Effect of Pulsed Electric Field on the Electrodialysis Performance of Phosphate-Containing Solutions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Membranes
2.2. Solutions
2.3. Electrodialysis Desalination of Phosphate-Containing Solutions
2.4. Scaling Characterization
3. Results
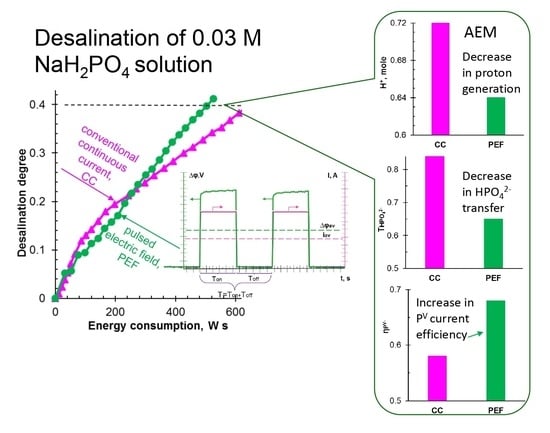
3.1. Desalination of NaH2PO4 Solution
3.2. Desalination of a Multicomponent Solution
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- An, W.; Zhao, J.; Lu, J.; Han, Y.; Li, D. Zero-liquid discharge technologies for desulfurization wastewater: A review. J. Environ. Manag. 2022, 321, 115953. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Q.-B.; Wang, J.; Li, P.-F.; Zhao, J. Fractionation of monovalent ions from seawater brine via softening nanofiltration and selective electrodialysis: Which is better? Desalination 2022, 533, 115717. [Google Scholar] [CrossRef]
- Wang, Z.; He, P.; Zhang, H.; Zhang, N.; Lü, F. Desalination, nutrients recovery, or products extraction: Is electrodialysis an effective way to achieve high-value utilization of liquid digestate? Chem. Eng. J. 2022, 446, 136996. [Google Scholar] [CrossRef]
- Robles, Á.; Aguado, D.; Barat, R.; Borrás, L.; Bouzas, A.; Giménez, J.B.; Martí, N.; Ribes, J.; Ruano, M.V.; Serralta, J.; et al. New frontiers from removal to recycling of nitrogen and phosphorus from wastewater in the Circular Economy. Bioresour. Technol. 2020, 300, 122673. [Google Scholar] [CrossRef] [PubMed]
- Faucher, M.; Thibodeau, J.; Nazim Ciftci, O.; Gaaloul, S.; Bazinet, L. Phospholipid recovery from sweet whey by combination of electrodialytic processes and understanding of specific mechanisms involved. Chem. Eng. J. 2022, 448, 137165. [Google Scholar] [CrossRef]
- Faucher, M.; Perreault, V.; Ciftci, O.N.; Gaaloul, S.; Bazinet, L. Phospholipid recovery from sweet whey and whey protein concentrate: Use of electrodialysis with bipolar membrane combined with a dilution factor as an ecoefficient method. Future Foods 2021, 4, 100052. [Google Scholar] [CrossRef]
- Aguero, R.; Bringas, E.; Román, M.F.S.; Ortiz, I.; Ibañez, R. Membrane Processes for Whey Proteins Separation and Purification. A Review. Curr. Org. Chem. 2017, 21, 1740–1752. [Google Scholar] [CrossRef]
- Eliseeva, T.; Kharina, A. Desalination of Neutral Amino Acid Solutions in an Electromembrane System. Membranes 2022, 12, 665. [Google Scholar] [CrossRef]
- Dufton, G.; Mikhaylin, S.; Gaaloul, S.; Bazinet, L. Positive Impact of Pulsed Electric Field on Lactic Acid Removal, Demineralization and Membrane Scaling during Acid Whey Electrodialysis. Int. J. Mol. Sci. 2019, 20, 797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moltedo, J.J.; Schwarz, A.; Gonzalez-Vogel, A. Evaluation of percrystallization coupled with electrodialysis for zero liquid discharge in the pulping industry. J. Environ. Manag. 2022, 303, 114104. [Google Scholar] [CrossRef]
- Ferrari, F.; Pijuan, M.; Molenaar, S.; Duinslaeger, N.; Sleutels, T.; Kuntke, P.; Radjenovic, J. Ammonia recovery from anaerobic digester centrate using onsite pilot scale bipolar membrane electrodialysis coupled to membrane stripping. Water Res. 2022, 218, 118504. [Google Scholar] [CrossRef] [PubMed]
- Mitko, K.; Turek, M.; Jaroszek, H.; Bernacka, E.; Sambor, M.; Skóra, P.; Dydo, P. Pilot studies on circular economy solution for the coal mining sector. Water Resour. Ind. 2021, 26, 100161. [Google Scholar] [CrossRef]
- Cifuentes-Araya, N.; Pourcelly, G.; Bazinet, L. Impact of pulsed electric field on electrodialysis process performance and membrane fouling during consecutive demineralization of a model salt solution containing a high magnesium/calcium ratio. J. Colloid Interface Sci. 2011, 361, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Sosa-Fernandez, P.A.; Post, J.W.; Ramdlan, M.S.; Leermakers, F.A.M.; Bruning, H.; Rijnaarts, H.H.M. Improving the performance of polymer-flooding produced water electrodialysis through the application of pulsed electric field. Desalination 2020, 484, 114424. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Bazinet, L. Fouling on ion-exchange membranes: Classification, characterization and strategies of prevention and control. Adv. Colloid Interface Sci. 2016, 229, 34–56. [Google Scholar] [CrossRef]
- Grossman, G.; Sonin, A.A. Membrane fouling in electrodialysis: A model and experiments. Desalination 1973, 12, 107–125. [Google Scholar] [CrossRef]
- Li, P.-F.; Chen, Q.-B.; Wang, J.; Xu, Y.; Dong, L.; Wang, J. Developing a reclamation strategy for softening nanofiltration brine: A scaling-free conversion approach via continuous two-stage electrodialysis metathesis. Sci. Total Environ. 2022, 807, 150374. [Google Scholar] [CrossRef]
- Chen, Q.-B.; Tian, Z.; Zhao, J.; Wang, J.; Li, P.-F.; Xu, Y. Near-zero liquid discharge and reclamation process based on electrodialysis metathesis for high-salinity wastewater with high scaling potential. Desalination 2022, 525, 115390. [Google Scholar] [CrossRef]
- Zhao, D.; Lee, L.Y.; Ong, S.L.; Chowdhury, P.; Siah, K.B.; Ng, H.Y. Electrodialysis reversal for industrial reverse osmosis brine treatment. Sep. Purif. Technol. 2019, 213, 339–347. [Google Scholar] [CrossRef]
- Shi, L.; Xie, S.; Hu, Z.; Wu, G.; Morrison, L.; Croot, P.; Hu, H.; Zhan, X. Nutrient recovery from pig manure digestate using electrodialysis reversal: Membrane fouling and feasibility of long-term operation. J. Membr. Sci. 2019, 573, 560–569. [Google Scholar] [CrossRef]
- Turek, M.; Mitko, K.; Piotrowski, K.; Dydo, P.; Laskowska, E.; Jakóbik-Kolon, A. Prospects for high water recovery membrane desalination. Desalination 2017, 401, 180–189. [Google Scholar] [CrossRef]
- Honarparvar, S.; Zhang, X.; Chen, T.; Alborzi, A.; Afroz, K.; Reible, D. Frontiers of Membrane Desalination Processes for Brackish Water Treatment: A Review. Membranes 2021, 11, 246. [Google Scholar] [CrossRef] [PubMed]
- Barros, K.S.; Martí-Calatayud, M.C.; Pérez-Herranz, V.; Espinosa, D.C.R. A three-stage chemical cleaning of ion-exchange membranes used in the treatment by electrodialysis of wastewaters generated in brass electroplating industries. Desalination 2020, 492, 114628. [Google Scholar] [CrossRef]
- Zhao, J.; Chen, Q.; Wang, J. Novel ecofriendly cation exchange membranes for low-cost electrodialysis of brackish water: Desalination and antiscaling performance. J. Membr. Sci. 2022, 661, 120908. [Google Scholar] [CrossRef]
- Shah, S.R.; Walter, S.L.; Winter, A.G. Using feed-forward voltage-control to increase the ion removal rate during batch electrodialysis desalination of brackish water. Desalination 2019, 457, 62–74. [Google Scholar] [CrossRef]
- Lemay, N.; Mikhaylin, S.; Mareev, S.; Pismenskaya, N.; Nikonenko, V.; Bazinet, L. How demineralization duration by electrodialysis under high frequency pulsed electric field can be the same as in continuous current condition and that for better performances? J. Membr. Sci. 2020, 603, 117878. [Google Scholar] [CrossRef]
- Karlin, Y.V.; Kropotov, V.N. Elektrodializnoye razdeleniye Na + i Ca2 + v rezhime impul’snogo toka. Ross. Elektrokhim. Z. 1995, 31, 472. [Google Scholar]
- Bazinet, L.; Geoffroy, T.R. Electrodialytic Processes: Market Overview, Membrane Phenomena, Recent Developments and Sustainable Strategies. Membranes 2020, 10, 221. [Google Scholar] [CrossRef]
- Mikhaylin, S.; Nikonenko, V.; Pourcelly, G.; Bazinet, L. Intensification of demineralization process and decrease in scaling by application of pulsed electric field with short pulse/pause conditions. J. Membr. Sci. 2014, 468, 389–399. [Google Scholar] [CrossRef]
- Mishchuk, N.A.; Koopal, L.K.; Gonzalez-Caballero, F. Intensification of electrodialysis by applying a non-stationary electric field. Colloids Surf. A Physicochem. Eng. Asp. 2001, 176, 195–212. [Google Scholar] [CrossRef]
- De Jaegher, B.; De Schepper, W.; Verliefde, A.; Nopens, I. A model-based analysis of electrodialysis fouling during pulsed electric field operation. J. Membr. Sci. 2022, 642, 119975. [Google Scholar] [CrossRef]
- Lemay, N.; Mikhaylin, S.; Bazinet, L. Voltage spike and electroconvective vortices generation during electrodialysis under pulsed electric field: Impact on demineralization process efficiency and energy consumption. Innov. Food Sci. Emerg. Technol. 2019, 52, 221–231. [Google Scholar] [CrossRef]
- Gorobchenko, A.; Mareev, S.; Nikonenko, V. Mathematical Modeling of the Effect of Pulsed Electric Field on the Specific Permselectivity of Ion-Exchange Membranes. Membranes 2021, 11, 115. [Google Scholar] [CrossRef]
- Gonzalez-Vogel, A.; Rojas, O.J. Exploiting electroconvective vortices in electrodialysis with high-frequency asymmetric bipolar pulses for desalination in overlimiting current regimes. Desalination 2020, 474, 114190. [Google Scholar] [CrossRef]
- Zhang, Y.; Van der Bruggen, B.; Pinoy, L.; Meesschaert, B. Separation of nutrient ions and organic compounds from salts in RO concentrates by standard and monovalent selective ion-exchange membranes used in electrodialysis. J. Membr. Sci. 2009, 332, 104–112. [Google Scholar] [CrossRef]
- Ghyselbrecht, K.; Jongbloet, A.; Pinoy, L.; Meesschaert, B. Optimization of the configuration of the anion selectrodialysis stack for fractionation of phosphate from UASB effluent in batch mode on lab scale and pilot scale. J. Environ. Chem. Eng. 2020, 8, 104492. [Google Scholar] [CrossRef]
- Shi, L.; Xiao, L.; Hu, Z.; Zhan, X. Nutrient recovery from animal manure using bipolar membrane electrodialysis: Study on product purity and energy efficiency. Water Cycle 2020, 1, 54–62. [Google Scholar] [CrossRef]
- Rybalkina, O.A.; Sharafan, M.V.; Nikonenko, V.V.; Pismenskaya, N.D. Two mechanisms of H+/OH− ion generation in anion-exchange membrane systems with polybasic acid salt solutions. J. Membr. Sci. 2022, 651, 120449. [Google Scholar] [CrossRef]
- Pismenskaya, N.; Rybalkina, O.; Moroz, I.; Mareev, S.; Nikonenko, V. Influence of Electroconvection on Chronopotentiograms of an Anion-Exchange Membrane in Solutions of Weak Polybasic Acid Salts. Int. J. Mol. Sci. 2021, 22, 13518. [Google Scholar] [CrossRef] [PubMed]
- Rotta, E.H.; Marder, L.; Pérez-Herranz, V.; Bernardes, A.M. Characterization of an anion-exchange membrane subjected to phosphate and sulfate separation by electrodialysis at overlimiting current density condition. J. Membr. Sci. 2021, 635, 119510. [Google Scholar] [CrossRef]
- Malek, P.; Ortiz, J.M.; Richards, B.S.; Schäfer, A.I. Electrodialytic removal of NaCl from water: Impacts of using pulsed electric potential on ion transport and water dissociation phenomena. J. Membr. Sci. 2013, 435, 99–109. [Google Scholar] [CrossRef]
- Butylskii, D.; Moroz, I.; Tsygurina, K.; Mareev, S. Effect of Surface Inhomogeneity of Ion-Exchange Membranes on the Mass Transfer Efficiency in Pulsed Electric Field Modes. Membranes 2020, 10, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Q.; Li, Z.; Lei, C.; Fu, R.; Wang, W.; Li, Q.; Liu, Z. Application of Pulsed Electric Field in Antifouling Treatment of Sodium Gluconate Mother Liquor by Electrodialysis. Materials 2020, 13, 2501. [Google Scholar] [CrossRef] [PubMed]
- Dufton, G.; Mikhaylin, S.; Gaaloul, S.; Bazinet, L. Systematic Study of the Impact of Pulsed Electric Field Parameters (Pulse/Pause Duration and Frequency) on ED Performances during Acid Whey Treatment. Membranes 2020, 10, 14. [Google Scholar] [CrossRef] [Green Version]
- Nefedova, G.Z.; Klimova, Z.G.; Sapozhnikova, G.S. Ion-Exchange Membranes: Granules and Powders (Catalog); Pashkov, A.B., Ed.; NIITEKhim: Moscow, Russia, 1977. [Google Scholar]
- Pismenskaya, N.D.; Pokhidnia, E.V.; Pourcelly, G.; Nikonenko, V.V. Can the electrochemical performance of heterogeneous ion-exchange membranes be better than that of homogeneous membranes? J. Membr. Sci. 2018, 566, 54–68. [Google Scholar] [CrossRef]
- Sarapulova, V.; Shkorkina, I.; Mareev, S.; Pismenskaya, N.; Kononenko, N.; Larchet, C.; Dammak, L.; Nikonenko, V. Transport Characteristics of Fujifilm Ion-Exchange Membranes as Compared to Homogeneous Membranes AMX and CMX and to Heterogeneous Membranes MK-40 and MA-41. Membranes 2019, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Volodina, E.; Pismenskaya, N.; Nikonenko, V.; Larchet, C.; Pourcelly, G. Ion transfer across ion-exchange membranes with homogeneous and heterogeneous surfaces. J. Colloid Interface Sci. 2005, 285, 247–258. [Google Scholar] [CrossRef]
- Gatapova, N.T.; Dzhubari, M.K.; Alekseeva, N.V. A Study of Diffusion Permissibility of MK-40 Membrane in Thermodynamic Conditions. Vestn. Tambovskogo Gos. Teh. Univ. 2020, 26, 619–628. [Google Scholar] [CrossRef]
- Laktionov, E.V.; Pismenskaya, N.D.; Nikonenko, V.V.; Zabolotsky, V.I. Method of electrodialysis stack testing with the feed solution concentration regulation. Desalination 2003, 151, 101–116. [Google Scholar] [CrossRef]
- Lévêque, M.A. The laws of heat transmission by convection. Ann. Mines Mem. 1928, 12, 201–299. [Google Scholar]
- Simons, R. Strong electric field effects on proton transfer between membrane-bound amines and water. Nature 1979, 280, 824–826. [Google Scholar] [CrossRef]
- Kumar, P.; Rubinstein, S.M.; Rubinstein, I.; Zaltzman, B. Mechanisms of hydrodynamic instability in concentration polarization. Phys. Rev. Res. 2020, 2, 033365. [Google Scholar] [CrossRef]
- Walton, H.F. Deutsche Ausführlichkeit: Ion Exchange. F. G. Helfferich. McGraw-Hill, New York, 1962. ix + 624 pp. Illus. $16. Science 1962, 138, 133. [Google Scholar] [CrossRef]
- Rotta, E.H.; Bitencourt, C.S.; Marder, L.; Bernardes, A.M. Phosphorus recovery from low phosphate-containing solution by electrodialysis. J. Memb. Sci. 2019, 573, 293–300. [Google Scholar] [CrossRef]
- Chandra, A.; Tadimeti, J.G.D.; Bhuvanesh, E.; Pathiwada, D.; Chattopadhyay, S. Switching selectivity of carboxylic acids and associated physico-chemical changes with pH during electrodialysis of ternary mixtures. Sep. Purif. Technol. 2018, 193, 327–344. [Google Scholar] [CrossRef]
- Martí-Calatayud, M.C.; Evdochenko, E.; Bär, J.; García-Gabaldón, M.; Wessling, M.; Pérez-Herranz, V. Tracking homogeneous reactions during electrodialysis of organic acids via EIS. J. Membr. Sci. 2020, 595, 117592. [Google Scholar] [CrossRef]
- Rybalkina, O.A.; Solonchenko, K.V.; Butylskii, D.Y.; Nikonenko, V.V.; Pismenskaya, N. Vliyaniye parametrov pul’siruyushchikh elektricheskikh poley na srednyuyu plotnost’ toka pri elektrodializnom obessolivanii fosfatsoderzhashchego rastvora. Membr. Membr. Tekhnol. 2022, 12, 438–451. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B.; Pundik, T. Ion-exchange funneling in thin-film coating modification of heterogeneous electrodialysis membranes. Phys. Rev. E 2002, 65, 041507. [Google Scholar] [CrossRef]
- Mishchuk, N.A. Concentration polarization of interface and non-linear electrokinetic phenomena. Adv. Colloid Interface Sci. 2010, 160, 16–39. [Google Scholar] [CrossRef]
- Rubinstein, I.; Zaltzman, B. Electro-osmotically induced convection at a permselective membrane. Phys. Rev. E 2000, 62, 2238–2251. [Google Scholar] [CrossRef]
- Strathmann, H. Electrodialysis, a mature technology with a multitude of new applications. Desalination 2010, 264, 268–288. [Google Scholar] [CrossRef]
- de Valença, J.C.; Kurniawan, A.; Wagterveld, R.M.; Wood, J.A.; Lammertink, R.G.H. Influence of Rayleigh-Bénard convection on electrokinetic instability in overlimiting current conditions. Phys. Rev. Fluids 2017, 2, 033701. [Google Scholar] [CrossRef]
- Karatay, E.; Andersen, M.B.; Wessling, M.; Mani, A. Coupling between Buoyancy Forces and Electroconvective Instability near Ion-Selective Surfaces. Phys. Rev. Lett. 2016, 116, 194501. [Google Scholar] [CrossRef] [Green Version]
- Nikonenko, V.V.; Kovalenko, A.V.; Urtenov, M.K.; Pismenskaya, N.D.; Han, J.; Sistat, P.; Pourcelly, G. Desalination at overlimiting currents: State-of-the-art and perspectives. Desalination 2014, 342, 85–106. [Google Scholar] [CrossRef]
- Rivera, R.; Chagnes, A.; Cathelineau, M.; Boiron, M.-C. Conditioning of poultry manure ash for subsequent phosphorous separation and assessment for a process design. Sustain. Mater. Technol. 2022, 31, e00377. [Google Scholar] [CrossRef]
- Cifuentes-Araya, N.; Pourcelly, G.; Bazinet, L. How pulse modes affect proton-barriers and anion-exchange membrane mineral fouling during consecutive electrodialysis treatments. J. Colloid Interface Sci. 2013, 392, 396–406. [Google Scholar] [CrossRef] [PubMed]











| Membrane | Ion-Exchange Resin | Fixed Groups | Thickness, µm (0.02 M NaCl) | Ion-Exchange Capacity of Wet Membrane, mol g−1 | Fraction of Conductive Surface, Θ |
|---|---|---|---|---|---|
| Anion-exchange membranes | |||||
| MA-41P | AV-17-2P [45] | –N+(CH3)3 and a small amount of secondary and tertiary amines | 510 ± 50 | 0.92 ± 0.05 [46] | 0.35 ± 0.01 [46] |
| MA-41 | AV-17-8 [45] | 450 ± 50 [47] | 1.22 ± 0.06 [47] | 0.22 ± 0.03 [48] | |
| Cation-exchange membrane | |||||
| MK-40 | KU-2-8 [45] | –SO3− | 520 ± 20 [47] | 1.43 ± 0.08 [47] | 0.55 ±0.05 [49] |
| Active membrane surface area, S, cm2 | 4.00 ± 0.01 |
| Intermembrane distance, h, cm | 0.66 ± 0.01 |
| Compartment length, L, cm | 2.00 ± 0.01 |
| Average linear flow velocity, V0, cm s−1 | 0.40 ± 0.01 |
| Temperature, °C | 25 ± 0.2 |
| Designation | iav, mA cm−2 | i, mA cm−2 | α | f,Hz | ||
|---|---|---|---|---|---|---|
| Experiment 1 | CC1 | 1 | 3.0 | 3.0 | 1 | 0 |
| PEF1 | 2 | 12.0 | 1/4 | 5 | ||
| 3 | 6.0 | 1/2 | 5 | |||
| 4 | 4.0 | 3/4 | 5 | |||
| Experiment 2 | CC2 | 1 | 3.5 | 3.5 | 1 | 0 |
| PEF2 | 2 | 14.0 | 1/4 | 5 | ||
| 3 | 7.0 | 1/2 | 5 | |||
| 4 | 4.7 | 3/4 | 5 | |||
| Designation | * H+, mmol | Q, C | W, W h | ||||
|---|---|---|---|---|---|---|---|
| CC1 | 1 | 0.72 | 0.16 | 0.84 | 0.58 | 191 | 0.18 |
| PEF1 | 2 (α = 1/4) | 0.95 | 0.17 | 0.83 | 0.59 | 183 | 0.18 |
| 3 (α = 1/2) | 0.64 | 0.35 | 0.65 | 0.68 | 162 | 0.14 | |
| 4 (α = 3/4) | 0.79 | 0.16 | 0.84 | 0.58 | 191 | 0.18 | |
| CC2 | 1 | 0.74 | 0.12 | 0.88 | 0.56 | 182 | 0.16 |
| PEF2 | 2 (α = 1/4) | 1.30 | 0.13 | 0.87 | 0.57 | 215 | 0.22 |
| 3 (α = 1/2) | 0.68 | 0.25 | 0.75 | 0.63 | 172 | 0.14 | |
| 4 (α = 3/4) | 1.16 | 0.1 | 0.9 | 0.55 | 197 | 0.21 | |
| Designations | i, mA cm−2 | iav, mA cm−2 | α | f, Hz | |
|---|---|---|---|---|---|
| CC1 | 1 | 4 | - | 1.0 | - |
| CC2 | 2 | 8 | - | 1.0 | - |
| PEF | 3 | 8 | 4 | 1/2 | 5 |
| The Current Mode | Demineralization Rate dκ/dt, mS cm−1 s−1 | The Degree of Desalination is Equal to 50% | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| i mA cm2 | i av mA cm2 | ** Q, C | W, W h | The Degree of Removal of Component *i, % | |||||||
| Cl− | PV | Na+ | K+ | Ca2+ | Mg2+ | ||||||
| CC1 | 4 | - | 0.20 | 500 | 0.100 | 57 | 20 | 41 | 42 | 61 | 63 |
| CC2 | 8 | - | 0.38 | 537 | 0.130 | 58 | 22 | 47 | 37 | 62 | 48 |
| PEF | 8 | 4 | 0.22 | 470 | 0.094 | 61 | 17 | 43 | 41 | 62 | 40 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rybalkina, O.; Solonchenko, K.; Chuprynina, D.; Pismenskaya, N.; Nikonenko, V. Effect of Pulsed Electric Field on the Electrodialysis Performance of Phosphate-Containing Solutions. Membranes 2022, 12, 1107. https://doi.org/10.3390/membranes12111107
Rybalkina O, Solonchenko K, Chuprynina D, Pismenskaya N, Nikonenko V. Effect of Pulsed Electric Field on the Electrodialysis Performance of Phosphate-Containing Solutions. Membranes. 2022; 12(11):1107. https://doi.org/10.3390/membranes12111107
Chicago/Turabian StyleRybalkina, Olesya, Ksenia Solonchenko, Daria Chuprynina, Natalia Pismenskaya, and Victor Nikonenko. 2022. "Effect of Pulsed Electric Field on the Electrodialysis Performance of Phosphate-Containing Solutions" Membranes 12, no. 11: 1107. https://doi.org/10.3390/membranes12111107