Epigallocatechin-3-Gallate Attenuates Leukocyte Infiltration in 67-kDa Laminin Receptor-Dependent and -Independent Pathways in the Rat Frontoparietal Cortex following Status Epilepticus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Chemicals
2.2. Surgical Procedures and SE Induction
2.3. 67LR Neutralization
2.4. Tissue Preparation and Immunohistochemistry
2.5. Western Blot and Quatitative Real-Time PCR (qRT-PCR)
2.6. Data Analysis
3. Results
3.1. EGCG Attenuates SE-Induced Leukocyte Infiltration in the FPC
3.2. EGCG Ameliorates MCP-1 and MIP-2 Expression in the FPC following SE
3.3. SE Reduces 67LR Expression in Astrocytes, but Not in Endothelial Cells, in the FPC
3.4. MCP-1 Neutralization Abolishes MIP-2 Expression, Leukocyte Infiltration and 67LR Downregulation Induced by SE
3.5. Neutralization of 67LR Leads to ERK1/2-MIP-2-Mediated Leukocyte Infiltration in the FPC under Physiological Conditions
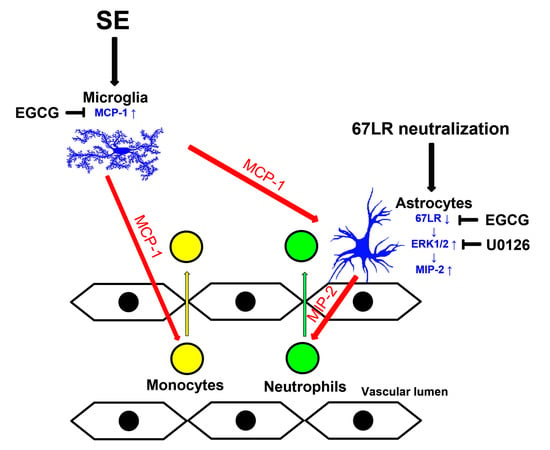
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ransohoff, R.M.; Kivisäkk, P.; Kidd, G. Three or more routes for leukocyte migration into the central nervous system. Nat. Rev. Immunol. 2003, 3, 569–581. [Google Scholar] [CrossRef]
- Babcock, A.A.; Kuziel, W.A.; Rivest, S.; Owens, T. Chemokine expression by glial cells directs leukocytes to sites of axonal injury in the CNS. J. Neurosci. 2003, 23, 7922–7930. [Google Scholar] [CrossRef] [PubMed]
- Bell, M.D.; Taub, D.D.; Kunkel, S.J.; Strieter, R.M.; Foley, R.; Gauldie, J.; Perry, V.H. Recombinant human adenovirus with rat MIP-2 gene insertion causes prolonged PMN recruitment to the murine brain. Eur. J. Neurosci. 1996, 8, 1803–1811. [Google Scholar] [CrossRef] [PubMed]
- Zwijnenburg, P.J.; Polfliet, M.M.; Florquin, S.; van den Berg, T.K.; Dijkstra, C.D.; van Deventer, S.J.; Roord, J.J.; van der Poll, T.; van Furth, A.M. CXC-chemokines KC and macrophage inflammatory protein-2 (MIP-2) synergistically induce leukocyte recruitment to the central nervous system in rats. Immunol. Lett. 2003, 85, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ravizza, T.; Gagliardi, B.; Noé, F.; Boer, K.; Aronica, E.; Vezzani, A. Innate and adaptive immunity during epileptogenesis and spontaneous seizures: Evidence from experimental models and human temporal lobe epilepsy. Neurobiol. Dis. 2008, 29, 142–160. [Google Scholar] [CrossRef]
- Buckley, M.W.; McGavern, D.B. Immune dynamics in the CNS and its barriers during homeostasis and disease. Immunol. Rev. 2022, 306, 58–75. [Google Scholar] [CrossRef]
- Kim, J.E.; Ryu, H.J.; Choi, S.Y.; Kang, T.C. Tumor necrosis factor-α-mediated threonine 435 phosphorylation of p65 nuclear factor-κB subunit in endothelial cells induces vasogenic edema and neutrophil infiltration in the rat piriform cortex following status epilepticus. J. Neuroinflammation 2012, 9, 6. [Google Scholar] [CrossRef]
- Kim, J.E.; Ryu, H.J.; Yeo, S.I.; Kang, T.C. P2X7 receptor regulates leukocyte infiltrations in rat frontoparietal cortex following status epilepticus. J. Neuroinflammation 2010, 7, 65. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, H.; Choi, S.H.; Kong, M.J.; Kang, T.C. Roscovitine Attenuates Microglia Activation and Monocyte Infiltration via p38 MAPK Inhibition in the Rat Frontoparietal Cortex Following Status Epilepticus. Cells 2019, 8, 746. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, H.; Lee, J.E.; Kang, T.C. CDDO-Me Inhibits Microglial Activation and Monocyte Infiltration by Abrogating NFκB- and p38 MAPK-Mediated Signaling Pathways Following Status Epilepticus. Cells 2020, 9, 1123. [Google Scholar] [CrossRef]
- Vézina, A.; Chokor, R.; Annabi, B. EGCG targeting efficacy of NF-κB downstream gene products is dictated by the monocytic/macrophagic differentiation status of promyelocytic leukemia cells. Cancer Immunol. Immunother. 2012, 61, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.F.; Wang, H.; Fan, Y.; Shi, H.J.; Wang, Q.M.; Chen, B.R.; Khurwolah, M.R.; Long, Q.Q.; Wang, S.B.; Wang, Z.M.; et al. Epigallocatechin-3-Gallate Inhibits Matrix Metalloproteinase-9 and Monocyte Chemotactic Protein-1 Expression Through the 67-κDa Laminin Receptor and the TLR4/MAPK/NF-κB Signalling Pathway in Lipopolysaccharide-Induced Macrophages. Cell Physiol. Biochem. 2017, 43, 926–936. [Google Scholar] [CrossRef] [PubMed]
- Katayama, T.; Kobayashi, H.; Okamura, T.; Yamasaki-Katayama, Y.; Kibayashi, T.; Kimura, H.; Ohsawa, K.; Kohsaka, S.; Minami, M. Accumulating microglia phagocytose injured neurons in hippocampal slice cultures: Involvement of p38 MAP kinase. PLoS ONE 2012, 7, e40813. [Google Scholar] [CrossRef]
- Morganti, J.M.; Goulding, D.S.; Van Eldik, L.J. Deletion of p38α MAPK in microglia blunts trauma-induced inflammatory responses in mice. J. Neuroinflammation 2019, 16, 98. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ren, Z.; Xu, Y.; Xiao, S.; Meydani, S.N.; Wu, D. Epigallocatechin-3-gallate ameliorates experimental autoimmune encephalomyelitis by altering balance among CD4+ T-cell subsets. Am. J. Pathol. 2012, 180, 221–234. [Google Scholar] [CrossRef] [PubMed]
- Gundimeda, U.; McNeill, T.H.; Fan, T.K.; Deng, R.; Rayudu, D.; Chen, Z.; Cadenas, E.; Gopalakrishna, R. Green tea catechins potentiate the neuritogenic action of brain-derived neurotrophic factor: Role of 67-kDa laminin receptor and hydrogen peroxide. Biochem. Biophys. Res. Commun. 2014, 445, 218–224. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, H.; Jeong, M.J.; Kang, T.C. Epigallocatechin-3-Gallate and PEDF 335 Peptide, 67LR Activators, Attenuate Vasogenic Edema, and Astroglial Degeneration Following Status Epilepticus. Antioxidants 2020, 9, 854. [Google Scholar] [CrossRef]
- Baloui, H.; von Boxberg, Y.; Vinh, J.; Weiss, S.; Rossier, J.; Nothias, F.; Stettler, O. Cellular prion protein/laminin receptor: Distribution in adult central nervous system and characterization of an isoform associated with a subtype of cortical neurons. Eur. J. Neurosci. 2004, 20, 2605–2616. [Google Scholar] [CrossRef]
- Park, H.; Choi, S.H.; Kong, M.J.; Kang, T.C. Dysfunction of 67-kDa Laminin Receptor Disrupts BBB Integrity via Impaired Dystrophin/AQP4 Complex and p38 MAPK/VEGF Activation Following Status Epilepticus. Front. Cell. Neurosci. 2019, 13, 236. [Google Scholar] [CrossRef]
- Kim, J.E.; Park, H.; Lee, J.E.; Kang, T.C. Blockade of 67-kDa Laminin Receptor Facilitates AQP4 Down-Regulation and BBB Disruption via ERK1/2-and p38 MAPK-Mediated PI3K/AKT Activations. Cells 2020, 9, 1670. [Google Scholar] [CrossRef]
- Pellegrini, R.; Martignone, S.; Ménard, S.; Colnaghi, M.I. Laminin receptor expression and function in small-cell lung carcinoma. Int. J. Cancer Suppl. 1994, 8, 116–120. [Google Scholar] [CrossRef] [PubMed]
- Ardini, E.; Pesole, G.; Tagliabue, E.; Magnifico, A.; Castronovo, V.; Sobel, M.E.; Colnaghi, M.I.; Ménard, S. The 67-kDa laminin receptor originated from a ribosomal protein that acquired a dual function during evolution. Mol. Biol. Evol. 1998, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Givant-Horwitz, V.; Davidson, B.; Reich, R. Laminin-induced signaling in tumor cells: The role of the M(r) 67,000 laminin receptor. Cancer Res. 2004, 64, 3572–3579. [Google Scholar] [CrossRef] [PubMed]
- Park, H.; Kang, T.C. The Regional Specific Alterations in BBB Permeability are Relevant to the Differential Responses of 67-kDa LR Expression in Endothelial Cells and Astrocytes Following Status Epilepticus. Int. J. Mol. Sci. 2019, 20, 6025. [Google Scholar] [CrossRef] [PubMed]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1997. [Google Scholar]
- Lund, H.; Pieber, M.; Parsa, R.; Han, J.; Grommisch, D.; Ewing, E.; Kular, L.; Needhamsen, M.; Espinosa, A.; Nilsson, E.; et al. Competitive repopulation of an empty microglial niche yields functionally distinct subsets of microglia-like cells. Nat. Commun. 2018, 9, 4845. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Frias, E.S.; Paladini, M.S.; Chen, D.; Boosalis, Z.; Becker, M.; Gupta, S.; Liu, S.; Gupta, N.; Rosi, S. Functional role of brain-engrafted macrophages against brain injuries. J. Neuroinflammation 2021, 18, 232. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, M.E.; Durham, S.K.; Swerdel, M.R.; Lewin, A.C.; Barton, D.S.; Megill, J.R.; Bravo, R.; Lira, S.A. Controlled recruitment of monocytes and macrophages to specific organs through transgenic expression of monocyte chemoattractant protein-1. J. Immunol. 1995, 155, 5769–5776. [Google Scholar] [CrossRef]
- Carr, M.W.; Roth, S.J.; Luther, E.; Rose, S.S.; Springer, T.A. Monocyte chemoattractant protein 1 acts as a T-lymphocyte chemoattractant. Proc. Natl. Acad. Sci. USA 1994, 91, 3652–3656. [Google Scholar] [CrossRef]
- Shaftel, S.S.; Carlson, T.J.; Olschowka, J.A.; Kyrkanides, S.; Matousek, S.B.; O’Banion, M.K. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J. Neurosci. 2007, 27, 9301–9309. [Google Scholar] [CrossRef]
- Schilling, M.; Strecker, J.K.; Schäbitz, W.R.; Ringelstein, E.B.; Kiefer, R. Effects of monocyte chemoattractant protein 1 on blood-borne cell recruitment after transient focal cerebral ischemia in mice. Neuroscience 2009, 161, 806–812. [Google Scholar] [CrossRef]
- Furuichi, K.; Wada, T.; Iwata, Y.; Kitagawa, K.; Kobayashi, K.; Hashimoto, H.; Ishiwata, Y.; Asano, M.; Wang, H.; Matsushima, K.; et al. CCR2 signaling contributes to ischemia-reperfusion injury in kidney. J. Am. Soc. Nephrol. 2003, 14, 2503–2515. [Google Scholar] [CrossRef] [PubMed]
- Busch-Petersen, J. Small molecule antagonists of the CXCR2 and CXCR1 chemokine receptors as therapeutic agents for the treatment of inflammatory diseases. Curr. Top. Med. Chem. 2006, 6, 1345–1352. [Google Scholar] [CrossRef] [PubMed]
- Otto, V.I.; Gloor, S.M.; Frentzel, S.; Gilli, U.; Ammann, E.; Hein, A.E.; Folkers, G.; Trentz, O.; Kossmann, T.; Morganti-Kossmann, M.C. The production of macrophage inflammatory protein-2 induced by soluble intercellular adhesion molecule-1 in mouse astrocytes is mediated by src tyrosine kinases and p42/44 mitogen-activated protein kinase. J. Neurochem. 2002, 80, 824–834. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.N.; Ho, Y.J.; Lai, C.C.; Chiu, C.T.; Wang, J.Y. 1,25-Dihydroxyvitamin D3 attenuates endotoxin-induced production of inflammatory mediators by inhibiting MAPK activation in primary cortical neuron-glia cultures. J. Neuroinflammation 2015, 12, 147. [Google Scholar] [CrossRef] [PubMed]
- Bae, H.B.; Li, M.; Kim, J.P.; Kim, S.J.; Jeong, C.W.; Lee, H.G.; Kim, W.M.; Kim, H.S.; Kwak, S.H. The effect of epigallocatechin gallate on lipopolysaccharide-induced acute lung injury in a murine model. Inflammation 2010, 33, 82–91. [Google Scholar] [CrossRef]
- Sansing, L.H.; Harris, T.H.; Kasner, S.E.; Hunter, C.A.; Kariko, K. Neutrophil depletion diminishes monocyte infiltration and improves functional outcome after experimental intracerebral hemorrhage. Acta Neurochir. Suppl. 2011, 111, 173–178. [Google Scholar]
- Sullivan, D.P.; Muller, W.A. Neutrophil and monocyte recruitment by PECAM, CD99, and other molecules via the LBRC. Semin. Immunopathol. 2014, 36, 193–209. [Google Scholar] [CrossRef]
- Yang, D.; Chen, Q.; Schmidt, A.P.; Anderson, G.M.; Wang, J.M.; Wooters, J.; Oppenheim, J.J.; Chertov, O. LL-37, the neutrophil granule- and epithelial cell-derived cathelicidin, utilizes formyl peptide receptor-like 1 (FPRL1) as a receptor to chemoattract human peripheral blood neutrophils, monocytes, and T cells. J. Exp. Med. 2000, 192, 1069–1074. [Google Scholar]
- Sun, R.; Iribarren, P.; Zhang, N.; Zhou, Y.; Gong, W.; Cho, E.H.; Lockett, S.; Chertov, O.; Bednar, F.; Rogers, T.J.; et al. Identification of neutrophil granule protein cathepsin G as a novel chemotactic agonist for the G protein-coupled formyl peptide receptor. J. Immunol. 2004, 173, 428–436. [Google Scholar] [CrossRef]
- Yoshimura, T.; Robinson, E.A.; Tanaka, S.; Appella, E.; Kuratsu, J.; Leonard, E.J. Purification and amino acid analysis of two human glioma-derived monocyte chemoattractants. J. Exp. Med. 1989, 169, 1449–1459. [Google Scholar] [CrossRef]
- Hoh, B.L.; Hosaka, K.; Downes, D.P.; Nowicki, K.W.; Fernandez, C.E.; Batich, C.D.; Scott, E.W. Monocyte chemotactic protein-1 promotes inflammatory vascular repair of murine carotid aneurysms via a macrophage inflammatory protein-1α and macrophage inflammatory protein-2-dependent pathway. Circulation 2011, 124, 2243–2252. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; St-Pierre, S.; Roy, P.; Morley, B.J.; Hao, J.; Simard, A.R. Infiltration of CCR2+Ly6Chigh Proinflammatory Monocytes and Neutrophils into the Central Nervous System Is Modulated by Nicotinic Acetylcholine Receptors in a Model of Multiple Sclerosis. J. Immunol. 2016, 196, 2095–2108. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Yu, L.; Kong, L.; Ma, R.; Zhang, J.; Zhu, Q.; Zhu, J.; Hao, D. Pyrroloquinoline quinone (PQQ) inhibits lipopolysaccharide induced inflammation in part via downregulated NF-κB and p38/JNK activation in microglial and attenuates microglia activation in lipopolysaccharide treatment mice. PLoS ONE 2014, 9, e109502. [Google Scholar] [CrossRef] [PubMed]
- Chuang, Y.C.; Chen, S.D.; Lin, T.K.; Chang, W.N.; Lu, C.H.; Liou, C.W.; Chan, S.H.; Chang, A.Y. Transcriptional upregulation of nitric oxide synthase II by nuclear factor-kappaB promotes apoptotic neuronal cell death in the hippocampus following experimental status epilepticus. J. Neurosci. Res. 2010, 88, 1898–1907. [Google Scholar] [PubMed]
- Hu, Q.P.; Mao, D.A. Histone deacetylase inhibitor SAHA attenuates post-seizure hippocampal microglia TLR4/MYD88 signaling and inhibits TLR4 gene expression via histone acetylation. BMC Neurosci. 2016, 17, 22. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, C.; Meng, X.; Li, Z.; Lv, C.; Cao, P. Neuroprotection of edaravone on the hippocampus of kainate-induced epilepsy rats through Nrf2/HO-1 pathway. Neurochem. Int. 2018, 112, 159–165. [Google Scholar] [CrossRef]
- Yang, Y.; Han, X.; Chen, Y.; Wu, J.; Li, M.; Yang, H.; Xu, W.; Wei, L. EGCG Induces Pro-inflammatory Response in Macrophages to Prevent Bacterial Infection through the 67LR/p38/JNK Signaling Pathway. J. Agric. Food Chem. 2021, 69, 5638–5651. [Google Scholar] [CrossRef]
- Kleibeuker, W.; Jurado-Pueyo, M.; Murga, C.; Eijkelkamp, N.; Mayor, F., Jr.; Heijnen, C.J.; Kavelaars, A. Physiological changes in GRK2 regulate CCL2-induced signaling to ERK1/2 and Akt but not to MEK1/2 and calcium. J. Neurochem. 2008, 104, 979–992. [Google Scholar] [CrossRef]
- Yu, M.; Zheng, N.; Jiang, D.; Wang, L.; Zhan, Q.; Zhao, J. Chemokine C-C motif ligand 2 suppressed the growth of human brain astrocytes under Ischemic/hypoxic conditions via regulating ERK1/2 pathway. Brain Inj. 2020, 34, 1277–1282. [Google Scholar] [CrossRef]
- Tachibana, H.; Koga, K.; Fujimura, Y.; Yamada, K. A receptor for green tea polyphenol EGCG. Nat. Struct. Mol. Biol. 2004, 11, 380–381. [Google Scholar] [CrossRef]
- Ku, H.C.; Chang, H.H.; Liu, H.C.; Hsiao, C.H.; Lee, M.J.; Hu, Y.J.; Hung, P.F.; Liu, C.W.; Kao, Y.H. Green tea (-)-epigallocatechin gallate inhibits insulin stimulation of 3T3-L1 preadipocyte mitogenesis via the 67-kDa laminin receptor pathway. Am. J. Physiol. Cell. Physiol. 2009, 297, C121–C132. [Google Scholar] [CrossRef] [PubMed]
- Ku, H.C.; Liu, H.S.; Hung, P.F.; Chen, C.L.; Liu, H.C.; Chang, H.H.; Tsuei, Y.W.; Shih, L.J.; Lin, C.L.; Lin, C.M.; et al. Green tea (-)-epigallocatechin gallate inhibits IGF-I and IGF-II stimulation of 3T3-L1 preadipocyte mitogenesis via the 67-kDa laminin receptor, but not AMP-activated protein kinase pathway. Mol. Nutr. Food Res. 2012, 56, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Jovanovic, K.; Chetty, C.J.; Khumalo, T.; Da Costa Dias, B.; Ferreira, E.; Malindisa, S.T.; Caveney, R.; Letsolo, B.T.; Weiss, S.F. Novel patented therapeutic approaches targeting the 37/67 kDa laminin receptor for treatment of cancer and Alzheimer’s disease. Expert Opin. Ther. Pat. 2015, 25, 567–582. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Sumida, M.; Sugihara, K.; Tsukamoto, S.; Yamada, K.; Tachibana, H. Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PLoS ONE 2012, 7, e37942. [Google Scholar]
- Shea, T.B.; Zheng, Y.L.; Ortiz, D.; Pant, H.C. Cyclin-dependent kinase 5 increases perikaryal neurofilament phosphorylation and inhibits neurofilament axonal transport in response to oxidative stress. J. Neurosci. Res. 2004, 76, 795–800. [Google Scholar] [CrossRef]
- He, M.; Wang, C.; Sun, J.H.; Liu, Y.; Wang, H.; Zhao, J.S.; Li, Y.F.; Chang, H.; Hou, J.M.; Song, J.N.; et al. Roscovitine attenuates intimal hyperplasia via inhibiting NF-κB and STAT3 activation induced by TNF-α in vascular smooth muscle cells. Biochem. Pharmacol. 2017, 137, 51–60. [Google Scholar] [CrossRef]
- Golub, V.M.; Reddy, D.S. Contusion brain damage in mice for modelling of post-traumatic epilepsy with contralateral hippocampus sclerosis: Comprehensive and longitudinal characterization of spontaneous seizures, neuropathology, and neuropsychiatric comorbidities. Exp. Neurol. 2022, 348, 113946. [Google Scholar] [CrossRef]
- Singh, T.; Joshi, S.; Williamson, J.M.; Kapur, J. Neocortical injury-induced status epilepticus. Epilepsia 2020, 61, 2811–2824. [Google Scholar] [CrossRef]
- Christian, F.; Smith, E.L.; Carmody, R.J. The Regulation of NF-κB Subunits by Phosphorylation. Cells 2016, 5, 12. [Google Scholar] [CrossRef]
- Schubert, S.Y.; Neeman, I.; Resnick, N. A novel mechanism for the inhibition of NF-kappaB activation in vascular endothelial cells by natural antioxidants. FASEB J. 2002, 16, 1931–1933. [Google Scholar] [CrossRef]
- Ahmad, R.; Raina, D.; Meyer, C.; Kharbanda, S.; Kufe, D. Triterpenoid CDDO-Me blocks the NF-kappaB pathway by direct inhibition of IKKbeta on Cys-179. J. Biol. Chem. 2006, 281, 35764–35769. [Google Scholar] [CrossRef]
- Aktas, O.; Prozorovski, T.; Smorodchenko, A.; Savaskan, N.E.; Lauster, R.; Kloetzel, P.M.; Infante-Duarte, C.; Brocke, S.; Zipp, F. Green tea epigallocatechin-3-gallate mediates T cellular NF-kappa B inhibition and exerts neuroprotection in autoimmune encephalomyelitis. J. Immunol. 2004, 173, 5794–5800. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Jing, D.; Shi, M.; Liu, Y.; Lin, T.; Xie, Z.; Zhu, Y.; Zhao, H.; Shi, X.; Du, F.; et al. Epigallocatechin gallate (EGCG) attenuates infrasound-induced neuronal impairment by inhibiting microglia-mediated inflammation. J. Nutr. Biochem. 2014, 25, 716–725. [Google Scholar] [CrossRef] [PubMed]
- Machova Urdzikova, L.; Ruzicka, J.; Karova, K.; Kloudova, A.; Svobodova, B.; Amin, A.; Dubisova, J.; Schmidt, M.; Kubinova, S.; Jhanwar-Uniyal, M.; et al. A green tea polyphenol epigallocatechin-3-gallate enhances neuroregeneration after spinal cord injury by altering levels of inflammatory cytokines. Neuropharmacology 2017, 126, 213–223. [Google Scholar] [CrossRef] [PubMed]








| Antigen | Isotype | Hose | Manufacturer (Catalog Number) | Dilution |
|---|---|---|---|---|
| 67LR | IgG | Rabbit | Abcam (ab133645) | 1:100 |
| CCR2 | IgG | Rabbit | Abcam (ab227015) | 1:100 |
| CD68 | IgG | Mouse | Abcam (ab31630) | 1:100 |
| GFAP | IgG | Mouse | Millipore, Burlington, MA, USA ($MAB3402) | 1:4000 |
| IB4 | lectin | - | Vector Laboratories, Inc. Newark, CA, USA (B-1205) | 1:200 |
| MCP-1 | IgG | Mouse | Abcam (ab25124) | 1:100 |
| MIP-2 | IgG | Rabbit | Invitrogen, Waltham, MA, USA (ARC1074) | 1:100 |
| MPO | IgG | Rabbit | Thermo Scientific, Waltham, MA, USA (#RB-373-A) | 1:100 |
| NeuN | IgG | Guinea pig | Millipore (#3238431) | 1:1000 |
| NF-κB p65 | IgG | Rabbit | Abcam (ab16502) | 1:2000 (WB *) |
| p-ERK1/2 | IgG | Rabbit | Millipore (#05-767R) | 1:100 |
| p-NF-κB p65 S276 | IgG | Rabbit | Abcam (ab30623) | 1:1000 (WB *) |
| SMI-71 (endothelial BBB marker) | IgM | Mouse | Covance, Princeton, NJ, USA (#SMI-71R) | 1:1000 |
| β-actin | IgG | Mouse | Sigma, St. Louis, MO, USA (#A5316) | 1:5000 (WB *) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.-E.; Lee, D.-S.; Kang, T.-C. Epigallocatechin-3-Gallate Attenuates Leukocyte Infiltration in 67-kDa Laminin Receptor-Dependent and -Independent Pathways in the Rat Frontoparietal Cortex following Status Epilepticus. Antioxidants 2023, 12, 969. https://doi.org/10.3390/antiox12040969
Kim J-E, Lee D-S, Kang T-C. Epigallocatechin-3-Gallate Attenuates Leukocyte Infiltration in 67-kDa Laminin Receptor-Dependent and -Independent Pathways in the Rat Frontoparietal Cortex following Status Epilepticus. Antioxidants. 2023; 12(4):969. https://doi.org/10.3390/antiox12040969
Chicago/Turabian StyleKim, Ji-Eun, Duk-Shin Lee, and Tae-Cheon Kang. 2023. "Epigallocatechin-3-Gallate Attenuates Leukocyte Infiltration in 67-kDa Laminin Receptor-Dependent and -Independent Pathways in the Rat Frontoparietal Cortex following Status Epilepticus" Antioxidants 12, no. 4: 969. https://doi.org/10.3390/antiox12040969