Graphene Oxide-Based Nanofiltration for Hg Removal from Wastewater: A Mini Review
Abstract
:1. Introduction
2. The Toxicity of Mercury and Its Removal
3. Membrane Separation for Mercury and Heavy Metals
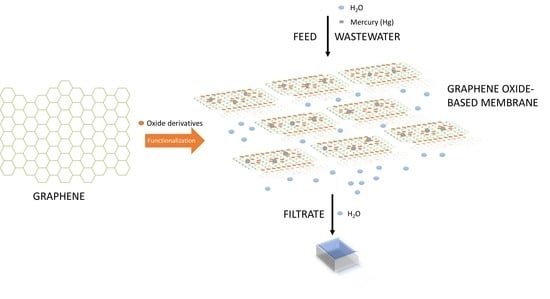
4. Graphene-Based Membranes
5. Graphene Oxide-Based Nanofiltration Membrane Preparation
6. Utilization of Graphene Oxide-Based Nanofiltration for Mercury Removal
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nies, D.H. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 1999, 51, 730–750. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Wagner-Döbler, I. Pilot plant for bioremediation of mercury-containing industrial wastewater. Appl. Microbiol. Biotechnol. 2003, 62, 124–133. [Google Scholar] [CrossRef]
- Barron-Zambrano, J.; Laborie, S.; Viers, P.; Rakib, M. Mercury removal from aqueous solutions by complexation—Ultrafiltration. Desalination 2002, 144, 201–206. [Google Scholar] [CrossRef]
- Jiang, G.-B.; Shi, J.-B.; Feng, X.-B. Mercury pollution in China. Environ. Sci. Technol. 2006, 40, 3672–3678. [Google Scholar] [CrossRef] [Green Version]
- Barringer, J.L.; Szabo, Z. Overview of investigations into mercury in ground water, soils, and septage. N. J. Coast. Plain Water Air Soil Pollut. 2006, 175, 193–221. [Google Scholar] [CrossRef]
- Lisha, K.; Pradeep, A.; Pradeep, T. Towards a practical solution for removing inorganic mercury from drinking water using gold nanoparticles. Gold Bull. 2009, 42, 144–152. [Google Scholar] [CrossRef] [Green Version]
- Heaven, S.; Ilyushchenko, M.; Tanton, T.; Ullrich, S. Mercury in the River Nura and its floodplain, Central Kazakhstan: I. River sediments and water. Sci. Total Environ. 2000, 260, 35–44. [Google Scholar] [CrossRef]
- Yan, H.; Feng, X.; Shang, L.; Qiu, G. The variations of mercury in sediment profiles from a historically mercury-contaminated reservoir, Guizhou province, China. Sci. Total Environ. 2008, 407, 497–506. [Google Scholar] [CrossRef]
- Liu, B.; Yan, H.; Wang, C.; Li, Q. Insights into low fish mercury bioaccumulation in a mercury-contaminated reservoir, Guizhou, China. Environ. Pollut. 2012, 160, 109–117. [Google Scholar] [CrossRef]
- Oehmen, A.; Vergel, D.; Fradinho, J.; Reis, M.A.M. Mercury removal from water streams through the ion exchange membrane bioreactor concept. J. Hazard. Mater. 2014, 264, 65–70. [Google Scholar] [CrossRef]
- Drioli, E.; Stankiewicz, A.I.; Macedonio, F. Membrane engineering in process intensification—An overview. J. Membr. Sci. 2011, 380, 1–8. [Google Scholar] [CrossRef]
- Strathmann, H.; Grabowski, A.; Eigenberger, G. Ion-exchange membranes in the chemical process industry. Ind. Eng. Chem. Res. 2013, 52, 10364–10379. [Google Scholar] [CrossRef]
- Wenten, I. Reverse osmosis applications: Prospect and challenges. Desalination 2016, 391, 112–125. [Google Scholar] [CrossRef]
- Himma, N.F.; Anisah, S.; Prasetya, N.; Wenten, I.G. Advances in preparation, modification, and application of polypropylene membrane. J. Polym. Eng. 2016, 36, 329–362. [Google Scholar] [CrossRef]
- Khoiruddin, K.; Hakim, A.; Wenten, I. Advances in electrodeionization technology for ionic separation—A review. Membr. Water Treat. 2014, 5, 87–108. [Google Scholar] [CrossRef]
- Wenten, I.G.; Victoria, A.V.; Tanukusuma, G.; Khoiruddin, K. Simultaneous clarification and dehydration of crude palm oil using superhydrophobic polypropylene membrane. J. Food Eng. 2019, 248, 23–27. [Google Scholar] [CrossRef]
- Makertihartha, I.; Dharmawijaya, P.T.; Zunita, M.; Wenten, I.G. Hydrogen Selective Layer for Dehydrogenation Membrane Reactor. Adv. Sci. Lett. 2017, 23, 5726–5728. [Google Scholar] [CrossRef]
- Wenten, I.G.; Syaifi, Y.S.; Saputra, F.A.; Zunita, M. Preparation of antibacterial and antifouling PSF/ZnO/eugenol membrane for peat water ultrafiltration. Water Supply 2019, 19, 2248–2255. [Google Scholar] [CrossRef]
- Criscuoli, A.; Basile, A.; Drioli, E.; Loiacono, O. An economic feasibility study for water gas shift membrane reactor. J. Membr. Sci. 2001, 181, 21–27. [Google Scholar] [CrossRef]
- Li, K. Ceramic Membranes for Separation and Reaction; John Wiley & Sons: Hoboken, NJ, USA, 2007. [Google Scholar]
- Zunita, M.; Makertihartha, I.; Saputra, F.; Syaifi, Y. Metal oxide based antibacterial membrane. IOP Conf. Ser. Mater. Sci. Eng. 2018, 395, 012021. [Google Scholar] [CrossRef]
- Makertihartha, I.; Zunita, M.; Rizki, Z.; Dharmawijaya, P. Solvent extraction of gold using ionic liquid based process. AIP Conf. Proc. 2017, 1805. [Google Scholar] [CrossRef] [Green Version]
- Makertihartha, I.; Zunita, M.; Dharmawijaya, P.; Wenten, I. Supported ionic liquid membrane in membrane reactor. AIP Conf. Proc. 2017, 1788. [Google Scholar] [CrossRef] [Green Version]
- Makertihartha, I.; Rizki, Z.; Zunita, M.; Dharmawijaya, P. Dyes removal from textile wastewater using graphene based nanofiltration. AIP Conf. Proc. 2017, 1840. [Google Scholar] [CrossRef]
- Tsetseris, L.; Pantelides, S.T. Graphene: An impermeable or selectively permeable membrane for atomic species? Carbon 2014, 67, 58–63. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Zunita, M.; Irawanti, R.; Koesmawati, T.A.; Lugito, G. Graphene Oxide (Go) Membrane in Removing Heavy Metals From Wastewater: A Review. Chem. Eng. Trans. 2020, 82, 415–420. [Google Scholar]
- Makertihartha, I.; Rizki, Z.; Zunita, M.; Dharmawijaya, P.T. Graphene Based Nanofiltration for Mercury Removal from Aqueous Solutions. Adv. Sci. Lett. 2017, 23, 5684–5686. [Google Scholar] [CrossRef]
- Makertiharta, I.; Dharmawijaya, P.; Zunita, M.; Wenten, I. Rare earth element enrichment using membrane based solvent extraction. AIP Conf. Proc. 2017, 1805. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Liu, Y.; Zhao, Y.; Zhang, X. Selective separation of metal ions via monolayer nanoporous graphene with carboxyl groups. Anal. Chem. 2016, 88, 10002–10010. [Google Scholar] [CrossRef]
- Jin, B.; Zhang, X.; Li, F.; Zhang, N. Influence of nanopore density on ethylene/acetylene separation by monolayer graphene. Phys. Chem. Chem. Phys. 2019, 21, 6126–6132. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, H.; Xia, J.; Zhang, F. Novel GO-blended PVDF ultrafiltration membranes. Desalination 2012, 299, 50–54. [Google Scholar] [CrossRef]
- Chae, H.-R.; Lee, J.; Lee, C.-H.; Kim, I.-C. Graphene oxide-embedded thin-film composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance. J. Membr. Sci. 2015, 483, 128–135. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Shao, X.; Liu, S. Dual fluorescence of graphene oxide: A time-resolved study. J. Phys. Chem. A 2012, 116, 7308–7313. [Google Scholar] [CrossRef]
- Wei, Y.; Zhang, Y.; Gao, X.; Yuan, Y. Declining flux and narrowing nanochannels under wrinkles of compacted graphene oxide nanofiltration membranes. Carbon 2016, 108, 568–575. [Google Scholar] [CrossRef]
- Namasivayam, C.; Kadirvelu, K. Uptake of mercury (II) from wastewater by activated carbon from an unwanted agricultural solid by-product: Coirpith. Carbon 1999, 37, 79–84. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Galbreath, K.C.; Zygarlicke, C.J. Mercury speciation in coal combustion and gasification flue gases. Environ. Sci. Technol. 1996, 30, 2421–2426. [Google Scholar] [CrossRef]
- Presto, A.A.; Granite, E.J. Survey of catalysts for oxidation of mercury in flue gas. Environ. Sci. Technol. 2006, 40, 5601–5609. [Google Scholar] [CrossRef]
- Lee, W.; Bae, G.-N. Removal of elemental mercury (Hg (0)) by nanosized V2O5/TiO2 catalysts. Environ. Sci. Technol. 2009, 43, 1522–1527. [Google Scholar] [CrossRef]
- Yang, S.; Guo, Y.; Yan, N.; Wu, D. Nanosized Cation-Deficient Fe− Ti Spinel: A Novel Magnetic Sorbent for Elemental Mercury Capture from Flue Gas. ACS Appl. Mater. Interfaces 2011, 3, 209–217. [Google Scholar] [CrossRef]
- Kellie, S.; Cao, Y.; Duan, Y.; Li, L. Factors affecting mercury speciation in a 100-MW coal-fired boiler with low-NO x burners. Energy Fuels 2005, 19, 800–806. [Google Scholar] [CrossRef]
- Eom, Y.; Jeon, S.H.; Ngo, T.A.; Kim, J. Heterogeneous mercury reaction on a selective catalytic reduction (SCR) catalyst. Catal. Lett. 2008, 121, 219–225. [Google Scholar] [CrossRef]
- Hu, C.-X.; Zhou, J.S.; Luo, Z.Y.; Sheng, H. Effect of oxidation treatment on the adsorption and the stability of mercury on activated carbon. J. Environ. Sci. 2006, 18, 1161–1166. [Google Scholar] [CrossRef]
- Mergler, D.; Anderson, H.A.; Chan, L.H.M.; Mahaffey, K.R. Methylmercury exposure and health effects in humans: A worldwide concern. AMBIO 2007, 36, 3–11. [Google Scholar] [CrossRef]
- Jin, L.; Zhang, L.; Li, Z.; Liu, J.M. Placental concentrations of mercury, lead, cadmium, and arsenic and the risk of neural tube defects in a Chinese population. Reprod. Toxicol. 2013, 35, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Fang, G.-C.; Yang, I.-L.; Liu, C.-K. Measure and modeling the ambient air particles and particle bound mercury Hg (p) at a traffic sampling site. Atmos. Res. 2010, 97, 97–105. [Google Scholar] [CrossRef]
- Chen, S.-J.; Lo, C.-T.; Fang, G.-C.; Huang, C.-S. Particulate-bound mercury (Hg [p]) size distributions in Central Taiwan. Environ. Forensics 2012, 13, 98–104. [Google Scholar] [CrossRef]
- Silvo, K.; Melanen, M.; Honkasalo, A.; Ruonala, S. Integrated pollution prevention and control—the Finnish approach. Resour. Conserv. Recycl. 2002, 35, 45–60. [Google Scholar] [CrossRef]
- Ritter, J.A.; Bibler, J. Removal of mercury from waste water: Large-scale performance of an ion exchange process. Water Sci. Technol. 1992, 25, 165–172. [Google Scholar] [CrossRef]
- Bailey, S.E.; Olin, T.J.; Bricka, R.M.; Adrian, D.D. A review of potentially low-cost sorbents for heavy metals. Water Res. 1999, 33, 2469–2479. [Google Scholar] [CrossRef]
- Zhang, H.-L.; Cai, H.; Xia, Y.; Zhang, P. An l-cystine/l-cysteine impregnated nanofiltration membrane with the superior performance of an anchoring heavy metal in wastewater. RSC Adv. 2020, 10, 3438–3449. [Google Scholar] [CrossRef]
- Fiskum, S.K.; Rapko, B.M.; Lumetta, G.J. Partitioning of mercury from actinides in the TRUEX process. Solvent Extr. Ion Exch. 2001, 19, 643–657. [Google Scholar] [CrossRef]
- Volchek, K.; Krentsel, E.; Zhilin, Y.; Shtereva, G. Polymer binding/ultrafiltration as a method for concentration and separation of metals. J. Membr. Sci. 1993, 79, 253–272. [Google Scholar] [CrossRef]
- Chaufer, B.; Deratani, A. Removal of metal ions by complexation-ultrafiltration using water-soluble macromolecules: Perspective of application to wastewater treatment. Nucl. Chem. Waste Manag. 1988, 8, 175–187. [Google Scholar] [CrossRef]
- Olson, E.; Miller, S.; Sharma, R.; Dunham, G. Catalytic effects of carbon sorbents for mercury capture. J. Hazard. Mater. 2000, 74, 61–79. [Google Scholar] [CrossRef]
- Granite, E.J.; Pennline, H.W.; Hargis, R.A. Novel sorbents for mercury removal from flue gas. Ind. Eng. Chem. Res. 2000, 39, 1020–1029. [Google Scholar] [CrossRef]
- Hall, B.; Schager, P.; Weesmaa, J. The homogeneous gas phase reaction of mercury with oxygen, and the corresponding heterogeneous reactions in the presence of activated carbon and fly ash. Chemosphere 1995, 30, 611–627. [Google Scholar] [CrossRef]
- Baldeck, C.M.; Kalb, G.W.; Crist, H.L. Determination of elemental mercury in an emission source having a high sulfur dioxide concentration by amalgamation with gold and ultraviolet spectrophotometry. Anal. Chem. 1974, 46, 1500–1505. [Google Scholar] [CrossRef]
- Aeschliman, D.B.; Norton, G.A. Collection and thermal evolution behaviors of different mercury species captured with gold. Environ. Sci. Technol. 1999, 33, 2278–2283. [Google Scholar] [CrossRef]
- Ghorishi, S.B.; Lee, C.W.; Jozewicz, W.S.; Kilgroe, J.D. Effects of fly ash transition metal content and flue gas HCl/SO2 ratio on mercury speciation in waste combustion. Environ. Eng. Sci. 2005, 22, 221–231. [Google Scholar] [CrossRef]
- Wilcox, J.; Rupp, E.; Ying, S.C.; Lim, D.-H. Mercury adsorption and oxidation in coal combustion and gasification processes. Int. J. Coal Geol. 2012, 90, 4–20. [Google Scholar] [CrossRef]
- Ling, L.; Fan, M.; Wang, B.; Zhang, R. Application of computational chemistry in understanding the mechanisms of mercury removal technologies: A review. Energy Environ. Sci. 2015, 8, 3109–3133. [Google Scholar] [CrossRef]
- Spencer, N.; Lambert, R. Chlorine chemisorption and surface chloride formation on Au (111). Surf. Sci. 1981, 107, 237–248. [Google Scholar] [CrossRef]
- Xu, W.; Tong, L.; Qi, H.; Zhou, X. Effect of Flue Gas Components on Hg0 Oxidation over Fe/HZSM-5 Catalyst. Ind. Eng. Chem. Res. 2014, 54, 146–152. [Google Scholar] [CrossRef]
- Fan, X.; Li, C.; Zeng, G.; Zhang, X. The effects of Cu/HZSM-5 on combined removal of Hg 0 and NO from flue gas. Fuel Process. Technol. 2012, 104, 325–331. [Google Scholar] [CrossRef]
- Li, Q.; Sun, L.; Zhang, Y.; Qian, Y. Characteristics of equilibrium, kinetics studies for adsorption of Hg(II) and Cr(VI) by polyaniline/humic acid composite. Desalination 2011, 266, 188–194. [Google Scholar] [CrossRef]
- Wang, J.; Deng, B.; Chen, H.; Wang, X. Removal of Aqueous Hg(II) by Polyaniline: Sorption Characteristics and Mechanisms. Environ. Sci. Technol. 2009, 43, 5223–5228. [Google Scholar] [CrossRef]
- Sgarlata, C.; Arena, G.; Longo, E.; Zhang, D. Heavy metal separation with polymer inclusion membranes. J. Membr. Sci. 2008, 323, 444–451. [Google Scholar] [CrossRef]
- Chakrabarty, K.; Saha, P.; Ghoshal, A.K. Simultaneous separation of mercury and lignosulfonate from aqueous solution using supported liquid membrane. J. Membr. Sci. 2010, 346, 37–44. [Google Scholar] [CrossRef]
- Li, R.; Liu, L.; Yang, F. Removal of aqueous Hg(II) and Cr(VI) using phytic acid doped polyaniline/cellulose acetate composite membrane. J. Hazard. Mater. 2014, 280, 20–30. [Google Scholar] [CrossRef]
- Pancharoen, U.; Somboonpanya, S.; Chaturabul, S.; Lothongkum, A.W. Selective removal of mercury as HgCl 4 2− from natural gas well produced water by TOA via HFSLM. J. Alloy. Compd. 2010, 489, 72–79. [Google Scholar] [CrossRef]
- Shamsipur, M.; Hashemi, O.R.; Lippolis, V. A supported liquid membrane system for simultaneous separation of silver (I) and mercury (II) from dilute feed solutions. J. Membr. Sci. 2006, 282, 322–327. [Google Scholar] [CrossRef]
- Zulfikar, M.; Maulina, D.; Nasir, M.; Alni, A. Poly (acrylic acid)/SiO2 composite nanofiber functionalized with mercapto groups for the removal of humic acid from aqueous solution. Desalin. Water Treat. 2019, 141, 115–123. [Google Scholar] [CrossRef]
- Boricha, A.G.; Murthy, Z. Acrylonitrile butadiene styrene/chitosan blend membranes: Preparation, characterization and performance for the separation of heavy metals. J. Membr. Sci. 2009, 339, 239–249. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, X.; Tan, H.; Qi, W. Combustion fabrication of nanoporous graphene for ionic separation membranes. Adv. Funct. Mater. 2018, 28, 1805026. [Google Scholar] [CrossRef]
- Tan, H.; Liu, T.; Zhang, X.; Shan, Q. Preparation of vortex porous graphene chiral membrane for enantioselective separation. Anal. Chem. 2020, 92, 13630–13633. [Google Scholar] [CrossRef]
- Tan, H.; Zhang, X.; Li, Z.; Liang, Q. Nitrogen-doped nanoporous graphene induced by a multiple confinement strategy for membrane separation of rare earth. Iscience 2021, 24, 101920. [Google Scholar] [CrossRef]
- Azamat, J.; Khataee, A.; Joo, S.W. Functionalized graphene as a nanostructured membrane for removal of copper and mercury from aqueous solution: A molecular dynamics simulation study. J. Mol. Graph. Model. 2014, 53, 112–117. [Google Scholar] [CrossRef]
- Mercader-Trejo, F.E.; Rodríguez de San Miguel, E.; de Gyves, J. Mercury (II) removal using polymer inclusion membranes containing Cyanex 471X. J. Chem. Technol. Biotechnol. 2009, 84, 1323–1330. [Google Scholar] [CrossRef]
- Brinchi, L.; Germani, R.; Mancini, M.V.; Savelli, G. Carrier-Mediated Transport of Toxic Heavy Metal Ions in Bulk Liquid Membranes. Eur. J. Org. Chem. 2004, 2004, 1330–1335. [Google Scholar] [CrossRef]
- Gupta, S.; Chakraborty, M.; Murthy, Z. Removal of mercury by emulsion liquid membranes: Studies on emulsion stability and scale up. J. Dispers. Sci. Technol. 2013, 34, 1733–1741. [Google Scholar] [CrossRef]
- Visser, H.C.; Reinhoudt, D.N.; de Jong, F. Carrier-mediated transport through liquid membranes. Chem. Soc. Rev. 1994, 23, 75–81. [Google Scholar] [CrossRef] [Green Version]
- Sirlin, C.; Burgard, M.; Leroy, M.; Prevost, M. Silver nitrate refining using supported liquid membranes. J. Membr. Sci. 1990, 54, 299–305. [Google Scholar] [CrossRef]
- Cahn, R.; Li, N. Separation of phenol from waste water by the liquid membrane technique. Sep. Sci. 1974, 9, 505–519. [Google Scholar] [CrossRef]
- Rajasimman, M.; Sangeetha, R.; Karthik, P. Statistical optimization of process parameters for the extraction of chromium (VI) from pharmaceutical wastewater by emulsion liquid membrane. Chem. Eng. J. 2009, 150, 275–279. [Google Scholar] [CrossRef]
- Matos, C.T.; Velizarov, S.; Crespo, J.G.; Reis, M.A.M. Simultaneous removal of perchlorate and nitrate from drinking water using the ion exchange membrane bioreactor concept. Water Res. 2006, 40, 231–240. [Google Scholar] [CrossRef]
- Hanif, Z.; Lee, S.; Qasim, G.H.; Ardiningsih, I. Polypyrrole multilayer-laminated cellulose for large-scale repeatable mercury ion removal. J. Mater. Chem. A 2016, 4, 12425–12433. [Google Scholar] [CrossRef]
- Khan, A.A.; Alam, M.M. New and novel organic–inorganic type crystalline ‘polypyrrolel/polyantimonic acid’composite system: Preparation, characterization and analytical applications as a cation-exchange material and Hg (II) ion-selective membrane electrode. Anal. Chim. Acta 2004, 504, 253–264. [Google Scholar] [CrossRef]
- Nakagawa, R.; Yumita, Y. Change and behavior of residual mercury in paddy soils and rice of Japan. Chemosphere 1998, 37, 1483–1487. [Google Scholar] [CrossRef]
- Han, D.S.; Orillano, M.; Khodary, A.; Duan, Y. Reactive iron sulfide (FeS)-supported ultrafiltration for removal of mercury (Hg(II)) from water. Water Res. 2014, 53, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Siva, S.; Sudharsan, S.; Kannan, R.S. Synthesis, characterization and ion-exchange properties of novel hybrid polymer nanocomposites for selective and effective mercury (ii) removal. RSC Adv. 2015, 5, 79665–79678. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, S.; Streets, D.G.; Hao, J. Trends in Anthropogenic Mercury Emissions in China from 1995 to 2003. Environ. Sci. Technol. 2006, 40, 5312–5318. [Google Scholar] [CrossRef]
- Koopman, C.; Witkamp, G. Extraction of heavy metals from industrial phosphoric acid in a transverse flow hollow fiber membrane contactor. Sep. Sci. Technol. 2002, 37, 1273–1290. [Google Scholar] [CrossRef]
- Guo, Y.; Yan, N.; Liu, P.; Yang, S. Removal of elemental mercury with Mn/Mo/Ru/Al2O3 membrane catalytic system. Front. Environ. Sci. Eng. 2013, 7, 464–473. [Google Scholar] [CrossRef]
- Huang, Y.; Du, J.R.; Zhang, Y.; Lawless, D. Removal of mercury (II) from wastewater by polyvinylamine-enhanced ultrafiltration. Sep. Purif. Technol. 2015, 154, 1–10. [Google Scholar] [CrossRef]
- Müslehiddinoğlu, J.; Uludağ, Y.; Özbelge, H.Ö.; Yilmaz, L. Effect of operating parameters on selective separation of heavy metals from binary mixtures via polymer enhanced ultrafiltration. J. Membr. Sci. 1998, 140, 251–266. [Google Scholar] [CrossRef]
- Spreti, N.; Brinchi, L.; Germani, R.; Mancini, M.V. A new carrier for selective removal of heavy metal ions from aqueous solutions through bulk liquid membranes. Eur. J. Org. Chem. 2004, 2004, 3865–3871. [Google Scholar] [CrossRef]
- Wang, B.; Yang, X.; Huang, W. Preparation and recovery of polysulfone affinity membrane with mercapto as chelating group for Hg2+ cations. J. Appl. Polym. Sci. 2007, 103, 2514–2522. [Google Scholar] [CrossRef]
- Meeks, N.D.; Davis, E.; Jain, M.; Skandan, G. Mercury removal by thiol-functionalized metal oxide–carbon black sorbent and mixed-matrix membranes. Environ. Prog. Sustain. Energy 2013, 32, 705–714. [Google Scholar] [CrossRef]
- Urgun-Demirtas, M.; Benda, P.L.; Gillenwater, P.S.; Negri, M.C. Achieving very low mercury levels in refinery wastewater by membrane filtration. J. Hazard. Mater. 2012, 215-216, 98–107. [Google Scholar] [CrossRef]
- Huang, X.; Yin, Z.; Wu, S.; Qi, X. Graphene-based materials: Synthesis, characterization, properties, and applications. Small 2011, 7, 1876–1902. [Google Scholar] [CrossRef]
- Li, H.; Song, Z.; Zhang, X.; Huang, Y.; Li, S.; Mao, Y.; Ploehn, H.J.; Bao, Y.; Yu, M. Ultrathin, Molecular-Sieving Graphene Oxide Membranes for Selective Hydrogen Separation. Science 2013, 342, 95–98. [Google Scholar] [CrossRef]
- Jankovský, O.; Marvan, P.; Nováček, M.; Luxa, J. Synthesis procedure and type of graphite oxide strongly influence resulting graphene properties. Appl. Mater. Today 2016, 4, 45–53. [Google Scholar] [CrossRef]
- Frank, I.W.; Tanenbaum, D.M.; Van Der Zande, A.M.; McEuen, P.L. Mechanical properties of suspended graphene sheets. J. Vac. Sci. Technol. B Microelectron. Nanometer Struct. 2007, 25, 2558–2561. [Google Scholar] [CrossRef] [Green Version]
- Falkovsky, L.A. Optical properties of graphene. J. Phys. Conf. Ser. 2008, 129, 012004. [Google Scholar] [CrossRef]
- Castro Neto, A.H.; Guinea, F.; Peres, N.M.R.; Novoselov, K.S. The electronic properties of graphene. Rev. Mod. Phys. 2009, 81, 109–162. [Google Scholar] [CrossRef] [Green Version]
- Petridis, C.; Konios, D.; Stylianakis, M.M.; Kakavelakis, G. Solution processed reduced graphene oxide electrodes for organic photovoltaics. Nanoscale Horiz. 2016, 1, 375–382. [Google Scholar] [CrossRef]
- Xu, C.; Cui, A.; Xu, Y.; Fu, X. Graphene oxide—TiO2 composite filtration membranes and their potential application for water purification. Carbon 2013, 62, 465–471. [Google Scholar] [CrossRef]
- Ai, L.; Zhang, C.; Chen, Z. Removal of methylene blue from aqueous solution by a solvothermal-synthesized graphene/magnetite composite. J. Hazard. Mater. 2011, 192, 1515–1524. [Google Scholar] [CrossRef]
- Galán-vidal, C.A.; Romero-romo, M.; Palomar-pardave, M. Mercury Ions Removal from Aqueous Solution Using an Activated Composite Membrane Mercury Ions Removal from Aqueous Solution Using an Activated Composite Membrane. Environ. Sci. Technol. 2005. [Google Scholar] [CrossRef]
- Park, S.; Lee, K.-S.; Bozoklu, G.; Cai, W. Graphene oxide papers modified by divalent ions-enhancing mechanical properties via chemical cross-linking. ACS Nano 2008, 2, 572–578. [Google Scholar] [CrossRef]
- Rafiee, M.A.; Rafiee, J.; Wang, Z.; Song, H. Enhanced Mechanical Properties of Nanocomposites at Low Graphene Content. ACS Nano 2009, 3, 3884–3890. [Google Scholar] [CrossRef]
- Edwards, R.S.; Coleman, K.S. Graphene synthesis: Relationship to applications. Nanoscale 2013, 5, 38–51. [Google Scholar] [CrossRef]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Shahriary, L.; Athawale, A.A. Graphene Oxide Synthesized by using Modified Hummers Approach. Int. J. Renew. Energy Environ. Eng. 2014, 02, 58–63. [Google Scholar]
- Kaniyoor, A.; Baby, T.T.; Ramaprabhu, S. Graphene synthesis via hydrogen induced low temperature exfoliation of graphite oxide. J. Mater. Chem. 2010, 20, 8467. [Google Scholar] [CrossRef]
- Ai, L.; Jiang, J. Removal of methylene blue from aqueous solution with self-assembled cylindrical graphene—carbon nanotube hybrid. Chem. Eng. J. 2012, 192, 156–163. [Google Scholar] [CrossRef]
- Jiao, T.; Guo, H.; Zhang, Q.; Peng, Q. Reduced Graphene Oxide-Based Silver Nanoparticle-Containing Composite Hydrogel as Highly Efficient Dye Catalysts for Wastewater Treatment. Nat. Publ. Group 2015. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.; Jin, W.; Xu, N. Graphene-Based Membranes. Chem. Soc. Rev. 2015, 44, 5016–5030. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Li, J.; Zhang, J.; Su, G. Separation of Hydrogen and Nitrogen Gases with Porous Graphene Membrane Separation of Hydrogen and Nitrogen Gases with Porous Graphene Membrane. J. Phys. Chem. C 2011, 115, 23261–23266. [Google Scholar] [CrossRef]
- Schrier, J. Helium separation using porous graphene membranes. J. Phys. Chem. Lett. 2010, 1, 2284–2287. [Google Scholar] [CrossRef]
- Blankenburg, S.; Bieri, M.; Fasel, R.; Müllen, K. Porous graphene as an atmospheric nanofilter. Nanoporous Mater. 2010, 6, 2266–2271. [Google Scholar] [CrossRef] [PubMed]
- Jiang, D.E.; Cooper, V.R.; Dai, S. Porous gaphene as the ultimate membrane for gas separation. Nano Lett. 2009, 9, 4019–4024. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, S.; Gao, J.; Chung, T.S. Layer-by-layer construction of graphene oxide (GO) framework composite membranes for highly ef fi cient heavy metal removal. J. Membr. Sci. 2016, 515, 230–237. [Google Scholar] [CrossRef]
- Schaepe, S. Engineering Graphene Oxide Membranes for Contaminant Removal and Bacterial Inactivation. Master’s Thesis, University of Nebraska Lincoln, Lincoln, NE, USA, 2015. [Google Scholar]
- Wang, J.; Tsuzuki, T.; Tang, B.; Sun, L.; Dai, X.J.; Rajmohan, G.D.; Li, J.; Wang, X. Recyclable textiles functionalized with reduced graphene oxide@ ZnO for removal of oil spills and dye pollutants. Aust. J. Chem. 2014, 67, 71–77. [Google Scholar] [CrossRef]
- Wei, N.; Peng, X.; Xu, Z. Understanding Water Permeation in Graphene Oxide Membranes. ACS Appl. Mater. Interfaces 2014, 6, 5877–5883. [Google Scholar] [CrossRef] [Green Version]
- Chong, J.Y.; Wang, B.; Li, K. Graphene oxide membranes in fluid separations. Sep. Eng. 2016, 12, 98–105. [Google Scholar] [CrossRef]
- Li, X.; Wang, Z.; Li, Q.; Ma, J. Preparation, characterization, and application of mesoporous silica-grafted graphene oxide for highly selective lead adsorption. Chem. Eng. J. 2015, 273, 630–637. [Google Scholar] [CrossRef]
- Hu, M.; Mi, B. Enabling Graphene Oxide Nanosheets as Water Separation Membranes. Environ. Sci. Technol. 2013, 47, 3715–3723. [Google Scholar] [CrossRef]
- Huang, H.; Ying, Y.; Peng, X. Graphene oxide nanosheet: An emerging star material for novel separation membranes. J. Mater. Chem. A Mater. Energy Sustain. 2014, 2, 13772–13782. [Google Scholar] [CrossRef]
- Chong, J.Y.; Aba, N.F.D.; Wang, B.; Mattevi, C. UV-Enhanced Sacrificial Layer Stabilised Graphene Oxide Hollow Fibre Membranes for Nanofiltration. Nature 2015. [Google Scholar] [CrossRef] [Green Version]
- Aba, N.F.D.; Yi, J.; Wang, B.; Mattevi, C. Graphene oxide membranes on ceramic hollow fibers—Microstructural stability and nano fi ltration performance. J. Membr. Sci. 2015, 484, 87–94. [Google Scholar] [CrossRef]
- Han, Y.; Xu, Z.; Gao, C. Ultrathin Graphene Nanofi ltration Membrane for Water Purifi cation. Anvanced Funct. Mater. 2013, 23, 3693–3700. [Google Scholar] [CrossRef]
- Han, Y.; Jiang, Y.; Gao, C. High-Flux Graphene Oxide Nano fi ltration Membrane Intercalated by Carbon Nanotubes. Appl. Mater. Interfaces 2015, 7, 8147–8155. [Google Scholar] [CrossRef]
- Nan, Q.; Li, P.; Cao, B. Applied Surface Science Fabrication of positively charged nanofiltration membrane via the layer-by-layer assembly of graphene oxide and polyethylenimine for desalination. Appl. Surf. Sci. 2016, 387, 521–528. [Google Scholar] [CrossRef]
- Aghigh, A.; Alizadeh, V.; Wong, H.Y.; Islam, S. Recent advances in utilization of graphene for fi ltration and desalination of water: A review. Desalination 2015, 365, 389–397. [Google Scholar] [CrossRef]
- Azamat, J.; Shirforush, B.; Khataee, A.; Woo, S. Removal of a hazardous heavy metal from aqueous solution using functionalized graphene and boron nitride nanosheets: Insights from simulations. J. Mol. Graph. Model. 2015, 61, 13–20. [Google Scholar] [CrossRef]
- Mahmoud, K.A.; Mansoor, B.; Mansour, A.; Khraisheh, M. Functional graphene nanosheets: The next generation membranes for water desalination. DES 2015, 356, 208–225. [Google Scholar] [CrossRef]
- Liu, F.; Chung, S.; Oh, G.; Seo, T.S. Three-Dimensional Graphene Oxide Nanostructure for Fast and Efficient Water-Soluble Dye Removal. Appl. Mater. Interfaces 2012, 4, 922–927. [Google Scholar] [CrossRef]
- Kumar, V.S.; Hariharan, K.S.; Mayya, K.S.; Han, S. Volume averaged reduced order Donnan Steric Pore Model for nano fi ltration membranes. DES 2013, 322, 21–28. [Google Scholar] [CrossRef]
- Jiao, T.; Liu, Y.; Wu, Y.; Zhang, Q. Facile and Scalable Preparation of Graphene Oxide-Based Magnetic Hybrids for Fast and Highly Efficient Removal of Organic Dyes. Nat. Publ. Group 2015. [Google Scholar] [CrossRef] [Green Version]
- Zunita, M.; Makertiharta, I.; Irawanti, R.; Prasetya, N. Graphene oxide-inorganic composite membrane: A review. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2018. [Google Scholar]
- Barron-Zambrano, J.; Laborie, S.; Viers, P.; Rakib, M. Mercury removal and recovery from aqueous solutions by coupled complexation-ultrafiltration and electrolysis. J. Membr. Sci. 2004, 229, 179–186. [Google Scholar] [CrossRef]
- Babu, C.M.; Vinodh, R.; Abidov, A. Removal of heavy metals using Amine crosslinked Reduced Graphene Oxide. Adv. Sci. Technol. Lett. 2015, 120, 430–433. [Google Scholar]
- Cui, L.; Guo, X.; Wei, Q.; Wang, Y. Removal of mercury and methylene blue from aqueous solution by xanthate functionalized magnetic graphene oxide: Sorption kinetic and uptake mechanism. J. Colloid Interface Sci. 2015, 439, 112–120. [Google Scholar] [CrossRef]
- Kabiri, S.; Tran, D.N.H.; Cole, M.A.; Losic, D. Functionalized three-dimensional (3D) graphene composite for high efficiency removal of mercury. Environ. Sci. Water Res. Technol. 2016, 2, 390–402. [Google Scholar] [CrossRef]
- Hartanto, Y.; Yaswari, Y.; Zunita, M.; Soerawidjaja, T.H. Decolorization of crude terpineol by adsorption. Sep. Sci. Technol. 2017, 52, 1967–1972. [Google Scholar] [CrossRef]
- Ziaei, E.; Mehdinia, A.; Jabbari, A. A novel hierarchical nanobiocomposite of graphene oxide-magnetic chitosan grafted with mercapto as a solid phase extraction sorbent for the determination of mercury ions in environmental water samples. Anal. Chim. Acta 2014, 850, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Henriques, B.; Goncalves, G.; Emami, N.; Pereira, E. Optimized graphene oxide foam with enhanced performance and high selectivity for mercury removal from water. J. Hazard. Mater. 2016, 301, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Kong, L.; Liu, J. Removal of mercury and fluoride from aqueous solutions by three-dimensional reduced-graphene oxide aerogel. Res. Chem. Intermed. 2016, 42, 4513–4530. [Google Scholar] [CrossRef]




| Membrane | Mixture Components | Separation Conditions | Rejection % or Removal % | Ref. |
|---|---|---|---|---|
| Two micro-porous PP supported membrane loaded with a mixed N/O/S-donor | Ag+ and Hg2+ | Na2S2O3 (0.04 M) and EDTA disodium salt (0.025 M) as stripping agents in 3.5 h | 95.3% Ag+ and 94.7% Hg2+ | [75] |
|
30% cellulose triacetate (CTA), 60% 2-nitrophenyl octyl ether (NPOE), and 10% w/w Cyanex 471 | HgCl2 |
Hg [2+] in HCl + NaCl at pH 12. | 81 % | [82] |
| D2EHPA (CAS No. 298-07-7) with 98.5% purity | HgCl2 | 1 M H2SO4 with 0.5 M thiourea | 92% | [84] |
| polyethyleneimine (PEI) | Hg in a heavy metal mixture | pH 5.5 cadmium/polymer ratio about 0.35 mercury/polymer ratio about 0.39 | 98% Hg and 97% Cd | [99] |
| 1,1,7,7-tetraethyl 4(tetradecyl)diethylenetriamine (TE14DT) |
Cd2+/Pb2+ and Hg2+/
Cu2+ mixtures | pH 2.5 | 90% | [100] |
| poly(benzylsulfone) | Hg2+ | Diluted in hydrochloric acid | >90% | [101] |
| Mixed-matrix membranes (sorbent particles and polysulfone) | Ca2+, Ag+, Hg2+ | Diluted in HCl at pH 4 | 95% Hg2+ | [102] |
| Cyanex 302 (bis(2,4,4-trimethyl- pentyl)thiophosphinic acid) in kerosene | Cu2+ and Hg2+ | Diluted in phosphoric acid slurry | 70% | [96] |
| Mn/Mo/Ru/Al2O3 membrane | Hg | Diluted in hydrochloric acid | 95% | [97] |
| Polyvinylamine | mercury - sodium chloride and sulfate | feed mercury concentration range tested (0–50 ppm) | 99% | [98] |
| Cross flow membrane filtration cell (CF 042, Sterlitech, California) | Hg2+ | higher operating pressures (≥34.5 bar) | 95% | [103] |
| GO Membrane Type | Components Mixture | Separation Conditions | Rejection % or Removal % of Mercury | Ref. |
|---|---|---|---|---|
| Xanthate functionalized magnetic graphene oxide (Fe3O4-xGO) | Hg2+ and methylene blue | pH: 7.5, 3 h, 298 K, 1 atm | 94.5% | [149] |
| Graphene-Diatom (GN-DE) Hydrogel Decorated with αFeOOH Nanoparticles | Hg2+ | pH: 10, 90 min, 298 K, 1 atm, pore size: 0.22 µm | 80% | [150] |
| mercapto-grafted graphene oxide–magnetic chitosan (GO–MC) | Hg2+ in environmental water samples | 60 mg of sorbent, pH of 6.5, 10 min for adsorption time, 3 mL of HCl (0.1 mol L−1)/thiourea (2% w/v) as the eluent , 298 K, 1 atm | 95% to 100% | [152] |
| GO foams | Hg2+ | A small dose of 3DGON (10 mg L−1), pH: 5 and 9, 24 h, 298 K, 1 atm | 96% | [153] |
| Three-dimensional reduced-graphene oxide (3-D RGO) hydrogel | Hg2+ and F− | pH: 6, 24 h, 298 K, 1 atm | 65% | [154] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zunita, M. Graphene Oxide-Based Nanofiltration for Hg Removal from Wastewater: A Mini Review. Membranes 2021, 11, 269. https://doi.org/10.3390/membranes11040269
Zunita M. Graphene Oxide-Based Nanofiltration for Hg Removal from Wastewater: A Mini Review. Membranes. 2021; 11(4):269. https://doi.org/10.3390/membranes11040269
Chicago/Turabian StyleZunita, Megawati. 2021. "Graphene Oxide-Based Nanofiltration for Hg Removal from Wastewater: A Mini Review" Membranes 11, no. 4: 269. https://doi.org/10.3390/membranes11040269