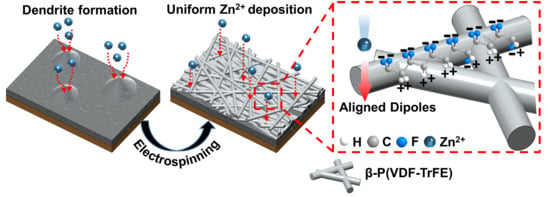
Inhibition of Zinc Dendrites Realized by a β-P(VDF-TrFE) Nanofiber Layer in Aqueous Zn-Ion Batteries
Abstract
:1. Introduction
2. Experimental Section
2.1. P(VDF-TrFE) and PVDF Nanofiber Fabrication
2.2. Preparation of MnO2 Electrode
2.3. Cell Assembly
2.4. Electrochemical Measurements
2.5. Material Characterization
3. Results and Discussion
3.1. Characterization of the PNF-Cu Electrode
3.2. Suppression of Side Reactions
3.3. Zn Deposition Morphology
3.4. Electrochemical Properties of the Half Cells and Symmetric Cells
3.5. Full-Cell Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, J.-K.; Mueller, F.; Kim, H.; Jeong, S.; Park, J.-S.; Passerini, S.; Kim, Y. Eco-friendly Energy Storage System: Seawater and Ionic Liquid Electrolyte. ChemSusChem 2016, 9, 42–49. [Google Scholar] [CrossRef]
- Alam, R.B.; Ahmad, M.H.; Islam, M.R. Bio-inspired gelatin/single-walled carbon nanotube nanocomposite for transient electrochemical energy storage: An approach towards eco-friendly and sustainable energy system. Heliyon 2021, 7, e07468. [Google Scholar] [CrossRef]
- Chang, N.; Li, T.; Li, R.; Wang, S.; Yin, Y.; Zhang, H.; Li, X. An aqueous hybrid electrolyte for low-temperature zinc-based energy storage devices. Energy Environ. Sci. 2020, 13, 3527–3535. [Google Scholar] [CrossRef]
- Mensah-Darkwa, K.; Zequine, C.; Kahol, P.K.; Gupta, R.K. Supercapacitor Energy Storage Device Using Biowastes: A Sustainable Approach to Green Energy. Sustainability 2019, 11, 414. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.W.; Aurbach, D. Promise and reality of post-lithium-ion batteries with high energy densities. Nat. Rev. Mater. 2016, 1, 16013. [Google Scholar] [CrossRef] [Green Version]
- Ogumi, Z.; Kostecki, R.; Guyomard, D.; Inaba, M. Lithium-Ion Batteries-The 25th Anniversary of Commercialization. Electrochem. Soc. Interface 2016, 25, 65. [Google Scholar] [CrossRef] [Green Version]
- Yoshino, A. The Birth of the Lithium-Ion Battery. Angew. Chem. Int. Ed. 2012, 51, 5798–5800. [Google Scholar] [CrossRef]
- Liu, J.; Xu, C.; Chen, Z.; Ni, S.; Shen, Z.X. Progress in aqueous rechargeable batteries. Green Energy Environ. 2018, 3, 20–41. [Google Scholar] [CrossRef]
- Balakrishnan, P.G.; Ramesh, R.; Prem Kumar, T. Safety mechanisms in lithium-ion batteries. J. Power Sources 2006, 155, 401–414. [Google Scholar] [CrossRef]
- Chawla, N.; Bharti, N.; Singh, S. Recent Advances in Non-Flammable Electrolytes for Safer Lithium-Ion Batteries. Batteries 2019, 5, 19. [Google Scholar] [CrossRef]
- Wang, F.; Borodin, O.; Ding, M.S.; Gobet, M.; Vatamanu, J.; Fan, X.; Gao, T.; Eidson, N.; Liang, Y.; Sun, W.; et al. Hybrid Aqueous/Non-aqueous Electrolyte for Safe and High-Energy Li-Ion Batteries. Joule 2018, 2, 927–937. [Google Scholar] [CrossRef] [Green Version]
- Dou, Q.; Wang, Y.; Wang, A.; Ye, M.; Hou, R.; Lu, Y.; Su, L.; Shi, S.; Zhang, H.; Yan, X. Water in salt/ionic liquid” electrolyte for 2.8 V aqueous lithium-ion capacitor. Sci. Bull. 2020, 65, 1812–1822. [Google Scholar] [CrossRef]
- Ren, J.; Li, C.; Li, H.; Li, Z.; Liu, S.; Luo, B.; Wang, L. Realizing highly stable zinc-ion batteries via electrolyte engineering with adsorbed molecular protective layer. Electrochim. Acta 2022, 427, 140876. [Google Scholar] [CrossRef]
- Sun, P.; Liu, W.; Yang, D.; Zhang, Y.; Xiong, W.; Li, S.; Chen, J.; Tian, J.; Zhang, L. Stable Zn anodes enabled by high-modulus agarose gel electrolyte with confined water molecule mobility. Electrochim. Acta 2022, 429, 140985. [Google Scholar] [CrossRef]
- Hoang Huy, V.P.; Hieu, L.T.; Hur, J. Zn Metal Anodes for Zn-Ion Batteries in Mild Aqueous Electrolytes: Challenges and Strategies. Nanomaterials 2021, 11, 2746. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Ang, E.H.; Yang, Y.; Zhang, Y.; Ye, M.; Du, W.; Li, C.C. High-Voltage Zinc-Ion Batteries: Design Strategies and Challenges. Adv. Funct. Mater. 2021, 31, 2010213. [Google Scholar] [CrossRef]
- Chen, D.; Lu, M.; Cai, D.; Yang, H.; Han, W. Recent advances in energy storage mechanism of aqueous zinc-ion batteries. J. Energy Chem. 2021, 54, 712–726. [Google Scholar] [CrossRef]
- Kim, E.; Choi, I.; Nam, K.W. Metal–organic framework for dendrite-free anodes in aqueous rechargeable zinc batteries. Electrochim. Acta 2022, 425, 140648. [Google Scholar] [CrossRef]
- Yang, Q.; Li, Q.; Liu, Z.; Wang, D.; Guo, Y.; Li, X.; Tang, Y.; Li, H.; Dong, B.; Zhi, C. Dendrites in Zn-Based Batteries. Adv. Mater. 2020, 48, 2001854. [Google Scholar] [CrossRef]
- Kumar, S.; Yoon, H.; Park, H.; Park, G.; Suh, S.; Kim, H.-J. A dendrite-free anode for stable aqueous rechargeable zinc-ion batteries. J. Ind. Eng. Chem. 2022, 108, 321–327. [Google Scholar] [CrossRef]
- Liu, B.; Wang, S.; Wang, Z.; Lei, H.; Chen, Z.; Mai, W. Novel 3D Nanoporous Zn–Cu Alloy as Long-Life Anode toward High-Voltage Double Electrolyte Aqueous Zinc-Ion Batteries. Small 2020, 16, 2001323. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xue, J.; Han, C.; Liu, N.; Ma, K.; Zhang, R.; Wu, X.; Dai, L.; Wang, L.; He, Z. A hafnium oxide-coated dendrite-free zinc anode for rechargeable aqueous zinc-ion batteries. J. Colloid Interface Sci. 2021, 599, 467–475. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Yuan, X.; Xia, Y.; Zhang, Y.; Fu, L.; Liu, L.; Yu, N.; Huang, Q.; Wang, B.; Hu, X.; et al. An Artificial Polyacrylonitrile Coating Layer Confining Zinc Dendrite Growth for Highly Reversible Aqueous Zinc-Based Batteries. Adv. Sci. 2021, 8, 2100309. [Google Scholar] [CrossRef] [PubMed]
- Jian, Q.; Wan, Y.; Sun, J.; Wu, M.; Zhao, T. A dendrite-free zinc anode for rechargeable aqueous batteries. J. Mater. Chem. 2020, 8, 20175–20184. [Google Scholar] [CrossRef]
- He, H.; Qin, H.; Wu, J.; Chen, X.; Huang, R.; Shen, F.; Wu, Z.; Chen, G.; Yin, S.; Liu, J. Engineering interfacial layers to enable Zn metal anodes for aqueous zinc-ion batteries. Energy Stor. Mater. 2021, 43, 317–336. [Google Scholar] [CrossRef]
- Pusty, M.; Shirage, P.M. Insights and perspectives on graphene-PVDF based nanocomposite materials for harvesting mechanical energy. J. Alloys Compd. 2022, 904, 164060. [Google Scholar] [CrossRef]
- Yu, Y.; Shao, W.; Zhong, J.; Ye, H.; Yang, L.; Zhen, L. Tuning the Energy Storage Efficiency in PVDF Nanocomposites Incorporated with Crumpled Core–Shell BaTiO3@Graphene Oxide Nanoparticles. ACS Appl. Energy Mater. 2021, 4, 9553–9562. [Google Scholar] [CrossRef]
- Zhang, M.; Tan, S.; Xiong, J.; Chen, C.; Zhang, Y.; Wei, X.; Zhang, Z. Tailoring Dielectric and Energy Storage Performance of PVDF-Based Relaxor Ferroelectrics with Hydrogen Bonds. ACS Appl. Energy Mater. 2021, 4, 8454–8464. [Google Scholar] [CrossRef]
- Yi, Z.; Wang, Z.; Nian, W.; Wang, T.; Chen, H.; Cheng, Z. High Energy Storage Density of Sandwich-Structured Na0.5Bi0.5TiO3/PVDF Nanocomposites Enhanced by Optimizing the Dimensions of Fillers. ACS Appl. Energy Mater. 2021, 4, 13528–13537. [Google Scholar] [CrossRef]
- Shepelin, N.A.; Sherrell, P.C.; Skountzos, E.N.; Goudeli, E.; Zhang, J.; Lussini, V.C.; Imtiaz, B.; Usman, K.A.S.; Dicinosk, G.W.; Shapter, J.G.; et al. Interfacial piezoelectric polarization locking in printable Ti3C2Tx MXene-fluoropolymer composites. Nat. Commun. 2021, 12, 3171. [Google Scholar] [CrossRef]
- Fortunato, M.; Cavallini, D.; Bellis, G.D.; Marra, F.A.; Tamburrano, F.; Sarto, M.S.; Sarto, F. Phase Inversion in PVDF Films with Enhanced Piezoresponse Through Spin-Coating and Quenching. Polymers 2019, 11, 1096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, M.; Lee, S.; Kim, Y.-I. Solvent-controlled crystalline beta-phase formation in electrospun P(VDF-TrFE) fibers for enhanced piezoelectric energy harvesting. APL Mater. 2020, 8, 071109. [Google Scholar] [CrossRef]
- Song, W.-J.; Joo, S.H.; Kim, D.H.; Hwang, C.; Jung, G.Y.; Bae, S.; Son, Y.; Cho, J.; Song, H.-K.; Kwak, S.K.; et al. Significance of ferroelectric polarization in poly (vinylidene difluoride) binder for high-rate Li-ion diffusion. Nano Energy 2017, 32, 255–262. [Google Scholar] [CrossRef]
- Hwang, C.; Song, W.-J.; Song, G.; Wu, Y.; Lee, S.; Son, H.B.; Kim, A.J.; Liu, N.; Park, S.; Song, H.-K. A Three-Dimensional Nano-web Scaffold of Ferroelectric Beta-PVDF Fibers for Lithium Metal Plating and Stripping. ACS Appl. Mater. Interfaces 2020, 12, 29235–29241. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Y.; Wang, H.; Dou, S.; Gan, W.; Ci, L.; Huang, Y.; Yuan, Q. MOF-based ionic sieve interphase for regulated Zn2+ flux toward dendrite-free aqueous zinc-ion batteries. J. Mater. Chem. 2022, 10, 4366–4375. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, T.; Feng, M.; Cui, Y.; Zhang, T.; Zhang, Y.; Feng, Y.; Zhang, Y.; Chi, Q.; Liu, X. Significantly Improved Energy Storage Performance of PVDF Ferroelectric Films by Blending PMMA and Filling PCBM. ACS Sustain. Chem. Eng. 2021, 9, 16291–16303. [Google Scholar] [CrossRef]
- Mayeen, A.; Kala, M.S.; Sunija, S.; Rouxel, D.; Bhowmik, R.N.; Thomas, S.; Kalarikkal, N. Flexible dopamine-functionalized BaTiO3/BaTiZrO3/BaZrO3-PVDF ferroelectric nanofibers for electrical energy storage. J. Alloys Compd. 2020, 837, 155492. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, T.; Yin, J.; Tian, Z.; Ma, Y.; Liu, Z.; Zhu, Y.; Alshareef, H.N. Controlled Deposition of Zinc-Metal Anodes via Selectively Polarized Ferroelectric Polymers. Adv. Mater. 2020, 34, 2106937. [Google Scholar] [CrossRef] [PubMed]
- Mai, M.; Ke, S.; Lin, P.; Zeng, X. Ferroelectric Polymer Thin Films for Organic Electronics. J. Nanomater. 2015, 2015, 812538. [Google Scholar] [CrossRef] [Green Version]
- Abrha, L.H.; Nikodimos, Y.; Weldeyohannes, H.H.; Hagos, T.T.; Wang, D.-Y.; Huang, C.-J.; Jiang, S.-K.; Wu, S.-H.; Su, W.-N.; Tsai, M.-C.; et al. Effects of a Thermally Electrochemically Activated β-PVDF Fiber on Suppression of Li Dendrite Growth for Anode-Free Batteries. ACS Appl. Energy Mater. 2021, 4, 3240–3248. [Google Scholar] [CrossRef]
- Li, C.; Qiu, M.; Li, R.; Li, X.; Wang, M.; He, J.; Lin, G.; Xiao, L.; Qian, Q.; Chen, Q.; et al. Electrospinning Engineering Enables High-Performance Sodium-Ion Batteries. Adv. Fiber Mater. 2022, 4, 43–65. [Google Scholar] [CrossRef]
- Li, R.; Wu, J.; He, J.; Li, X.; Mai, Y.; Chen, Y.; Li, X. Embedding amorphous SnS in electrospun porous carbon nanofibers for efficient potassium storage with ultralong cycle life. Compos. B Eng. 2022, 243, 110132. [Google Scholar] [CrossRef]
- Li, X.; Chen, W.; Qian, Q.; Huang, H.; Chen, Y.; Wang, Z.; Chen, Q.; Yang, J.; Li, J.; Mai, Y. Electrospinning-Based Strategies for Battery Materials. Adv. Energy Mater. 2021, 11, 2000845. [Google Scholar] [CrossRef]
- Xie, S.; Li, Y.; Li, X.; Zhou, Y.; Dang, Z.; Rong, J.; Dong, L. Stable Zinc Anodes Enabled by Zincophilic Cu Nanowire Networks. Nanomicro Lett 2021, 14, 39. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Yang, F.; Zeng, S.; Gao, X.; Liu, X.; Cao, X.; Yu, P.; Lu, X. Zincophilic Cu Sites Induce Dendrite-Free Zn Anodes for Robust Alkaline/Neutral Aqueous Batteries. Adv. Funct. Mater. 2022, 32, 2110829. [Google Scholar] [CrossRef]
- Xie, C.; Li, Y.; Wang, Q.; Sun, D.; Tang, T.; Wang, H. Issues and solutions toward zinc anode in aqueous zinc-ion batteries: A mini review. Carbon Energy 2020, 2, 540–560. [Google Scholar] [CrossRef]
- Ruan, L.; Yao, X.; Chang, Y.; Zhou, L.; Qin, G.; Zhang, X. Properties and Applications of the β Phase Poly(vinylidene fluoride). Polymers 2018, 10, 228. [Google Scholar] [CrossRef] [Green Version]
- Satthiyaraju, M.; Ramesh, T. Effect of annealing treatment on PVDF nanofibers for mechanical energy harvesting applications. Mater. Res. Express 2019, 6, 105366. [Google Scholar] [CrossRef]
- Poudel, A.; Fernandez, M.A.; Tofail, S.A.M.; Biggs, M.J.P. Boron Nitride Nanotube Addition Enhances the Crystallinity and Cytocompatibility of PVDF-TrFE. Front. Chem. 2019, 7, 364. [Google Scholar] [CrossRef]
- Wu, Y.; Du, X.; Gao, R.; Li, J.; Li, W.; Yu, H.; Jiang, Z.; Wang, Z.; Tai, H. Self-Polarization of PVDF Film Triggered by Hydrophilic Treatment for Pyroelectric Sensor with Ultra-Low Piezoelectric Noise. Nanoscale Res. Lett. 2019, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Yang, Z.; Ji, H.; Zeng, X.; Tang, Y.; Sun, D.; Wang, H. Issues and rational design of aqueous electrolyte for Zn-ion batteries. SusMat 2021, 1, 432–447. [Google Scholar] [CrossRef]
- Yang, J.; Cao, J.; Peng, Y.; Yang, W.; Barg, S.; Liu, Z.; Kinloch, I.A.; Bissett, M.A.; Dryfe, R.A.W. Unravelling the Mechanism of Rechargeable Aqueous Zn–MnO2 Batteries: Implementation of Charging Process by Electrodeposition of MnO2. ChemSusChem 2020, 13, 4103–4110. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Hoang, T.K.A.; Zhi, J.; Han, M.; Li, S.; Chen, P. Functioning Mechanism of the Secondary Aqueous Zn-β-MnO2 Battery. ACS Appl. Mater. Interfaces 2020, 12, 12834–12846. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhang, X.; Huang, Y.; Jiang, B.; Chang, Z.; Xu, C.; Kang, F. β-MnO2 with proton conversion mechanism in rechargeable zinc ion battery. J. Energy Chem. 2021, 56, 365–373. [Google Scholar] [CrossRef]
- Cui, Y.; Zhao, Q.; Wu, X.; Chen, X.; Yang, J.; Wang, Y.; Qin, R.; Ding, S.; Song, Y.; Wu, J.; et al. An Interface-Bridged Organic–Inorganic Layer That Suppresses Dendrite Formation and Side Reactions for Ultra-Long-Life Aqueous Zinc Metal Anodes. Angew. Chem. 2020, 132, 16737–16744. [Google Scholar]
- Cui, M.; Xiao, Y.; Kang, L.; Du, W.; Gao, Y.; Sun, X.; Zhou, Y.; Li, X.; Li, H.; Jiang, F.; et al. Quasi-Isolated Au Particles as Heterogeneous Seeds to Guide Uniform Zn Deposition for Aqueous Zinc-Ion Batteries. ACS Appl. Energy Mater. 2019, 2, 6490–6496. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhao, J.; Hu, Z.; Li, J.; Li, J.; Zhang, Y.; Wang, C.; Cui, G. Long-Life and Deeply Rechargeable Aqueous Zn Anodes Enabled by a Multifunctional Brightener-Inspired Interphase. Energy Environ. Sci. 2019, 12, 1938–1949. [Google Scholar] [CrossRef]
- Cai, Z.; Ou, Y.; Wang, J.; Xiao, R.; Fu, L.; Yuan, Z.; Zhan, R.; Sun, Y. Chemically Resistant Cu–Zn/Zn Composite Anode for Long Cycling Aqueous Batteries. Energy Storage Mater. 2020, 27, 205–211. [Google Scholar] [CrossRef]
- Hieu, L.T.; So, S.; Kim, I.T.; Hur, J. Zn Anode with Flexible β-PVDF Coating for Aqueous Zn-Ion Batteries with Long Cycle Life. Chem. Eng. J. 2021, 411, 128584. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, S.; Fang, G.; Sun, H.; Cao, X.; Zhou, J.; Pan, A.; Liang, S. Suppressing by-Product via Stratified Adsorption Effect to Assist Highly Reversible Zinc Anode in Aqueous Electrolyte. J. Energy Chem. 2021, 55, 549–556. [Google Scholar] [CrossRef]
- Xie, X.; Liang, S.; Gao, J.; Guo, S.; Guo, J.; Wang, C.; Xu, G.; Wu, X.; Chen, G.; Zhou, J. Manipulating the Ion-Transfer Kinetics and Interface Stability for High-Performance Zinc Metal Anodes. Energy Environ. Sci. 2020, 13, 503–510. [Google Scholar] [CrossRef]
- Cao, Z.; Zhu, X.; Xu, D.; Dong, P.; Chee, M.O.; Li, X.; Zhu, K.; Ye, M.; Shen, J. Eliminating Zn Dendrites by Commercial Cyanoacrylate Adhesive for Zinc Ion Battery. Energy Storage Mater. 2021, 36, 132–138. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.; Lv, Z.; Yang, H.; Zhang, Y.; Ye, M.; Chen, L.; Zhao, J.; Li, C.C. Synergistic Manipulation of Zn2+ Ion Flux and Desolvation Effect Enabled by Anodic Growth of a 3D ZnF2 Matrix for Long-Lifespan and Dendrite-Free Zn Metal Anodes. Adv. Mater. 2021, 33, 2007388. [Google Scholar] [CrossRef] [PubMed]
- Miao, Z.; Du, M.; Li, H.; Zhang, F.; Jiang, H.; Sang, Y.; Li, Q.; Liu, H.; Wang, S. Constructing Nano-Channeled Tin Layer on Metal Zinc for High-Performance Zinc-Ion Batteries Anode. EcoMat 2021, 3, e12125. [Google Scholar] [CrossRef]
- Ma, C.; Wang, X.; Lu, W.; Wang, C.; Yue, H.; Sun, G.; Zhang, D.; Du, F. Achieving Stable Zn Metal Anode via a Simple Nico Layered Double Hydroxides Artificial Coating for High Performance Aqueous Zn-Ion Batteries. Chem. Eng. J. 2022, 429, 132576. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, C.; Lv, Z.; Yang, H.; Cheng, X.; Zhang, S.; Ye, M.; Zhang, Y.; Chen, L.; Zhao, J.; et al. Redistributing Zn-Ion Flux by Interlayer Ion Channels in MG-Al Layered Double Hydroxide-Based Artificial Solid Electrolyte Interface for Ultra-Stable and Dendrite-Free Zn Metal Anodes. Energy Storage Mater. 2021, 41, 230–239. [Google Scholar] [CrossRef]
- Guo, Z.; Fan, L.; Zhao, C.; Chen, A.; Liu, N.; Zhang, Y.; Zhang, N. A Dynamic and Self-Adapting Interface Coating for Stable Zn-Metal Anodes. Adv. Mater. 2021, 34, 2105133. [Google Scholar] [CrossRef]
- Cao, J.; Zhang, D.; Gu, C.; Wang, X.; Wang, S.; Zhang, X.; Qin, J.; Wu, Z.S. Manipulating Crystallographic Orientation of Zinc Deposition for Dendrite-Free Zinc Ion Batteries. Adv. Energy Mater. 2021, 11, 2101299. [Google Scholar] [CrossRef]
- Wu, C.; Xie, K.; Ren, K.; Yang, S.; Wang, Q. Dendrite-Free Zn Anodes Enabled by Functional Nitrogen-Doped Carbon Protective Layers for Aqueous Zinc-Ion Batteries. Dalton Trans. 2020, 49, 17629–17634. [Google Scholar] [CrossRef]
- Zou, P.; Zhang, R.; Yao, L.; Qin, J.; Kisslinger, K.; Zhuang, H.; Xin, H.L. Ultrahigh-Rate and Long-Life Zinc–Metal Anodes Enabled by Self-Accelerated Cation Migration. Adv. Energy Mater. 2021, 11, 2100982. [Google Scholar] [CrossRef]
- Cao, P.; Zhou, X.; Wei, A.; Meng, Q.; Ye, H.; Liu, W.; Tang, J.; Yang, J. Fast-Charging and Ultrahigh-Capacity Zinc Metal Anode for High-Performance Aqueous Zinc-Ion Batteries. Adv. Funct. Mater. 2021, 31, 2100398. [Google Scholar] [CrossRef]
- Hao, J.; Li, B.; Li, X.; Zeng, X.; Zhang, S.; Yang, F.; Liu, S.; Li, D.; Wu, C.; Guo, Z. An in-Depth Study of Zn Metal Surface Chemistry for Advanced Aqueous Zn-Ion Batteries. Adv. Mater. 2020, 32, 2003021. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, J.; Liu, D.; Liu, M.; Zhou, T.; Qi, K.; Shi, L.; Zhu, Y.; Qian, Y. Ultra-Long-Life and Highly Reversible Zn Metal Anodes Enabled by a desolvation and deanionization Interface Layer. Energy Environ. Sci. 2021, 14, 3120–3129. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, G.; Park, H.; Seol, W.; Suh, S.; Jo, J.Y.; Kumar, S.; Kim, H.-J. Inhibition of Zinc Dendrites Realized by a β-P(VDF-TrFE) Nanofiber Layer in Aqueous Zn-Ion Batteries. Membranes 2022, 12, 1014. https://doi.org/10.3390/membranes12101014
Park G, Park H, Seol W, Suh S, Jo JY, Kumar S, Kim H-J. Inhibition of Zinc Dendrites Realized by a β-P(VDF-TrFE) Nanofiber Layer in Aqueous Zn-Ion Batteries. Membranes. 2022; 12(10):1014. https://doi.org/10.3390/membranes12101014
Chicago/Turabian StylePark, Geumyong, Hyeonghun Park, WooJun Seol, Seokho Suh, Ji Young Jo, Santosh Kumar, and Hyeong-Jin Kim. 2022. "Inhibition of Zinc Dendrites Realized by a β-P(VDF-TrFE) Nanofiber Layer in Aqueous Zn-Ion Batteries" Membranes 12, no. 10: 1014. https://doi.org/10.3390/membranes12101014