Effects of Citrus kawachiensis Peel in Frailty-like Model Mice Induced by Low Protein Nutrition Disorders
Abstract
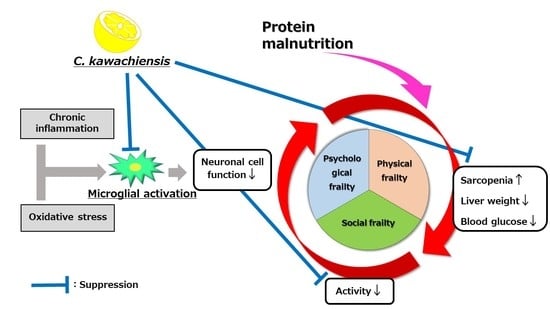
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Sample Treatment
2.3. Food Intake
2.4. The Y-maze Test
2.5. Dissection
2.6. Blood Glucose Measurement
2.7. Immunohistochemistry for Optical Microscopy
2.8. Immunofluorescence for Confocal Microscopy
2.9. Image Analysis
2.10. Statistical Analysis
3. Results
3.1. Changes in Body Weight and Food Intake in Different Experimental Groups
3.2. Percent Weight Loss 2 or 8 Days after the Administration of LPS
3.3. Ameliorative Effects of C. kawachiensis on Peripheral Dysfunction 8 Days after the Administration of LPS
3.4. Ameliorative Effects of C. kawachiensis on Spontaneous Behavior
3.5. Suppressive Effects of C. kawachiensis on Microglial Activation
3.6. Protective Effects of C. kawachiensis on Neuronal Cell Function in the Hippocampus
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sacha, J.; Sacha, M.; Sobon, J.; Borysiuk, Z.; Feusette, P. Is It Time to Being a Public Campaign Concerning Frailty and Pre-Frailty? A Review Article. Front. Physiol. 2017, 8, 484. [Google Scholar] [CrossRef]
- Shinkai, S.; Yoshida, H.; Taniguchi, Y.; Murayama, H.; Nishi, M.; Amano, H.; Nofuji, Y.; Seino, S.; Fujiwara, Y. Public health approach to preventing frailty in the community and its effect on healthy aging in Japan. Geriatr. Gerontol. Int. 2016, 16, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Panza, F.; Lozupone, M.; Solfrizzi, V.; Sardone, R.; Dibello, V.; Lena, L.D.; D’Urso, F.; Stallone, R.; Petruzzi, M.; Giannelli, G.; et al. Different Cognitive Frailty Models and Health-and Cognitive-related Outcomes in Older Age: From Epidemiology to Prevention. J. Alzheimer’s Dis. 2018, 62, 993–1012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smallwood, M.J.; Nissim, A.; Knight, A.R.; Whiteman, M.; Haigh, R.; Winyard, P.G. Oxidative stress in autoimmune rheumatic disease. Free Radic. Biol. Med. 2018, 125, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Reuter, S.; Gupta, S.C.; Chaturvedi, M.M.; Aggarwal, B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010, 49, 1603–1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moylan, J.S.; Reid, M.B. Oxidative stress, chronic disease, and muscle wasting. Muscle Nerve 2007, 35, 411–429. [Google Scholar] [CrossRef]
- Fischer, R.; Maier, O. Interrelation of Oxidative Stress and Inflammation in Neurodegenerative Disease: Role of TNF. Oxid. Med. Cell. Longev. 2015, 18, 610813. [Google Scholar] [CrossRef] [Green Version]
- Melrose, J. The Potential of Flavonoids and Flavonoid Metabolites in the Treatment of Neurodegenerative Pathology in Disorders of Cognitive Decline. Antioxidants 2023, 12, 663. [Google Scholar] [CrossRef]
- Furukawa, Y.; Okuyama, S.; Amakura, Y.; Watanabe, S.; Fukata, T.; Nakajima, M.; Yoshimura, M.; Yoshida, T. Isolation and Characterization of Activators of ERK/MAPK from Citrus Plants. Int. J. Mol. Sci. 2012, 13, 1832–1845. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Yamamoto, K.; Mori, H.; Toyoda, N.; Yoshimura, M.; Amakura, Y.; Yoshida, T.; Sugawara, K.; Sudo, M.; Nakajima, M.; et al. Auraptene in the Peels of Citrus kawachiensis (Kawachi bankan) Ameliorates Lipopolysaccharide-Induced Inflammation in the Mouse Brain. Evid. Based Complement. Altern. Med. 2014, 2014, 408503. [Google Scholar] [CrossRef] [Green Version]
- Amakura, Y.; Yoshimura, M.; Ouchi, K.; Okuyama, S.; Furukawa, Y.; Yoshida, T. Characterization of Constituents in the Peel of Citrus kawachiensis (Kawachi bankan). Biosci. Biotechnol. Biochem. 2013, 77, 1977–1980. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Yamamoto, K.; Mori, H.; Sawamoto, A.; Amakura, Y.; Yoshimura, Y.; Tamanaha, A.; Ohkubo, Y.; Sugawara, K.; Sudo, M.; et al. Neuroprotective effect of Citrus kawachiensis (Kawachi Bankan) peels, a rich source of naringin, against global cerebral ischemia/reperfusion injury in mice. Biosci. Biotechnol. Biochem. 2018, 82, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Okuyama, S.; Shinoka, W.; Nakamura, K.; Kotani, M.; Sawamoto, A.; Sugawara, K.; Sudo, M.; Nakajima, M.; Furukawa, Y. Suppressive effects of the peel of Citrus kawachiensis (Kawachi bankan) on astroglial activation, tau phosphorylation, and inhibition of neurogenesis in the hippocampus of type 2 diabetic db/db mice. Biosci. Biotechnol. Biochem. 2018, 82, 1384–1395. [Google Scholar] [CrossRef]
- Okuyama, S.; Kotani, Y.; Yamamoto, K.; Sawamoto, A.; Sugawara, K.; Sudo, M.; Ohkubo, Y.; Tamanaha, A.; Nakajima, M.; Furukawa, Y. The peel of Citrus kawachiensis (Kawachi Bankan) ameliorates microglial activation, tau hyper-phosphorylation, and suppression of neurogenesis in the hippocampus of senescence-accelerated mice. Biosci. Biotechnol. Biochem. 2018, 82, 869–878. [Google Scholar] [CrossRef]
- Boban, P.J.; Corbett, D.; Saucier, D.M.; Noyan-Ashraf, M.H.; Juurlink, B.H.J.; Paterson, P.G. Protein-energy malnutrition impairs functional outcome in global ischemia. Exp. Neurol. 2005, 196, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Feoli, A.M.; Siqueira, I.R.; Almeida, L.; Tramontina, A.C.; Vanzella, C.; Sbaraini, S.; Schweigert, I.D.; Netto, C.A.; Perry, M.L.S.; Gonḉalves, C.A. Effects of protein malnutrition on oxidative status in rat brain. Nutrition 2006, 22, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Noworyta-Sokołowska, K.; Gόrska, A.; Gołembiowska, K. LPS-induced oxidative stress and inflammatory reaction in the rat striatum. Pharmacol. Rep. 2013, 65, 863–869. [Google Scholar] [CrossRef]
- Okuyama, S.; Shimada, N.; Kaji, M.; Morita, M.; Miyoshi, K.; Minami, S.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Watanabe, S.; et al. Heptamethoxyflavone, a citrus flavonoid, enhances brain-derived neurotrophic factor production and neurogenesis in the hippocampus following cerebral global ischemia in mice. Neurosci. Lett. 2012, 528, 190–195. [Google Scholar] [CrossRef]
- Sawamoto, A.; Okuyama, S.; Yamamoto, K.; Amakura, Y.; Yoshimura, M.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a Citrus Flavonoid, Ameliorates Corticosterone-Induced Depression-like Behavior and Restores Brain-Derived Neurotrophic Factor Expression, Neurogenesis, and Neuroplasticity in the Hippocampus. Molecules 2016, 21, 541. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Morita, M.; Miyoshi, K.; Nishigawa, Y.; Kaji, M.; Sawamoto, A.; Terugo, T.; Toyoda, N.; Makihata, N.; Amakura, Y.; et al. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a citrus flavonoid, on protection against memory impairment and neuronal cell death in a global cerebral ischemia mouse model. Neurochem. Int. 2014, 70, 30–38. [Google Scholar] [CrossRef] [Green Version]
- Sawamoto, A.; Okuyama, S.; Amakura, Y.; Yoshimura, M.; Yamada, T.; Yokogoshi, H.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone Ameliorates Depression-Like Behavior and Hippocampal Neurochemical Changes in Chronic Unpredictable Mild Stressed Mice by Regulating the Brain-Derived Neurotrophic Factor: Requirement for ERK Activation. Int. J. Mol. Sci. 2017, 18, 2133. [Google Scholar] [CrossRef] [Green Version]
- Okuyama, S.; Miyoshi, K.; Tsumura, Y.; Amakura, Y.; Yoshimura, M.; Yoshida, T.; Nakajima, M.; Furukawa, Y. 3,5,6,7,8,3′,4′-Heptamethoxyflavone, a Citrus Polymethoxylated Flavone, Attenuates Inflammtion in the Mouse Hippocampus. Brain Sci. 2015, 5, 118–129. [Google Scholar] [CrossRef] [Green Version]
- Ihara, H.; Yamamoto, H.; Ida, T.; Tsutsuki, H.; Sakamoto, T.; Fujita, T.; Okada, T.; Kozaki, S. Inhibition of Nitric Oxide Production and Inducible Nitric Oxide Synthase Expression by a Polymethoxyflavone from Young Fruits of Citrus unshiu in Rat Primary Astrocytes. Biosci. Biotechnol. Biochem. 2012, 76, 1843–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawamoto, A.; Okuyama, S.; Nakajima, M.; Furukawa, Y. Citrus flavonoid 3,5,6,7,8,3′,4′-heptamethoxyflavone induces BDNF via cAMP/ERK/CREB signaling and reduces phosphodiesterase activity in C6 cells. Pharmacol. Rep. 2019, 71, 653–658. [Google Scholar] [CrossRef]
- Okuyama, S.; Semba, T.; Toyoda, N.; Epifano, F.; Genovese, S.; Fiorito, S.; Taddeo, V.A.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Auraptene and Other Prenyloxyphenylpropanoids Suppress Microglial Activation and Dopaminergic Neuronal Cell Death in a Lipopolysaccharide-Induced Model of Parkinson’s Disease. Int. J. Mol. Sci. 2016, 17, 1716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.J.; Jang, Y.; Zhu, J.; Namgung, E.; Go, D.; Seo, C.; Ju, X.; Cui, J.; Lee, Y.L.; Kang, H.; et al. Auraptene Enhances Junction Assembly in Cerebrovascular Endothelial Cells by Promoting Resilience to Mitochondrial Stress through Activation of Antioxidant Enzymes and mtUPR. Antioxidants 2021, 10, 475. [Google Scholar] [CrossRef] [PubMed]
- Amini-Khoei, H.; Boroujeni, S.N.; Maghsoudi, F.; Rahimi-Madiseh, M.; Bijad, E.; Moradi, M.; Lorigooini, Z. Possible involvement of L-arginine-nitric oxide pathway in the antidepressant activity of Auraptene in mice. Behav. Brain Funct. 2022, 18, 4. [Google Scholar] [CrossRef]
- Okuyama, S.; Nakashima, T.; Nakamura, K.; Shinoka, W.; Kotani, M.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Inhibitory Effects of Auraptene and Naringin on Astroglial Activation, Tau Hyperphosphorylation, and Suppression of Neurogenesis in the Hippocampus of Streptozotocin-Induced Hyperglycemic Mice. Antioxidants 2018, 7, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okuyama, S.; Katoh, M.; Kanzaki, T.; Kotani, Y.; Amakura, Y.; Yoshimura, M.; Fukuda, N.; Tamai, T.; Sawamoto, A.; Nakajima, M.; et al. Auraptene/Naringin-Rich Fruit Juice of Citrus kawachiensis (Kawachi Bankan) Prevents Ischemia-Induced Neuronal Cell Death in Mouse Brain through Anti-Inflammatory Responces. J. Nutr. Sci. Vitaminol. 2019, 65, 66–71. [Google Scholar] [CrossRef] [Green Version]
- Golechha, M.; Chaudhry, U.; Bhatia, J.; Saluja, D.; Arya, D.S. Naringin Protects against Kainic Acid-Induced Status Epilepticus in Rats: Evidence for an Antioxidant, Anti-inflammatory and Neuroprotective Intervention. Biol. Pharm. Bull. 2011, 34, 360–365. [Google Scholar] [CrossRef] [Green Version]
- Yamada, C.; Saegusa, Y.; Nakagawa, K.; Ohnishi, S.; Muto, S.; Nahata, M.; Sadakane, C.; Hattori, T.; Sakamoto, N.; Takeda, H. Rikkunshito, a Japanese Kampo Medicine, Ameliorates Decreased Feeding Behavior via Ghrelin and Serotonin 2B Receptor Signaling in a Novelty Stress Murine Model. BioMed Res. Int. 2013, 2013, 792940. [Google Scholar] [CrossRef] [PubMed]
- Fujitsuka, N.; Uezono, Y. Rikkunshito, a ghrelin potentiator, ameliorates anorexia-cachexia syndrome. Front. Pharmacol. 2014, 5, 271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Uto Sameshima, N.; Amitani, H.; Atobe, Y.; Sameshima, Y.; Sakaki, M.; Rokot, N.; Ataka, K.; Amitani, M.; Inui, A. Herbal Medicine Ninjin’yoeito in the Treatment of Sarcopenia and Frailty. Front. Nutr. 2018, 5, 126. [Google Scholar]
- Takeda, H.; Sadakane, C.; Hattori, T.; Katsurada, T.; Ohkawara, T.; Nagai, K.; Asaka, M. Rikkunshito, an Herbal Medicine, Suppresses Cisplatin-Induced Anorexia in Rats Via 5-HT2 Receptor Antagonism. Gastroenterology 2008, 134, 2004–2013. [Google Scholar] [CrossRef]
- Guillory, B.; Splenser, A.; Garcia, J. The Role of Ghrelin in Anorexia-Cachexia Syndromes. Vitam. Horm. 2013, 92, 61–106. [Google Scholar]
- Amitani, M.; Amitani, H.; Cheng, K.; Kairupan, T.S.; Sameshima, N.; Shimoshikiryo, I.; Mizuma, K.; Rokot, N.T.; Nerome, Y.; Owaki, T.; et al. The Role of Ghrelin and Ghrelin Signaling in Aging. Int. J. Mol. Sci. 2017, 18, 1511. [Google Scholar] [CrossRef]
- Amitani, M.; Asakawa, A.; Amitani, H.; Inui, A. Control of food intake and muscle wasting in cachexia. Int. J. Biochem. Cell Biol. 2013, 45, 2179–2185. [Google Scholar] [CrossRef] [PubMed]
- Bandsma, R.H.J.; Mendel, M.; Spoelstra, M.N.; Reijngoud, D.; Boer, T.; Stellaard, F.; Brabin, B.; Schellekens, R.; Senga, E.; Heikens, G.T. Mechanisms Behind Decreased Endogenous Glucose Production in Malnourished Children. Pediatr. Res. 2010, 68, 423–428. [Google Scholar] [CrossRef] [Green Version]
- Heard, C.R.C.; Frangi, S.M.; Wright, P.M.; McCartney, P.R. Biochemical characteristic of different forms of protein-energy malnutrition: An experimental model using young rats. Br. J. Nutr. 1977, 37, 1–21. [Google Scholar] [CrossRef] [Green Version]
- Biesmans, S.; Meert, T.F.; Bouwknecht, J.A.; Acton, P.D.; Davoodi, N.; Haes, P.D.; Kuijlaars, J.; Langlois, X.; Matthews, L.J.R.; Donck, L.V.; et al. Systemic Immune Activation Leads to Neuroinflammation and Sickness Behavior in Mice. Mediat. Inflamm. 2013, 2013, 271359. [Google Scholar] [CrossRef]
- Zhao, X.; Cao, F.; Liu, Q.; Li, X.; Xu, G.; Liu, G.; Zhang, Y.; Yang, X.; Yi, S.; Xu, F.; et al. Behavioral, inflammatory and neurochemical disturbances in LPS and UCMS-induced mouse models of depression. Behav. Brain Res. 2019, 364, 494–502. [Google Scholar] [CrossRef] [PubMed]
- Martone, A.M.; Onder, G.; Vetrano, D.L.; Ortolani, E.; Tosato, M.; Marzetti, E.; Landi, F. Anorexia of Aging: A Modifiable Risk Factor for Frailty. Nutrients 2013, 5, 4126–4133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onozuka, H.; Nakajima, A.; Matsuzaki, K.; Shin, R.; Ogino, K.; Saigusa, D.; Tetsu, N.; Yokosuka, A.; Sashida, Y.; Mimaki, Y.; et al. Nobiletin, a Citrus Flavonoid, Improves Memory Impairment and Aβ Pathology in a Transgenic Mouse Model of Alzheimer’s Disease. J. Pharmacol. Exp. Ther. 2008, 326, 739–744. [Google Scholar] [CrossRef]
- Nakajima, A.; Ohizumi, Y.; Yamada, K. Anti-dementia Activity of Nobiletin, a Citrus Flavonoid: A Review of Animal Studies. Clin. Psychopharmacol. Neurosci. 2014, 12, 75–82. [Google Scholar] [CrossRef] [Green Version]
- Choi, M.; Lee, E.; Park, J.; Kim, S.; Park, E.; Kim, H. Anti-inflammatory mechanism of galangin in lipopolysaccharide-stimulated microglia: Critical role of PPAR-γ signaling pathway. Biochem. Pharmacol. 2017, 144, 120–131. [Google Scholar] [CrossRef] [PubMed]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Omasa, T.; Okuyama, S.; Sawamoto, A.; Nakajima, M.; Furukawa, Y. Effects of Citrus kawachiensis Peel in Frailty-like Model Mice Induced by Low Protein Nutrition Disorders. Antioxidants 2023, 12, 779. https://doi.org/10.3390/antiox12030779
Omasa T, Okuyama S, Sawamoto A, Nakajima M, Furukawa Y. Effects of Citrus kawachiensis Peel in Frailty-like Model Mice Induced by Low Protein Nutrition Disorders. Antioxidants. 2023; 12(3):779. https://doi.org/10.3390/antiox12030779
Chicago/Turabian StyleOmasa, Toshiki, Satoshi Okuyama, Atsushi Sawamoto, Mitsunari Nakajima, and Yoshiko Furukawa. 2023. "Effects of Citrus kawachiensis Peel in Frailty-like Model Mice Induced by Low Protein Nutrition Disorders" Antioxidants 12, no. 3: 779. https://doi.org/10.3390/antiox12030779