholar] [CrossRef]
- Son, W.-I.; Hong, J.-M.; Kim, B.-S. Polypyrrole composite membrane with high permeability prepared by interfacial polymerization. Korean J. Chem. Eng. 2005, 22, 285–290. [Google Scholar] [CrossRef]
- Gulsen, D.; Hacarloglu, P.; Toppare, L.; Yilmaz, L. Effect of preparation parameters on the performance of conductive composite gas separation membranes. J. Membr. Sci. 2001, 182, 29–39. [Google Scholar] [CrossRef]
- Hacarlioglu, P.; Toppare, L.; Yilmaz, L. Polycarbonate–polypyrrole mixed matrix gas separation membranes. J. Membr. Sci. 2003, 225, 51–62. [Google Scholar] [CrossRef]
- Wang, X.; Ding, X.; Zhao, H.; Fu, J.; Xin, Q.; Zhang, Y. Pebax-based mixed matrix membranes containing hollow polypyrrole nanospheres with mesoporous shells for enhanced gas permeation performance. J. Membr. Sci. 2020, 602, 117968. [Google Scholar] [CrossRef]
- Asghari, M.; Saadatmandi, S.; Parnian, M.J. Polypyrrole-aided surface decoration of graphene oxide nanosheets as fillers for poly(ether-b-amid) mixed matrix membranes to enhance CO2 capture. Int. J. Energy Res. 2021, 45, 10843–10857. [Google Scholar] [CrossRef]
- Musselman, I.H.; Li, L.; Washmon, L.; Varadarajan, D.; Riley, S.J.; Hmyene, M.; Ferraris, J.P.; Balkus, K.J. Poly(3-dodecylthiophene) membranes for gas separations. J. Membr. Sci. 1999, 152, 1–18. [Google Scholar] [CrossRef]
- Reid, B.D.; Ebron, V.H.M.; Musselman, I.H.; Ferraris, J.P.; Balkus, K.J. Enhanced gas selectivity in thin film composite membranes of poly(3-(2-acetoxyethyl)thiophene). J. Membr. Sci. 2002, 195, 181–192. [Google Scholar] [CrossRef]
- Zhang, M.; Jing, X.; Zhao, S.; Shao, P.; Zhang, Y.; Yuan, S.; Li, Y.; Gu, C.; Wang, X.; Ye, Y. Electropolymerization of molecular-sieving polythiophene membranes for H2 separation. Angew. Chem. Int. Ed. 2019, 58, 8768–8772. [Google Scholar] [CrossRef]
- Norris, I.D.; Fadeev, A.G.; Pellegrino, J.; Mattes, B.R. Development of integrally skinned asymmetric polyaniline hollow fibers for membrane applications. Synth. Met. 2005, 153, 57–60. [Google Scholar] [CrossRef]
- Mattes, B.R. Transport and Other Physicochemical Properties of Polyaniline. In Proceedings of the 8th Annual Meeeting of the North American Membrane Society, Ottawa, ONT, Canada, 18–22 May 1996. [Google Scholar]
- Mattes, B.R.; Wang, H.-L.; Yang, D.; Zhua, Y.T.; Blumenthala, W.R.; Hundleya, M.F. Formation of conductive polyaniline fibers derived from highly concentrated emeraldine base solutions. Synth. Met. 1997, 84, 45–49. [Google Scholar] [CrossRef]
- Mattes, B.R.; Wang, H.-L.; Yang, D. Electrically conductive polyaniline fibers prepared by dry-wet spinning techniques. In Conductive Polymers and Plastics; Rupprecht, L., Ed.; Elsevier: Amsterdam, The Netherlands, 1999; pp. 135–141. [Google Scholar]
- Wang, H.-L.; Romero, R.J.; Mattes, B.R.; Zhu, Y.; Winokur, M.J. Effect of processing conditions on the properties of high molecular weight conductive polyaniline fiber. J. Polym. Sci. B Polym. Phys. 2000, 38, 194–204. [Google Scholar] [CrossRef]
- Yang, D.; Fadeev, A.; Adams, P.N.; Mattes, B.R. Controlling Macrovoid Formation in Wet-Spun Polyaniline Fibers. In Smart Structures and Materials 2001: Electroactive Polymer Actuators and Devices; SPIE: Newport Beach, CA, USA, 2001; pp. 59–71. [Google Scholar]
- Adams, P.N.; Bowman, D.; Brown, L.; Yang, D.; Mattes, B.R. Molecular weight dependence of the physical properties of protonated polyaniline films and fibers. In Smart Structures and Materials 2001: Electroactive Polymer Actuators and Devices; International Society for Optics and Photonics: Newport Beach, CA, USA, 2001; pp. 475–481. [Google Scholar]
- Mattes, B.R.; Adams, P.N.; Yang, D.; Brown, L.A.; Fadeev, A.G.; Norris, I.D. Spinning, Doping, Dedoping and Redoping Polyaniline Fiber. U.S. Patent 8,425,822, 23 April 2013. [Google Scholar]
- Benjamin, R.M.; Wang, H.-L. Method for Preparing Polyaniline Fibers. U.S. Patent 6,123,883, 26 September 2000. [Google Scholar]
- Hadi, A.; Karimi-Sabet, J.; Nikkho, S.; Dastbaz, A. Fabrication of ZIF-8/polyethersulfone (PES) mixed matrix hollow fiber membranes for O2/N2 separation. Chem. Pap. 2021, 75, 4129–4145. [Google Scholar] [CrossRef]
- Park, S.; Jeong, H.-K. Transforming polymer hollow fiber membrane modules to mixed-matrix hollow fiber membrane modules for propylene/propane separation. J. Membr. Sci. 2020, 612, 118429. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, K.; Xu, L.; Labreche, Y.; Kraftschik, B.; Koros, W.J. Highly scalable ZIF-based mixed-matrix hollow fiber membranes for advanced hydrocarbon separations. AIChE J. 2014, 60, 2625–2635. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Chew, T.L.; Lau, K.K. Comparison of post-treatment methods on the performance of hollow fiber membranes containing metal organic framework in gases separation. Ind. Eng. Chem. Res. 2019, 58, 7120–7130. [Google Scholar] [CrossRef]
- Zhu, H.; Jie, X.; Wang, L.; Kang, G.; Liu, D.; Cao, Y. Enhanced gas separation performance of mixed matrix hollow fiber membranes containing post-functionalized S-MIL-53. J. Energy Chem. 2018, 27, 781–790. [Google Scholar] [CrossRef] [Green Version]
- Li, G.; Kujawski, W.; Knozowska, K.; Kujawa, J. Thin film mixed matrix hollow fiber membrane fabricated by incorporation of amine functionalized metal-organic framework for CO2/N2 separation. Materials 2021, 14, 3366. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Chew, T.L.; Lau, K.K. Optimization of spinning parameters on the fabrication of NH2-MIL-53(Al)/cellulose acetate (CA) hollow fiber mixed matrix membrane for CO2 separation. Sep. Purif. Technol. 2019, 215, 32–43. [Google Scholar] [CrossRef]
- Mubashir, M.; Yin, F.Y.; Leng, C.T.; Keong, L.K.; Jusoh, N. Study on the effect of process parameters on CO2/CH4 binary gas separation performance over NH2-MIL-53(Al)/cellulose acetate hollow fiber mixed matrix membrane. Polym. Test. 2020, 81, 106223. [Google Scholar] [CrossRef]
- Wijiyanti, R.; Ubaidillah, A.N.; Gunawan, T.; Karim, Z.A.; Ismail, A.F.; Smart, S.; Lin, R.; Widiastuti, N. Polysulfone mixed matrix hollow fiber membranes using zeolite templated carbon as a performance enhancement filler for gas separation. Chem. Eng. Res. Des. 2019, 150, 274–288. [Google Scholar] [CrossRef]
- Park, H.B.; Jung, C.H.; Lee, Y.M.; Hill, A.J.; Pas, S.J.; Mudie, S.T.; Van Wagner, E.; Freeman, B.D.; Cookson, D.J. Polymers with cavities tuned for fast selective transport of small molecules and ions. Science 2007, 318, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Han, S.H.; Lee, Y.M. Thermally rearranged (TR) polybenzoxazole hollow fiber membranes for CO2 capture. J. Membr. Sci. 2012, 403–404, 169–178. [Google Scholar] [CrossRef]
- Woo, K.T.; Lee, J.; Dong, G.; Kim, J.S.; Do, Y.S.; Hung, W.-S.; Lee, K.-R.; Barbieri, G.; Drioli, E.; Lee, Y.M. Fabrication of thermally rearranged (TR) polybenzoxazole hollow fiber membranes with superior CO2/N2 separation performance. J. Membr. Sci. 2015, 490, 129–138. [Google Scholar] [CrossRef]
- Woo, K.T.; Lee, J.; Dong, G.; Kim, J.S.; Do, Y.S.; Jo, H.J.; Lee, Y.M. Thermally rearranged poly(benzoxazole-co-imide) hollow fiber membranes for CO2 capture. J. Membr. Sci. 2016, 498, 125–134. [Google Scholar] [CrossRef]
- Woo, K.T.; Dong, G.; Lee, J.; Kim, J.S.; Do, Y.S.; Lee, W.H.; Lee, H.S.; Lee, Y.M. Ternary mixed-gas separation for flue gas CO2 capture using high performance thermally rearranged (TR) hollow fiber membranes. J. Membr. Sci. 2016, 510, 472–480. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.S.; Kim, J.F.; Jo, H.J.; Park, H.; Seong, J.G.; Lee, Y.M. Densification-induced hollow fiber membranes using crosslinked thermally rearranged (XTR) polymer for CO2 capture. J. Membr. Sci. 2019, 573, 393–402. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.S.; Moon, S.-y.; Park, C.Y.; Kim, J.F.; Lee, Y.M. Dimensionally-controlled densification in crosslinked thermally rearranged (XTR) hollow fiber membranes for CO2 capture. J. Membr. Sci. 2020, 595, 117535. [Google Scholar] [CrossRef]
- Brunetti, A.; Cersosimo, M.; Kim, J.S.; Dong, G.; Fontananova, E.; Lee, Y.M.; Drioli, E.; Barbieri, G. Thermally rearranged mixed matrix membranes for CO2 separation: An aging study. Int. J. Greenh. Gas Control 2017, 61, 16–26. [Google Scholar] [CrossRef]
- Smith, S.J.D.; Hou, R.; Lau, C.H.; Konstas, K.; Kitchin, M.; Dong, G.; Lee, J.; Lee, W.H.; Seong, J.G.; Lee, Y.M.; et al. Highly permeable thermally rearranged mixed matrix membranes (TR-MMM). J. Membr. Sci. 2019, 585, 260–270. [Google Scholar] [CrossRef]
- Nocoń-Szmajda, K.; Wolińska-Grabczyk, A.; Jankowski, A.; Szeluga, U.; Wójtowicz, M.; Konieczkowska, J.; Hercog, A. Gas transport properties of mixed matrix membranes based on thermally rearranged poly(hydroxyimide)s filled with inorganic porous particles. Sep. Purif. Technol. 2020, 242, 116778. [Google Scholar] [CrossRef]
- Soto, C.; Aguilar Lugo, C.; Rodríguez, S.; Palacio, L.; Lozano, Á.E.; Prádanos, P.; Hernandez, A. Enhancement of CO2/CH4 permselectivity via thermal rearrangement of mixed matrix membranes made from an o-hydroxy polyamide with an optimal load of a porous polymer network. Sep. Purif. Technol. 2020, 247, 116895. [Google Scholar] [CrossRef]
- Japip, S.; Erifin, S.; Chung, T.-S. Reduced thermal rearrangement temperature via formation of zeolitic imidazolate framework (ZIF)-8-based nanocomposites for hydrogen purification. Sep. Purif. Technol. 2019, 212, 965–973. [Google Scholar] [CrossRef]
- Kim, J.S.; Moon, S.J.; Wang, H.H.; Kim, S.; Lee, Y.M. Mixed matrix membranes with a thermally rearranged polymer and ZIF-8 for hydrogen separation. J. Membr. Sci. 2019, 582, 381–390. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, W.; Qu, Z.; Wan, C.; Yao, Z.; Xu, J.; Kang, X.; Bai, Y.; Zhang, C. Cardo-type porous organic nanospheres: Tailoring interfacial compatibility in thermally rearranged mixed matrix membranes for improved hydrogen purification. J. Membr. Sci. 2020, 612, 118414. [Google Scholar] [CrossRef]
- Patel, A.K.; Acharya, N.K. Thermally rearranged (TR) HAB-6FDA nanocomposite membranes for hydrogen separation. Int. J. Hydrog. Energy 2020, 45, 18685–18692. [Google Scholar] [CrossRef]
- Yang, S.; Kwon, Y.H.; Koh, D.-Y.; Min, B.; Liu, Y.; Nair, S. Highly selective SSZ-13 zeolite hollow fiber membranes by ultraviolet activation at near-ambient temperature. ChemNanoMat 2019, 5, 61–67. [Google Scholar] [CrossRef]
- Yang, S.; Chiang, Y.; Nair, S. Scalable one-step gel conversion route to high-performance CHA zeolite hollow fiber membranes and modules for CO2 separation. Energy Technol. 2019, 7, 1900494. [Google Scholar] [CrossRef]
- Sihar, A.S.; Othman, N.H.; Alias, N.H.; Shahruddin, M.Z.; Asghrar Syed-Hassan, S.S.; Rahman, M.A.; Ismail, A.F.; Wu, Z. Fabrication of lanthanum-based perovskites membranes on porous alumina hollow fibre (AHF) substrates for oxygen enrichment. Ceram. Int. 2019, 45, 13086–13093. [Google Scholar] [CrossRef] [Green Version]
- Lei, L.; Lindbråthen, A.; Hillestad, M.; Sandru, M.; Favvas, E.P.; He, X. Screening cellulose spinning parameters for fabrication of novel carbon hollow fiber membranes for gas separation. Ind. Eng. Chem. Res. 2019, 58, 13330–13339. [Google Scholar] [CrossRef]
- Lei, L.; Lindbråthen, A.; Zhang, X.; Favvas, E.P.; Sandru, M.; Hillestad, M.; He, X. Preparation of carbon molecular sieve membranes with remarkable CO2/CH4 selectivity for high-pressure natural gas sweetening. J. Membr. Sci. 2020, 614, 118529. [Google Scholar] [CrossRef]
- Karousos, D.S.; Lei, L.; Lindbråthen, A.; Sapalidis, A.A.; Kouvelos, E.P.; He, X.; Favvas, E.P. Cellulose-based carbon hollow fiber membranes for high-pressure mixed gas separations of CO2/CH4 and CO2/N2. Sep. Purif. Technol. 2020, 253, 117473. [Google Scholar] [CrossRef]
- Yang, R.; Chen, M.Y.; Li, P. Carbon molecular sieve hollow fiber composite membrane derived from PMDA-ODA polyimide for gas separation. High Perform. Polym. 2022, 444–454. [Google Scholar] [CrossRef]
- Wey, M.-Y.; Chen, H.-H.; Lin, Y.-T.; Tseng, H.-H. Thin carbon hollow fiber membrane with Knudsen diffusion for hydrogen/alkane separation: Effects of hollow fiber module design and gas flow mode. Int. J. Hydrog. Energy 2020, 45, 7290–7302. [Google Scholar] [CrossRef]
- Kim, S.-J.; Lee, P.S.; Chang, J.-S.; Nam, S.-E.; Park, Y.-I. Preparation of carbon molecular sieve membranes on low-cost alumina hollow fibers for use in C3H6/C3H8 separation. Sep. Purif. Technol. 2018, 194, 443–450. [Google Scholar] [CrossRef]
- Rungta, M.; Wenz, G.B.; Zhang, C.; Xu, L.; Qiu, W.; Adams, J.S.; Koros, W.J. Carbon molecular sieve structure development and membrane performance relationships. Carbon 2017, 115, 237–248. [Google Scholar] [CrossRef] [Green Version]
- Bhuwania, N.; Labreche, Y.; Achoundong, C.S.K.; Baltazar, J.; Burgess, S.K.; Karwa, S.; Xu, L.; Henderson, C.L.; Williams, P.J.; Koros, W.J. Engineering substructure morphology of asymmetric carbon molecular sieve hollow fiber membranes. Carbon 2014, 76, 417–434. [Google Scholar] [CrossRef]
- Song, J.; Feng, B.; Tan, X.; Han, N.; Sunarso, J.; Liu, S. Oxygen selective perovskite hollow fiber membrane bundles. J. Membr. Sci. 2019, 581, 393–400. [Google Scholar] [CrossRef]
- Leo, A.; Smart, S.; Liu, S.; Diniz da Costa, J.C. High performance perovskite hollow fibres for oxygen separation. J. Membr. Sci. 2011, 368, 64–68. [Google Scholar] [CrossRef]
- Han, N.; Shen, Z.; Zhao, X.; Chen, R.; Thakur, V.K. Perovskite oxides for oxygen transport: Chemistry and material horizons. Sci. Total Environ. 2022, 806, 151213. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; An, R.; Chu, Y.; Tan, X.; Sunarso, J.; Wang, S.; Liu, S. Perovskite hollow fiber membranes supported in a porous and catalytically active perovskite matrix for air separation. Sep. Purif. Technol. 2018, 192, 435–440. [Google Scholar] [CrossRef]
- Wang, T.; Liu, Z.; Xu, X.; Zhu, J.; Zhang, G.; Jin, W. Insights into the design of nineteen-channel perovskite hollow fiber membrane and its oxygen transport behaviour. J. Membr. Sci. 2020, 595, 117600. [Google Scholar] [CrossRef]
- Li, Y.; Li, M.; Gao, J.; Lun, Y.; Li, C.; Sunarso, J.; Tan, X. Characterization of La0.6Sr0.4CoO3−δ oxygen selective hollow fiber made from acetate precursor-derived powder. Ceram. Int. 2020, 46, 3744–3749. [Google Scholar] [CrossRef]
- Buck, F.; Feldhoff, A.; Caro, J.; Schiestel, T. Permeation improvement of LCCF hollow fiber membranes by spinning and sintering optimization. Sep. Purif. Technol. 2021, 259, 118023. [Google Scholar] [CrossRef]
- Buck, F.; Bunjaku, O.; Caro, J.; Schiestel, T. High-flux CO2-stable oxygen transport hollow fiber membranes through surface engineering. J. Eur. Ceram. Soc. 2022, 42, 1537–1547. [Google Scholar] [CrossRef]
- Wei, Y.; Tang, J.; Zhou, L.; Li, Z.; Wang, H. Oxygen permeation through U-shaped K2NiF4-type oxide hollow-fiber membranes. Ind. Eng. Chem. Res. 2011, 50, 12727–12734. [Google Scholar] [CrossRef]
- Han, N.; Zhang, S.; Meng, X.; Yang, N.; Meng, B.; Tan, X.; Liu, S. Effect of enhanced oxygen reduction activity on oxygen permeation of La0.6Sr0.4Co0.2Fe0.8O3−δ membrane decorated by K2NiF4-type oxide. J. Alloys Compd. 2016, 654, 280–289. [Google Scholar] [CrossRef]
- Han, N.; Zhang, S.; Meng, B.; Tan, X. The effect of microstructure and surface decoration with K2NiF4-type oxide upon the oxygen permeability of perovskite-type La0.7Sr0.3FeO3−δ hollow fiber membranes. RSC Adv. 2015, 5, 88602–88611. [Google Scholar] [CrossRef]
- Han, N.; Meng, B.; Yang, N.; Sunarso, J.; Zhu, Z.; Liu, S. Enhancement of oxygen permeation fluxes of La0.6Sr0.4CoO3−δ hollow fiber membrane via macrostructure modification and (La0.5Sr0.5)2CoO4+δ decoration. Chem. Eng. Res. Des. 2018, 134, 487–496. [Google Scholar] [CrossRef] [Green Version]
- Han, N.; Chen, R.; Chang, T.; Li, L.; Wang, H.; Zeng, L. A novel lanthanum strontium cobalt iron composite membrane synthesised through beneficial phase reaction for oxygen separation. Ceram. Int. 2019, 45, 18924–18930. [Google Scholar] [CrossRef]
- Ma, T.; Han, N.; Meng, B.; Yang, N.; Zhu, Z.; Liu, S. Enhancing oxygen permeation via the incorporation of silver inside perovskite oxide membranes. Processes 2019, 7, 199. [Google Scholar] [CrossRef] [Green Version]
- Gao, J.; Lun, Y.; Han, N.; Tan, X.; Fan, C.; Liu, S. Influence of nitric oxide on the oxygen permeation behavior of La0.6Sr0.4Co0.2Fe0.8O3−δ perovskite membranes. Sep. Purif. Technol. 2019, 210, 900–906. [Google Scholar] [CrossRef]
- Alqaheem, Y.; Alomair, A.A. Sealing perovskite membranes for long-term oxygen separation from air. Chem. Pap. 2020, 74, 4159–4168. [Google Scholar] [CrossRef]
- Yeo, J.Y.J.; Li, C.; Sunarso, J. Modeling and simulation study of oxygen permeation in La0.8Ca0.2Fe0.95O3−δ-Ag hollow fiber membrane module. Mater. Today Proc. 2021, in press. [Google Scholar] [CrossRef]
- Zhang, S.; Yeo, J.Y.J.; Li, C.; Meng, X.; Yang, N.; Sunarso, J.; Liu, S. Oxygen permeation simulation of La0.8Ca0.2Fe0.95O3−δ-Ag hollow fiber membrane at different modes and flow configurations. AIChE J. 2022, 68, e17508. [Google Scholar] [CrossRef]
- Song, J.; Feng, B.; Chu, Y.; Tan, X.; Gao, J.; Han, N.; Liu, S. One-step thermal processing to prepare BaCo0.95−xBi0.05ZrxO3−δ membranes for oxygen separation. Ceram. Int. 2019, 45, 12579–12585. [Google Scholar] [CrossRef]
- Abdel-Karim, A.; Leaper, S.; Skuse, C.; Zaragoza, G.; Gryta, M.; Gorgojo, P. Membrane cleaning and pretreatments in membrane distillation—A review. Chem. Eng. J. 2021, 422, 129696. [Google Scholar] [CrossRef]
- Leaper, S.; Abdel-Karim, A.; Gad-Allah, T.A.; Gorgojo, P. Air-gap membrane distillation as a one-step process for textile wastewater treatment. Chem. Eng. J. 2019, 360, 1330–1340. [Google Scholar] [CrossRef] [Green Version]
- Laqbaqbi, M.; García-Payo, M.; Khayet, M.; El Kharraz, J.; Chaouch, M. Application of direct contact membrane distillation for textile wastewater treatment and fouling study. Sep. Purif. Technol. 2019, 209, 815–825. [Google Scholar] [CrossRef]
- Yang, G.; Zhang, J.; Peng, M.; Du, E.; Wang, Y.; Shan, G.; Ling, L.; Ding, H.; Gray, S.; Xie, Z. A mini review on antiwetting studies in membrane distillation for textile wastewater treatment. Processes 2021, 9, 243. [Google Scholar] [CrossRef]
- Hubadillah, S.K.; Othman, M.H.D.; Matsuura, T.; Rahman, M.A.; Jaafar, J.; Ismail, A.; Amin, S.Z.M. Green silica-based ceramic hollow fiber membrane for seawater desalination via direct contact membrane distillation. Sep. Purif. Technol. 2018, 205, 22–31. [Google Scholar] [CrossRef]
- Gopi, G.; Arthanareeswaran, G.; Ismail, A. Perspective of renewable desalination by using membrane distillation. Chem. Eng. Res. Des. 2019, 144, 520–537. [Google Scholar]
- Jiang, L.; Chen, L.; Zhu, L. Fouling process of membrane distillation for seawater desalination: An especial focus on the thermal-effect and concentrating-effect during biofouling. Desalination 2020, 485, 114457. [Google Scholar] [CrossRef]
- Reis, B.G.; Araújo, A.L.B.; Amaral, M.C.S.; Ferraz, H.C. Comparison of Nanofiltration and Direct Contact Membrane Distillation as an alternative for gold mining effluent reclamation. Chem. Eng. Process. Process. Intensif. 2018, 133, 24–33. [Google Scholar] [CrossRef]
- Silva, M.; Reis, B.; Grossi, L.; Amaral, M. Improving the energetic efficiency of direct-contact membrane distillation in mining effluent by using the waste-heat-and-water process as the cooling fluid. J. Clean. Prod. 2020, 260, 121035. [Google Scholar] [CrossRef]
- Ghaffour, N.; Soukane, S.; Lee, J.-G.; Kim, Y.; Alpatova, A. Membrane distillation hybrids for water production and energy efficiency enhancement: A critical review. Appl. Energy 2019, 254, 113698. [Google Scholar] [CrossRef]
- Wang, W.; Shi, Y.; Zhang, C.; Hong, S.; Shi, L.; Chang, J.; Li, R.; Jin, Y.; Ong, C.; Zhuo, S. Simultaneous production of fresh water and electricity via multistage solar photovoltaic membrane distillation. Nat. Commun. 2019, 10, 3012. [Google Scholar] [CrossRef]
- Li, W.; Yang, Z.; Zhang, G.; Meng, Q. Heat-treated polyacrylonitrile (PAN) hollow fiber structured packings in isopropanol (IPA)/water distillation with improved thermal and chemical stability. Ind. Eng. Chem. Res. 2013, 52, 6492–6501. [Google Scholar] [CrossRef]
- Su, P.; Zhang, X.; Li, Y.; Chen, H.; Meng, Q.; Zhang, G. Distillation of alcohol/water solution in hybrid metal–organic framework hollow fibers. AIChE J. 2019, 65, e16693. [Google Scholar] [CrossRef]
- Chang, J.; Zuo, J.; Zhang, L.; O’Brien, G.S.; Chung, T.-S. Using green solvent, triethyl phosphate (TEP), to fabricate highly porous PVDF hollow fiber membranes for membrane distillation. J. Membr. Sci. 2017, 539, 295–304. [Google Scholar] [CrossRef]
- Li, H.; Liu, H.; Shi, W.; Zhang, H.; Zhou, R.; Qin, X. Preparation of hydrophobic zeolitic imidazolate framework-71 (ZIF-71)/PVDF hollow fiber composite membrane for membrane distillation through dilute solution coating. Sep. Purif. Technol. 2020, 251, 117348. [Google Scholar] [CrossRef]
- Khayet, M.; Essalhi, M.; Qtaishat, M.; Matsuura, T. Robust surface modified polyetherimide hollow fiber membrane for long-term desalination by membrane distillation. Desalination 2019, 466, 107–117. [Google Scholar] [CrossRef]
- Liu, Z.; Pan, Q.; Xiao, C. Preparation and vacuum membrane distillation performance of a silane coupling agent-modified polypropylene hollow fiber membrane. Desalination 2019, 468, 114060. [Google Scholar] [CrossRef]
- Song, L.; Huang, Q.; Huang, Y.; Bi, R.; Xiao, C. An electro-thermal braid-reinforced PVDF hollow fiber membrane for vacuum membrane distillation. J. Membr. Sci. 2019, 591, 117359. [Google Scholar] [CrossRef]
- Suga, Y.; Takagi, R.; Matsuyama, H. Effect of hollow fiber membrane properties and operating conditions on preventing scale precipitation in seawater desalination with vacuum membrane distillation. Desalination 2022, 527, 115578. [Google Scholar] [CrossRef]
- Davenport, D.M.; Gui, M.; Ormsbee, L.R.; Bhattacharyya, D. Development of PVDF membrane nanocomposites via various functionalization approaches for environmental applications. Polymers 2016, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Qu, D.; Wang, J.; Hou, D.; Luan, Z.; Fan, B.; Zhao, C. Experimental study of arsenic removal by direct contact membrane distillation. J. Hazard. Mater. 2009, 163, 374–879. [Google Scholar] [CrossRef]
- Wang, J.-W.; Li, L.; Zhang, J.-W.; Xu, X.; Chen, C.-S. β-Sialon ceramic hollow fiber membranes with high strength and low thermal conductivity for membrane distillation. J. Eur. Ceram. Soc. 2016, 36, 59–65. [Google Scholar] [CrossRef]
- Gabelman, A.; Hwang, S.-T. Hollow fiber membrane contactors. J. Membr. Sci. 1999, 159, 21–106. [Google Scholar] [CrossRef]
- Yang, M.C.; Cussler, E. Designing hollow-fiber contactors. AIChE J. 1986, 32, 1910–1916. [Google Scholar] [CrossRef]
- Bazhenov, S.D.; Bildyukevich, A.V.; Volkov, A.V. Gas-liquid hollow fiber membrane contactors for different applications. Fibers 2018, 6, 76. [Google Scholar] [CrossRef] [Green Version]
- Bakeri, G.; Naeimifard, S.; Matsuura, T.; Ismail, A. A porous polyethersulfone hollow fiber membrane in a gas humidification process. RSC Adv. 2015, 5, 14448–14457. [Google Scholar] [CrossRef]
- Huang, X.; Wang, W.; Zheng, Z.; Fan, W.; Mao, C.; Shi, J.; Li, L. Surface monofunctionalized polymethyl pentene hollow fiber membranes by plasma treatment and hemocompatibility modification for membrane oxygenators. Appl. Surf. Sci. 2016, 362, 355–363. [Google Scholar] [CrossRef]
- Su, J.; Wei, Y. Novel tri-bore PVDF hollow fiber membranes for the control of dissolved oxygen in aquaculture water. J. Water Process. Eng. 2019, 30, 100584. [Google Scholar] [CrossRef]
- Merle, T.; Pronk, W.; Von Gunten, U. MEMBRO3X, a novel combination of a membrane contactor with advanced oxidation (O3/H2O2) for simultaneous micropollutant abatement and bromate minimization. Environ. Sci. Technol. Lett. 2017, 4, 180–185. [Google Scholar] [CrossRef]
- Tilahun, E.; Bayrakdar, A.; Sahinkaya, E.; Çalli, B. Performance of polydimethylsiloxane membrane contactor process for selective hydrogen sulfide removal from biogas. Waste Manag. 2017, 61, 250–257. [Google Scholar] [CrossRef]
- Zhang, N.; Pan, Z.; Zhang, L.; Zhang, Z. Decarburization characteristics of coalbed methane by membrane separation technology. Fuel 2019, 242, 470–478. [Google Scholar] [CrossRef]
- Abdolahi-Mansoorkhani, H.; Seddighi, S. CO2 capture by modified hollow fiber membrane contactor: Numerical study on membrane structure and membrane wettability. Fuel Process. Technol. 2020, 209, 106530. [Google Scholar] [CrossRef]
- Qazi, S.; Gómez-Coma, L.; Albo, J.; Druon-Bocquet, S.; Irabien, A.; Sanchez-Marcano, J. CO2 capture in a hollow fiber membrane contactor coupled with ionic liquid: Influence of membrane wetting and process parameters. Sep. Purif. Technol. 2020, 233, 115986. [Google Scholar] [CrossRef]
- Nakhjiri, A.T.; Heydarinasab, A.; Bakhtiari, O.; Mohammadi, T. The effect of membrane pores wettability on CO2 removal from CO2/CH4 gaseous mixture using NaOH, MEA and TEA liquid absorbents in hollow fiber membrane contactor. Chin. J. Chem. Eng. 2018, 26, 1845–1861. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, F.; Rezakazemi, M.; Zhang, W.; Lu, C.; Chang, H.; Quan, X. Modeling of a CO2-piperazine-membrane absorption system. Chem. Eng. Res. Des. 2018, 131, 375–384. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, Y.; Zhang, L.; Chen, Y.; Ran, J.; Pu, G.; Qin, C. Theoretical study on CO2 absorption from biogas by membrane contactors: Effect of operating parameters. Ind. Eng. Chem. Res. 2014, 53, 14075–14083. [Google Scholar] [CrossRef]
- Bazhenov, S.; Lyubimova, E. Gas-liquid membrane contactors for carbon dioxide capture from gaseous streams. Pet. Chem. 2016, 56, 889–914. [Google Scholar] [CrossRef]
- Zhao, S.; Feron, P.H.; Deng, L.; Favre, E.; Chabanon, E.; Yan, S.; Hou, J.; Chen, V.; Qi, H. Status and progress of membrane contactors in post-combustion carbon capture: A state-of-the-art review of new developments. J. Membr. Sci. 2016, 511, 180–206. [Google Scholar] [CrossRef]
- Jin, P.; Huang, C.; Shen, Y.; Zhan, X.; Hu, X.; Wang, L.; Wang, L. Simultaneous separation of H2S and CO2 from biogas by gas-liquid membrane contactor using single and mixed absorbents. Energy Fuels 2017, 31, 11117–11126. [Google Scholar] [CrossRef]
- Al-Marzouqi, M.H.; Marzouk, S.A.; Abdullatif, N. High pressure removal of acid gases using hollow fiber membrane contactors: Further characterization and long-term operational stability. J. Nat. Gas Sci. Eng. 2017, 37, 192–198. [Google Scholar] [CrossRef]
- Tilahun, E.; Sahinkaya, E.; Çalli, B. A hybrid membrane gas absorption and bio-oxidation process for the removal of hydrogen sulfide from biogas. Int. Biodeterior. Biodegrad. 2018, 127, 69–76. [Google Scholar] [CrossRef]
- Hosseinzadeh, A.; Hosseinzadeh, M.; Vatani, A.; Mohammadi, T. Mathematical modeling for the simultaneous absorption of CO2 and SO2 using MEA in hollow fiber membrane contactors. Chem. Eng. Process. Process. Intensif. 2017, 111, 35–45. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, Y.; Wood, D.A.; Zhang, W.; Li, L.; Zhang, L.; Van der Bruggen, B. Influence of the membrane module geometry on SO2 removal: A numerical study. Ind. Eng. Chem. Res. 2015, 54, 11619–11627. [Google Scholar] [CrossRef]
- Dai, Z.; Ansaloni, L.; Deng, L. Precombustion CO2 capture in polymeric hollow fiber membrane contactors using ionic liquids: Porous membrane versus nonporous composite membrane. Ind. Eng. Chem. Res. 2016, 55, 5983–5992. [Google Scholar] [CrossRef]
- Bazhenov, S.; Malakhov, A.; Bakhtin, D.; Khotimskiy, V.; Bondarenko, G.; Volkov, V.; Ramdin, M.; Vlugt, T.J.; Volkov, A. CO2 stripping from ionic liquid at elevated pressures in gas-liquid membrane contactor. Int. J. Greenh. Gas Control 2018, 71, 293–302. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Yu, H.; Thé, J.; Tan, Z.; Feng, X. Modeling SO2 absorption into water accompanied with reversible reaction in a hollow fiber membrane contactor. Chem. Eng. Sci. 2016, 156, 136–146. [Google Scholar] [CrossRef]
- Li, Y.; Wang, L.A.; Zhang, Z.; Hu, X.; Cheng, Y.; Zhong, C. Carbon dioxide absorption from biogas by amino acid salt promoted potassium carbonate solutions in a hollow fiber membrane contactor: A numerical study. Energy Fuels 2018, 32, 3637–3646. [Google Scholar] [CrossRef]
- Taheri, M.; Mohebbi, A.; Hashemipour, H.; Rashidi, A.M. Simultaneous absorption of carbon dioxide (CO2) and hydrogen sulfide (H2S) from CO2-H2S-CH4 gas mixture using amine-based nanofluids in a wetted wall column. J. Nat. Gas Sci. Eng. 2016, 28, 410–417. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Darabi, M.; Soroush, E.; Mesbah, M. CO2 absorption enhancement by water-based nanofluids of CNT and SiO2 using hollow-fiber membrane contactor. Sep. Purif. Technol. 2019, 210, 920–926. [Google Scholar] [CrossRef]
- Stowe, H.M.; Hwang, G.S. Molecular insights into the enhanced rate of CO2 absorption to produce bicarbonate in aqueous 2-amino-2-methyl-1-propanol. Phys. Chem. Chem. Phys. 2017, 19, 32116–32124. [Google Scholar] [CrossRef]
- Yuan, S.; Yang, Z.; Ji, X.; Chen, Y.; Sun, Y.; Lu, X. CO2 absorption in mixed aqueous solution of MDEA and cholinium glycinate. Energy Fuels 2017, 31, 7325–7333. [Google Scholar] [CrossRef]
- Martić, I.; Maslarević, A.; Mladenović, S.; Lukić, U.; Budimir, S. Water deoxygenation using hollow fiber membrane module with nitrogen as inert gas. Desalin. Water Treat. 2015, 54, 1563–1567. [Google Scholar] [CrossRef]
- Kattan, O.; Ebbers, K.; Koolaard, A.; Vos, H.; Bargeman, G. Membrane contactors: An alternative for de-aeration of salt solutions? Sep. Purif. Technol. 2018, 205, 231–240. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, K.; Wang, J.; Hou, D.; Liu, H. Ozone mass transfer behaviors on physical and chemical absorption for hollow fiber membrane contactors. Water Sci. Technol. 2017, 76, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Stylianou, S.; Sklari, S.; Zamboulis, D.; Zaspalis, V.; Zouboulis, A. Development of bubble-less ozonation and membrane filtration process for the treatment of contaminated water. J. Membr. Sci. 2015, 492, 40–47. [Google Scholar] [CrossRef]
- Stylianou, S.K.; Katsoyiannis, I.A.; Mitrakas, M.; Zouboulis, A.I. Application of a ceramic membrane contacting process for ozone and peroxone treatment of micropollutant contaminated surface water. J. Hazard. Mater. 2018, 358, 129–135. [Google Scholar] [CrossRef]
- Dalane, K.; Svendsen, H.F.; Hillestad, M.; Deng, L. Membrane contactor for subsea natural gas dehydration: Model development and sensitivity study. J. Membr. Sci. 2018, 556, 263–276. [Google Scholar] [CrossRef]
- Qu, M.; Abdelaziz, O.; Gao, Z.; Yin, H. Isothermal membrane-based air dehumidification: A comprehensive review. Renew. Sust. Energ. Rev. 2018, 82, 4060–4069. [Google Scholar] [CrossRef]
- Fakharnezhad, A.; Keshavarz, P. Experimental investigation of gas dehumidification by tri-ethylene glycol in hollow fiber membrane contactors. J. Ind. Eng. Chem. 2016, 34, 390–396. [Google Scholar] [CrossRef]
- Bettahalli, N.S.; Lefers, R.; Fedoroff, N.; Leiknes, T.; Nunes, S.P. Triple-bore hollow fiber membrane contactor for liquid desiccant based air dehumidification. J. Membr. Sci. 2016, 514, 135–142. [Google Scholar] [CrossRef] [Green Version]
- Pantelic, J.; Teitelbaum, E.; Bozlar, M.; Kim, S.; Meggers, F. Development of moisture absorber based on hydrophilic nonporous membrane mass exchanger and alkoxylated siloxane liquid desiccant. Energy Build. 2018, 160, 34–43. [Google Scholar] [CrossRef]
- Kirsch, V.; Roldugin, V.; Ovcharova, A.; Bildyukevich, A. Modeling of ethylene absorption from an ethylene-ethane mixture by silver nitrate aqueous solution in a hollow-fiber membrane contactor. Pet. Chem. 2017, 57, 1242–1249. [Google Scholar] [CrossRef]
- Ovcharova, A.; Vasilevsky, V.; Borisov, I.; Bazhenov, S.; Volkov, A.; Bildyukevich, A.; Volkov, V. Polysulfone porous hollow fiber membranes for ethylene-ethane separation in gas-liquid membrane contactor. Sep. Purif. Technol. 2017, 183, 162–172. [Google Scholar] [CrossRef]
- Smith, K.R.; Frumkin, H.; Balakrishnan, K.; Butler, C.D.; Chafe, Z.A.; Fairlie, I.; Kinney, P.; Kjellstrom, T.; Mauzerall, D.L.; McKone, T.E. Energy and human health. Annu. Rev. Public Health 2013, 34, 159–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-J.; Yoon, T.-U.; Kim, A.-R.; Kim, S.-Y.; Cho, K.-H.; Hwang, Y.K.; Yeon, J.-W.; Bae, Y.-S. Adsorptive separation of xenon/krypton mixtures using a zirconium-based metal-organic framework with high hydrothermal and radioactive stabilities. J. Hazard. Mater. 2016, 320, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Stern, S.; Leone, S. Separation of krypton and xenon by selective permeation. AIChE J. 1980, 26, 881–890. [Google Scholar] [CrossRef]
- Stern, S.; Wang, S.C. Permeation cascades for the separation of krypton and xenon from nuclear reactor atmospheres. AIChE J. 1980, 26, 891–901. [Google Scholar] [CrossRef]
- Kwon, Y.H.; Min, B.; Yang, S.; Koh, D.-Y.; Bhave, R.R.; Nair, S. Ion-exchanged SAPO-34 membranes for krypton-xenon separation: Control of permeation properties and fabrication of hollow fiber membranes. ACS Appl. Mater. Interfaces 2018, 10, 6361–6368. [Google Scholar] [CrossRef]
- Wang, X.; Zhou, T.; Zhang, P.; Yan, W.; Li, Y.; Peng, L.; Veerman, D.; Shi, M.; Gu, X.; Kapteijn, F. High-silica CHA zeolite membrane with ultra-high selectivity and irradiation stability for krypton/xenon separation. Angew. Chem. Int. Ed. 2021, 133, 9114–9119. [Google Scholar] [CrossRef]
- Kandwal, P.; Ansari, S.; Mohapatra, P. A highly efficient supported liquid membrane system for near quantitative recovery of radio-strontium from acidic feeds. Part II: Scale up and mass transfer modeling in hollow fiber configuration. J. Membr. Sci. 2012, 405, 85–91. [Google Scholar] [CrossRef]
- Chaudhury, S.; Bhattacharyya, A.; Ansari, S.A.; Goswami, A. A new approach for selective Cs+ separation from simulated nuclear waste solution using electrodriven cation transport through hollow fiber supported liquid membranes. J. Membr. Sci. 2018, 545, 75–80. [Google Scholar] [CrossRef]
- Jagasia, P.; Ansari, S.A.; Raut, D.R.; Dhami, P.S.; Gandhi, P.M.; Kumar, A.; Mohapatra, P.K. Hollow fiber supported liquid membrane studies using a process compatible solvent containing calix [4] arene-mono-crown-6 for the recovery of radio-cesium from nuclear waste. Sep. Purif. Technol. 2016, 170, 208–216. [Google Scholar] [CrossRef]
- Song, J.; Chen, G.; Li, X.; He, T.; Jiang, B. Membrane chemical exchange for lithium isotope enrichment (II): Multistage cascade process. Fusion Eng. Des. 2020, 160, 111821. [Google Scholar] [CrossRef]
- Kaleekkal, N.J. Heparin immobilized graphene oxide in polyetherimide membranes for hemodialysis with enhanced hemocompatibility and removal of uremic toxins. J. Membr. Sci. 2021, 623, 119068. [Google Scholar] [CrossRef]
- Verma, S.K.; Modi, A.; Singh, A.K.; Teotia, R.; Bellare, J. Improved hemodialysis with hemocompatible polyethersulfone hollow fiber membranes: In vitro performance. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 1286–1298. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, Z.; Qiu, M.; He, C. Heparin-mimicking semi-interpenetrating composite membrane with multiple excellent performances for promising hemodialysis. J. Membr. Sci. 2021, 618, 118740. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Liu, F.; Li, T.; Zhong, Y.; Lin, H.; He, J. Enhanced hemocompatibility of flat and hollow fiber membranes via a heparin free surface crosslinking strategy. React. Funct. Polym. 2018, 124, 104–114. [Google Scholar] [CrossRef]
- Narayanaswamy, R.; Torchilin, V.P. Hydrogels and their applications in targeted drug delivery. Molecules 2019, 24, 603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luraghi, A.; Peri, F.; Moroni, L. Electrospinning for drug delivery applications: A review. J. Control. Release 2021, 334, 463–484. [Google Scholar] [CrossRef] [PubMed]
- Al-Baadani, M.A.; Yie, K.H.R.; Al-Bishari, A.M.; Alshobi, B.A.; Zhou, Z.; Fang, K.; Dai, B.; Shen, Y.; Ma, J.; Liu, J. Co-electrospinning polycaprolactone/gelatin membrane as a tunable drug delivery system for bone tissue regeneration. Mater. Des. 2021, 209, 109962. [Google Scholar] [CrossRef]
- Yang, Y.; Li, W.; Yu, D.-G.; Wang, G.; Williams, G.R.; Zhang, Z. Tunable drug release from nanofibers coated with blank cellulose acetate layers fabricated using tri-axial electrospinning. Carbohydr. Polym. 2019, 203, 228–237. [Google Scholar] [CrossRef]
- Wu, H.; Shi, C.; Zhu, Q.; Li, Y.; Xu, Z.; Wei, C.; Chen, D.; Huang, X. Capillary-driven blood separation and in-situ electrochemical detection based on 3D conductive gradient hollow fiber membrane. Biosens. Bioelectron. 2021, 171, 112722. [Google Scholar] [CrossRef]
- Wu, H.; Li, T.; Bao, Y.; Zhang, X.; Wang, C.; Wei, C.; Xu, Z.; Tong, W.; Chen, D.; Huang, X. MOF-enzyme hybrid nanosystem decorated 3D hollow fiber membranes for in-situ blood separation and biosensing array. Biosens. Bioelectron. 2021, 190, 113413. [Google Scholar] [CrossRef] [PubMed]
- Razali, M.; Kim, J.F.; Attfield, M.; Budd, P.M.; Drioli, E.; Lee, Y.M.; Szekely, G. Sustainable wastewater treatment and recycling in membrane manufacturing. Green Chem. 2015, 17, 5196–5205. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.; Salazar, O.R.; Nunes, S.P. Membrane manufacture for peptide separation. Green Chem. 2016, 18, 5151–5159. [Google Scholar] [CrossRef] [Green Version]
- Jung, J.T.; Wang, H.H.; Kim, J.F.; Jeon, S.M.; Park, S.H.; Lee, W.H.; Moon, S.J.; Drioli, E.; Lee, Y.M. Microfiber aligned hollow fiber membranes from immiscible polymer solutions by phase inversion. J. Membr. Sci. 2021, 617, 118654. [Google Scholar] [CrossRef]
- Zhao, J.; Chong, J.Y.; Shi, L.; Wang, R. Explorations of combined nonsolvent and thermally induced phase separation (N-TIPS) method for fabricating novel PVDF hollow fiber membranes using mixed diluents. J. Membr. Sci. 2019, 572, 210–222. [Google Scholar] [CrossRef]
- Fang, C.; Jeon, S.; Rajabzadeh, S.; Cheng, L.; Fang, L.; Matsuyama, H. Tailoring the surface pore size of hollow fiber membranes in the TIPS process. J. Mater. Chem. A 2018, 6, 535–547. [Google Scholar] [CrossRef]
- Lee, J.; Park, B.; Kim, J.; Park, S.B. Effect of PVP, lithium chloride, and glycerol additives on PVDF dual-layer hollow fiber membranes fabricated using simultaneous spinning of TIPS and NIPS. Macromol. Res. 2015, 23, 291–299. [Google Scholar] [CrossRef]
- Russo, F.; Tiecco, M.; Galiano, F.; Mancuso, R.; Gabriele, B.; Figoli, A. Launching deep eutectic solvents (DESs) and natural deep eutectic solvents (NADESs), in combination with different harmless co-solvents, for the preparation of more sustainable membranes. J. Membr. Sci. 2022, 649, 120387. [Google Scholar] [CrossRef]
- Sanguineti, A.; Di Nicolo, E.; Lee, Y.M.; Drioli, E.; Zhaoliang, C.; Hassankiadeh, N.T.; Lee, S.Y. Process for Manufacturing Fluoropolymer Membranes. U.S. Patent 10,688,447B2, 23 June 2020. [Google Scholar]
- Hassankiadeh, N.T.; Cui, Z.; Kim, J.H.; Shin, D.W.; Lee, S.Y.; Sanguineti, A.; Arcella, V.; Lee, Y.M.; Drioli, E. Microporous poly (vinylidene fluoride) hollow fiber membranes fabricated with PolarClean as water-soluble green diluent and additives. J. Membr. Sci. 2015, 479, 204–212. [Google Scholar] [CrossRef]
- Ursino, C.; Russo, F.; Ferrari, R.; De Santo, M.; Di Nicolò, E.; He, T.; Galiano, F.; Figoli, A. Polyethersulfone hollow fiber membranes prepared with Polarclean® as a more sustainable solvent. J. Membr. Sci. 2020, 608, 118216. [Google Scholar] [CrossRef]
- Matveev, D.; Vasilevsky, V.; Volkov, V.; Plisko, T.; Shustikov, A.; Volkov, A.; Bildyukevich, A. Fabrication of ultrafiltration membranes from non-toxic solvent dimethylsulfoxide: Benchmarking of commercially available acrylonitrile co-polymers. J. Environ. Chem. Eng. 2022, 10, 107061. [Google Scholar] [CrossRef]
- Lu, F.; Liu, H.; Xiao, C.; Wang, X.; Chen, K.; Huang, H. Effect of on-line stretching treatment on the structure and performance of polyvinyl chloride hollow fiber membranes. RSC Adv. 2019, 9, 6699–6707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelzer, M.; Vad, T.; Becker, A.; Gries, T.; Markova, S.; Teplyakov, V. Melt spinning and characterization of hollow fibers from poly (4-methyl-1-pentene). J. Appl. Polym. Sci. 2021, 138, 49630. [Google Scholar] [CrossRef]
- Lv, D.; Zhu, M.; Jiang, Z.; Jiang, S.; Zhang, Q.; Xiong, R.; Huang, C. Green electrospun nanofibers and their application in air filtration. Macromol. Mater. Eng. 2018, 303, 1800336. [Google Scholar] [CrossRef]
- Chandavasu, C.; Xanthos, M.; Sirkar, K.; Gogos, C. Fabrication of microporous polymeric membranes by melt processing of immiscible blends. J. Membr. Sci. 2003, 211, 167–175. [Google Scholar] [CrossRef]
- Ji, D.; Xiao, C.; An, S.; Chen, K.; Gao, Y.; Zhou, F.; Zhang, T. Completely green and sustainable preparation of PVDF hollow fiber membranes via melt-spinning and stretching method. J. Hazard. Mater. 2020, 398, 122823. [Google Scholar] [CrossRef]
- Ji, D.; Xiao, C.; Chen, K.; Zhou, F.; Gao, Y.; Zhang, T.; Ling, H. Solvent-free green fabrication of PVDF hollow fiber MF membranes with controlled pore structure via melt-spinning and stretching. J. Membr. Sci. 2021, 621, 118953. [Google Scholar] [CrossRef]
- He, H.-W.; Wang, L.; Yan, X.; Zhang, L.-H.; Yu, M.; Yu, G.-F.; Dong, R.-H.; Xia, L.-H.; Ramakrishna, S.; Long, Y.-Z. Solvent-free electrospinning of UV curable polymer microfibers. RSC Adv. 2016, 6, 29423–29427. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Y.; Li, H.; Chen, H.; Ding, Y.; Yang, W. Effect of oriented fiber membrane fabricated via needleless melt electrospinning on water filtration efficiency. Desalination 2014, 344, 266–273. [Google Scholar] [CrossRef]
- Markova, S.Y.; Dukhov, A.V.; Pelzer, M.; Shalygin, M.G.; Vad, T.; Gries, T.; Teplyakov, V.V. Designing 3D membrane modules for gas separation based on hollow fibers from poly (4-methyl-1-pentene). Membranes 2021, 12, 36. [Google Scholar] [CrossRef]
- Tocci, E.; Rizzuto, C.; Macedonio, F.; Drioli, E. Effect of green solvents in the production of PVDF-specific polymorphs. Ind. Eng. Chem. Res. 2020, 59, 5267–5275. [Google Scholar] [CrossRef]
- Fam, W.; Mansouri, J.; Li, H.; Chen, V. Improving CO2 separation performance of thin film composite hollow fiber with Pebax® 1657/ionic liquid gel membranes. J. Membr. Sci. 2017, 537, 54–68. [Google Scholar] [CrossRef]
- Gebreyohannes, A.Y.; Upadhyaya, L.; Silva, L.P.; Falca, G.; Carvalho, P.J.; Nunes, S.P. Hollow fibers with encapsulated green amino acid-based ionic liquids for dehydration. ACS Sustain. Chem. Eng. 2020, 8, 17763–17771. [Google Scholar] [CrossRef]
- Li, M.-S.; Zhao, Z.-P.; Wang, M.-X. Green hydrophilic modification of PE hollow fiber membranes in a module scale via long-distance and dynamic low-temperature H2O plasma flow. Appl. Surf. Sci. 2016, 386, 187–195. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, L.; Pramoda, K.P.; Gai, W.; Zhang, S. Highly permeable and fouling-resistant hollow fiber membranes for reverse osmosis. Chem. Eng. Sci. 2019, 207, 903–910. [Google Scholar] [CrossRef]
- Schneiderman, D.K.; Hillmyer, M.A. 50th anniversary perspective: There is a great Future in sustainable polymers. Macromolecules 2017, 50, 3733–3749. [Google Scholar] [CrossRef]
- Papageorgiou, G.Z. Thinking green: Sustainable polymers from renewable resources. Polymers 2018, 10, 952. [Google Scholar] [CrossRef] [Green Version]
- Zou, D.; Nunes, S.P.; Vankelecom, I.F.J.; Figoli, A.; Lee, Y.M. Recent advances in polymer membranes employing non-toxic solvents and materials. Green Chem. 2021, 23, 9815–9843. [Google Scholar] [CrossRef]
- Loeb, S.; Sourirajan, S. Sea water demineralization by means of an osmotic membrane. In Saline Water Conversion—II; Gould, R.F., Ed.; American Chemical Society: Washington, DC, USA, 1963; Volume 38, pp. 117–132. [Google Scholar]
- Chen, K.; Xiao, C.; Liu, H.; Ling, H.; Chu, Z.; Hu, Z. Design of robust twisted fiber bundle-reinforced cellulose triacetate hollow fiber reverse osmosis membrane with thin separation layer for seawater desalination. J. Membr. Sci. 2019, 578, 1–9. [Google Scholar] [CrossRef]
- Kumar, M.; RaoT, S.; Isloor, A.M.; Ibrahim, G.P.S.; Inamuddin; Ismail, N.; Ismail, A.F.; Asiri, A.M. Use of cellulose acetate/polyphenylsulfone derivatives to fabricate ultrafiltration hollow fiber membranes for the removal of arsenic from drinking water. Int. J. Biol. Macromol. 2019, 129, 715–727. [Google Scholar] [CrossRef]
- Kumar, M.; Isloor, A.M.; Somasekhara Rao, T.; Ismail, A.F.; Farnood, R.; Nambissan, P.M.G. Removal of toxic arsenic from aqueous media using polyphenylsulfone/cellulose acetate hollow fiber membranes containing zirconium oxide. Chem. Eng. J. 2020, 393, 124367. [Google Scholar] [CrossRef]
- Kumar, M.; Isloor, A.M.; Todeti, S.R.; Nagaraja, H.S.; Ismail, A.F.; Susanti, R. Effect of binary zinc-magnesium oxides on polyphenylsulfone/cellulose acetate derivatives hollow fiber membranes for the decontamination of arsenic from drinking water. Chem. Eng. J. 2021, 405, 126809. [Google Scholar] [CrossRef]
- Falca, G.; Musteata, V.-E.; Behzad, A.R.; Chisca, S.; Nunes, S.P. Cellulose hollow fibers for organic resistant nanofiltration. J. Membr. Sci. 2019, 586, 151–161. [Google Scholar] [CrossRef]
- Mubashir, M.; Yeong, Y.F.; Lau, K.K.; Chew, T.L. Effect of spinning conditions on the fabrication of cellulose acetate hollow fiber membrane for CO2 separation from N2 and CH4. Polym. Test. 2019, 73, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Z.; Morisato, A.; Bhuwania, N.; Chinn, D.; Koros, W.J. Natural gas sweetening using a cellulose triacetate hollow fiber membrane illustrating controlled plasticization benefits. J. Membr. Sci. 2020, 601, 117910. [Google Scholar] [CrossRef]
- Aseri, N.S.; Lau, W.J.; Goh, P.S.; Hasbullah, H.; Othman, N.H.; Ismail, A.F. Preparation and characterization of polylactic acid-modified polyvinylidene fluoride hollow fiber membranes with enhanced water flux and antifouling resistance. J. Water Process. Eng. 2019, 32, 100912. [Google Scholar] [CrossRef]
- Shen, P.; Tu, K.; Yang, C.Y.; Li, J.; Du, R.X. Preparation of anti-fouling poly(lactic acid) (PLA) hollow fiber membranes via non-solvent induced phase separation. Adv. Mat. Res. 2014, 884–885, 112–116. [Google Scholar] [CrossRef]
- Xiao, C.; Xiao, C.; Chen, M.; Huang, H. Study on structure and properties of tubular braid-reinforced poly(lactic acid) (PLA) hollow fiber membranes. Desalin. Water Treat. 2019, 161, 116–125. [Google Scholar] [CrossRef]
- Zhu, L.; Liu, F.; Yu, X.; Xue, L. Poly(lactic acid) hemodialysis membranes with poly(lactic acid)-block-poly(2-hydroxyethyl methacrylate) copolymer as additive: Preparation, characterization, and performance. ACS Appl. Mater. Interfaces 2015, 7, 17748–17755. [Google Scholar] [CrossRef]
- Rajesh, S.; Murthy, Z.V.P. Ultrafiltration membranes from waste polyethylene terephthalate and additives: Synthesis and characterization. Quím. Nova 2014, 37, 653–657. [Google Scholar] [CrossRef]
- Kusumocahyo, S.P.; Ambani, S.K.; Kusumadewi, S.; Sutanto, H.; Widiputri, D.I.; Kartawiria, I.S. Utilization of used polyethylene terephthalate (PET) bottles for the development of ultrafiltration membrane. J. Environ. Chem. Eng. 2020, 8, 104381. [Google Scholar] [CrossRef]
- Kusumocahyo, S.P.; Ambani, S.K.; Marceline, S. Improved permeate flux and rejection of ultrafiltration membranes prepared from polyethylene terephthalate (PET) bottle waste. Sustain. Environ. Res. 2021, 31, 19. [Google Scholar] [CrossRef]
- Doan, H.N.; Phong Vo, P.; Hayashi, K.; Kinashi, K.; Sakai, W.; Tsutsumi, N. Recycled PET as a PDMS-functionalized electrospun fibrous membrane for oil-water separation. J. Environ. Chem. Eng. 2020, 8, 103921. [Google Scholar] [CrossRef]
- Baggio, A.; Doan, H.N.; Vo, P.P.; Kinashi, K.; Sakai, W.; Tsutsumi, N.; Fuse, Y.; Sangermano, M. Chitosan-functionalized recycled polyethylene terephthalate nanofibrous membrane for sustainable on-demand oil-water separation. Glob. Chall. 2021, 5, 2000107. [Google Scholar] [CrossRef]
- Kiani, S.; Mousavi, S.M.; Bidaki, A. Preparation of polyethylene terephthalate/xanthan nanofiltration membranes using recycled bottles for removal of diltiazem from aqueous solution. J. Clean. Prod. 2021, 314, 128082. [Google Scholar] [CrossRef]
- Bonfim, D.P.F.; Cruz, F.G.S.; Bretas, R.E.S.; Guerra, V.G.; Aguiar, M.L. A sustainable recycling alternative: Electrospun PET-membranes for air nanofiltration. Polymers 2021, 13, 1166. [Google Scholar] [CrossRef]
- Zhuang, G.-L.; Tseng, H.-H.; Wey, M.-Y. Feasibility of using waste polystyrene as a membrane material for gas separation. Chem. Eng. Res. Des. 2016, 111, 204–217. [Google Scholar] [CrossRef]
- Zhuang, G.-L.; Wey, M.-Y.; Tseng, H.-H. A novel technique using reclaimed tire rubber for gas separation membranes. J. Membr. Sci. 2016, 520, 314–325. [Google Scholar] [CrossRef]
- Tseng, H.-H.; Lin, Z.-Y.; Chen, S.-H.; Lai, W.-H.; Wey, M.-Y. Reuse of reclaimed tire rubber for gas-separation membranes prepared by hot-pressing. J. Clean. Prod. 2019, 237, 117739. [Google Scholar] [CrossRef]
- Lai, W.-H.; Wang, D.K.; Wey, M.-Y.; Tseng, H.-H. Recycling waste plastics as hollow fiber substrates to improve the anti-wettability of supported ionic liquid membranes for CO2 separation. J. Clean. Prod. 2020, 276, 124194. [Google Scholar] [CrossRef]
- Jamalludin, M.R.; Harun, Z.; Othman, M.H.D.; Hubadillah, S.K.; Yunos, M.Z.; Ismail, A.F. Morphology and property study of green ceramic hollow fiber membrane derived from waste sugarcane bagasse ash (WSBA). Ceram. Int. 2018, 44, 18450–18461. [Google Scholar] [CrossRef]
- Tai, Z.S.; Hubadillah, S.K.; Othman, M.H.D.; Dzahir, M.I.H.M.; Koo, K.N.; Tendot, N.I.S.T.I.; Ismail, A.F.; Rahman, M.A.; Jaafar, J.; Aziz, M.H.A. Influence of pre-treatment temperature of palm oil fuel ash on the properties and performance of green ceramic hollow fiber membranes towards oil/water separation application. Sep. Purif. Technol. 2019, 222, 264–277. [Google Scholar] [CrossRef]


















| Membrane a | Pressure | Permeance | Rejection (%) | Reference | |
|---|---|---|---|---|---|
| (bar) | (LMH/bar) | Salts | Pollutants b | ||
| ZrO2-P84® PI | 10 | 22.7 | Na2SO4: 93.4 | Rifampicin, puerarin, tetracycline, and tea polyphenols: >90.0 | [242] |
| CL/PES | 4 | 2.6 | ZnCl2: 95.7 MgCl2: 95.1 | - | [243] |
| PA/(PES/PVDF) TFC | 5 | 13.8 | Na2SO4: 98.2 | - | [86] |
| PPA/PMIA TFC | 6 | 17.3 | Na2SO4: 98.5 MgSO4: 98.0 CaCl2: 96.0 MgCl2: 95.7 | CFB, RY3, and B2RL: >97.0 | [248] |
| GO/PSF TFC | 5 | 8.39 | Na2SO4: 96.1 | RB: >99.0 ST: 92.9 | [249] |
| MXene/PAN | 1 | ~5.9 | Na2SO4: ~70.0 | - | [251] |
| Polyester-reinforced PES | 6 | 8.7 | NaCl: <7.0 | CR: 99.9 | [254] |
| PEI-70K/PVDF/SMA | 1 | 10.4 | - | MO: 97.1 | [255] |
| PEI-70K & PEI-10K/PVDF/SMA | 1 | 6.4 | - | MO: 99.9 | [255] |
| PPTA/PSf-PA | 7 | 5.46 | MgSO4: 98.1 | MO, AR88, EBT, CR, RB5, and RR195: >99.0 MB, GTL, GRL, and RhB: >98.0 | [256] |
| Membrane a | Pressure (bar) | Solvent | Permeance (LMH/bar) | Solute b | Solute MW (Da) | Rejection (%) | Reference |
|---|---|---|---|---|---|---|---|
| PMDA-ODA PI | 10 | DMF | 2.5 | RB | 1017 | 96.7 | [261] |
| PMDA-MDA PI | 10 | THF | 7.2 | RB | 1017 | 95.7 | [262] |
| PEI/PIP-based TFC/PI | 2 | Acetone | 11.6 | MO AF | 327 585 | 46.5 91.8 | [263] |
| MPD-based TFC/PI | 2 | Ethanol Acetone Acetonitrile | 2.33 24.2 10.58 | MO AF MR Levofloxacin | 327 585 269 361 | 99.4 98.6 90.1 98.2 | [264] |
| K2S2O8-PBI | 5 | Acetone Ethanol Isopropanol | ~3 ~2 ~1 | MB RBB | 374 626 | ~99 ~96 ~99 | [108] |
| GO TFN | 5 | Ethanol Methanol | 2.0 5.8 | RDB RB | 479 1017 | 100 99 | [283] |
| TiO2@rGO TFN | 8 | Ethanol | 4.1 | BTB | 624 | 95 | [284] |
| NH2-MWCNT/PI | 5 | Acetone Ethanol Isopropanol | 4.31 1.17 0.53 | BBR Tetracycline MB | 826 444 320 | 99.9 97.4 99.8 | [285] |
| Polymer Precursor a | Pyrolysis Treatment | Pyrolysis Temperature (°C) | T/P (°C/atm) | Permeance (GPU) b | Selectivity | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| H2 | O2 | N2 | CH4 | CO2 | C2H4 | C2H6 | C3H6 | C3H8 | ||||||
| 6FDA/DETDA-DABA | VTMS c | 550 | 35/2 | - | - | - | - | 1000.0 | - | - | - | - | CO2/CH4: >25 | [142] |
| PIM-1 | - | 575 | 35/6.8 | - | 4.0 | 0.4 | 0.3 | 17.8 | 0.9 | 0.2 | - | - | O2/N2: 10.0 CO2/N2: 44.5 CO2/CH4: 59.3 C2H4/C2H6: 4.5 | [148] |
| PI/LPSQ | - | 675 | 35/1 | - | - | - | 19.0 | 956.0 | - | - | - | - | CO2/CH4: 50.2 | [145] |
| Cellulose | - | 600 | 25/2 | - | - | - | 0.02 g | 3.6 g | - | - | - | - | CO2/CH4: 186.0 | [529] |
| 25/28 | - | - | - | 0.03 f,g | 2.3 f,g | - | - | - | - | CO2/CH4: 75.0 f | [529] | |||
| 60/8 | - | - | - | 0.09 f,g | 4.5 f,g | - | - | - | - | CO2/CH4: 50.0 f | [529] | |||
| Cellulose | - | 600 | 25/2 | - | - | - | 0.001 g | 1.1 g | - | - | - | - | CO2/CH4: 917.0 | [530] |
| 60/50 | - | - | - | - | 1.5 f,g | - | - | - | - | CO2/CH4: 131.0 f | [530] | |||
| Cellulose | - | 600 | 25/8 | - | - | - | 0.03 f,g | 1.3 f,g | - | - | - | - | CO2/N2: 42.0 f | [531] |
| - | - | - | 0.03 f,g | 0.5 f,g | - | - | - | - | CO2/CH4: 15.0 f | [531] | ||||
| PMDA-ODA | Cross-linking with PEI c | 600 | 25/7 | - | 19.6 | 6.5 | 4.7 | 93.4 | - | - | - | - | O2/N2: 3.0 CO2/N2: 14.4 CO2/CH4: 19.8 | [532] |
| 6FDA/BPDA-DAM | - | 675 | NR e/3.5 | 117.0 | - | - | - | - | 0.4 | - | - | - | H2/C2H4: 297.0 | [141] |
| PEI | - | 600 | NR e/5 | 711.6 | - | - | - | 254.1 | - | 151.4 | - | 134.3 | H2/CO2: 2.8 H2/C2H6: 4.7 H2/C3H8: 5.3 | [533] |
| Matrimid | VTMS c | 675 | 600/1 | 430.0 f | - | - | - | - | - | - | - | - | H2/C3H8: 511.0 f | [138] |
| Cellulose | - | 850 | 130/2 | 148.2 | - | 0.2 | 0.03 | 1.8 | - | - | - | - | H2/N2: >800.0 H2/CH4: >5700.0 H2/CO2: 83.9 | [137] |
| PBI | - | 750 | 25/1 | - | 0.8 | - | - | - | - | - | - | O2/N2: 13.7 | [144] | |
| 6FDA/BPDA-DAM | Super-hyperaging thermal treatment d | 675 | 35/3.5 | - | - | - | - | - | - | - | 25.0 | 2.5 | C3H6/C3H8: 10.0 | [140] |
| Matrimid coated alumina | - | 650 | 25/2 | - | - | - | - | - | - | - | 45.0 | 2.7 | C3H6/C3H8: 16.5 | [534] |
| Ceramic a | Modification | Temperature (°C) | O2 Flux (mL cm−2 min−1) | Reference |
|---|---|---|---|---|
| BSCF | - | 900 | 7.05 | [541] |
| LSC | - | 1000 | 1.20 | [542] |
| LCCF | - | 1000 | 6.20 b | [543] |
| LCCF | - | 1000 | 5.10 b | [544] |
| BCBZ | - | 1000 | 5.75 | [555] |
| LSCF | LSCF bundle of 5 single LSCF hollow fiber | 950 | 0.46 | [540] |
| BCBZ | BCBZ bundle of 7 single BCBZ hollow fiber | 1000 | 7.06 | [537] |
| LSC | LSC214 Ruddlesden–Popper decoration | 850 | 0.40 | [548] |
| LSC | LSC214 Ruddlesden–Popper decoration | 900 | 0.60 | [548] |
| LSCF-LSC | LSC Ruddlesden-Popper phase | 950 | 4.52 | [549] |
| Wrinkled LSCF | Ag deposition via dip coating on outer surface | 950 | 1.48 | [127] |
| LCF | Ag deposition via wet complexation | 950 | 1.46 | [550] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lau, H.S.; Lau, S.K.; Soh, L.S.; Hong, S.U.; Gok, X.Y.; Yi, S.; Yong, W.F. State-of-the-Art Organic- and Inorganic-Based Hollow Fiber Membranes in Liquid and Gas Applications: Looking Back and Beyond. Membranes 2022, 12, 539. https://doi.org/10.3390/membranes12050539
Lau HS, Lau SK, Soh LS, Hong SU, Gok XY, Yi S, Yong WF. State-of-the-Art Organic- and Inorganic-Based Hollow Fiber Membranes in Liquid and Gas Applications: Looking Back and Beyond. Membranes. 2022; 12(5):539. https://doi.org/10.3390/membranes12050539
Chicago/Turabian StyleLau, Hui Shen, Siew Kei Lau, Leong Sing Soh, Seang Uyin Hong, Xie Yuen Gok, Shouliang Yi, and Wai Fen Yong. 2022. "State-of-the-Art Organic- and Inorganic-Based Hollow Fiber Membranes in Liquid and Gas Applications: Looking Back and Beyond" Membranes 12, no. 5: 539. https://doi.org/10.3390/membranes12050539