Exploring the Long-Term Tissue Accumulation and Excretion of 3 nm Cerium Oxide Nanoparticles after Single Dose Administration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model of the Effects of Protein Corona on Nanoceria Internalization and Intracellular Trafficking
2.2. CeO2NPs Adsorption by Human Hepatocyte Cancer Cells
2.3. Nanoceria Synthesis
2.4. Nanoceria Conjugation
MSA Conjugation
2.5. Nanoceria Characterization
2.5.1. Bacterial Endotoxin (LAL) Test
2.5.2. Transmission Electron Microscopy (TEM)
2.5.3. UV-Visible Spectra
2.5.4. Dynamic Light Scattering (DLS) and ζ-Potential
2.5.5. X-ray Diffraction
2.6. In Vivo Study Design
2.6.1. Long-Term Biodistribution in Healthy Mice
2.6.2. Nanoceria Excretion in CCl4-Treated Rats
2.6.3. Confocal Imaging of Nanoparticles in Mice Liver
2.6.4. Organ Distribution of Fe3O4NPs in CCl4-Treated Rats
2.6.5. Organ Biodistribution after Oral Administration in Healthy Rats
2.7. Cerium Content Determination
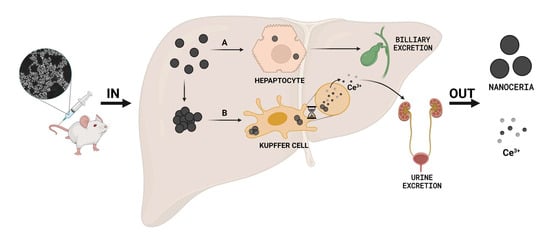
3. Results
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- O’Neill, L.A.J.; Kishton, R.J.; Rathmell, J. A Guide to Immunometabolism for Immunologists. Nat. Rev. Immunol. 2016, 16, 553. [Google Scholar] [CrossRef] [Green Version]
- Ernst, L.M.; Puntes, V. How Does Immunomodulatory Nanoceria Work? ROS and Immunometabolism. Front. Immunol. 2022, 13, 974. [Google Scholar] [CrossRef]
- Auten, R.L.; Davis, J.M. Oxygen Toxicity and Reactive Oxygen Species: The Devil Is in the Details. Pediatr. Res. 2009, 66, 121–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.; Gevezova, M.; Sarafian, V.; Maes, M. Redox Regulation of the Immune Response. Cell. Mol. Immunol. 2022, 19, 1079–1101. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Huang, Z.; Liu, J.; Huang, Z.; Liu, Y.; Liu, Q.; Yang, Z.; Li, R.; Wu, X.; Shi, Z.; et al. Neuroprotective Effect of Ketone Metabolism on Inhibiting Inflammatory Response by Regulating Macrophage Polarization after Acute Cervical Spinal Cord Injury in Rats. Front. Neurosci. 2020, 14, 583611. [Google Scholar] [CrossRef] [PubMed]
- Kesarwani, P.; Kant, S.; Zhao, Y.; Miller, C.R.; Chinnaiyan, P. The Influence of the Ketogenic Diet on the Immune Tolerant Microenvironment in Glioblastoma. Cancers 2022, 14, 5550. [Google Scholar] [CrossRef] [PubMed]
- Bai, B.; Chen, H. Metformin: A Novel Weapon Against Inflammation. Front. Pharmacol. 2021, 12, 622262. [Google Scholar] [CrossRef]
- Pauling, L. Vitamin C and the Common Cold. Can. Med. Assoc. J. 1971, 105, 448. [Google Scholar]
- Mannucci, C.; Casciaro, M.; Sorbara, E.E.; Calapai, F.; di Salvo, E.; Pioggia, G.; Navarra, M.; Calapai, G.; Gangemi, S. Nutraceuticals against Oxidative Stress in Autoimmune Disorders. Antioxidants 2021, 10, 261. [Google Scholar] [CrossRef]
- Arulselvan, P.; Fard, M.T.; Tan, W.S.; Gothai, S.; Fakurazi, S.; Norhaizan, M.E.; Kumar, S.S. Role of Antioxidants and Natural Products in Inflammation. Oxid. Med. Cell Longev. 2016, 2016, 5276130. [Google Scholar] [CrossRef] [Green Version]
- Morén, C.; de Souza, R.M.; Giraldo, D.M.; Uff, C. Antioxidant Therapeutic Strategies in Neurodegenerative Diseases. Int. J. Mol. Sci. 2022, 23, 9328. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic Inflammation and Oxidative Stress as a Major Cause of Age-Related Diseases and Cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Chen, L.; Liang, J.; Chen, Z.; Zhang, C.; Zhang, Z.; Yang, J. Potential Role of Superoxide Dismutase 3 (SOD3) in Resistance to Influenza A Virus Infection. Antioxidants 2023, 12, 354. [Google Scholar] [CrossRef] [PubMed]
- Firuzi, O.; Miri, R.; Tavakkoli, M.; Saso, L. Antioxidant Therapy: Current Status and Future Prospects. Curr. Med. Chem. 2012, 18, 3871–3888. [Google Scholar] [CrossRef]
- Benfeito, S.; Oliveira, C.; Soares, P.; Fernandes, C.; Silva, T.; Teixeira, J.; Borges, F. Antioxidant Therapy: Still in Search of the “Magic Bullet”. Mitochondrion 2013, 13, 427–435. [Google Scholar] [CrossRef]
- Nguyen, D.D.; Lai, J.Y. Synthesis, Bioactive Properties, and Biomedical Applications of Intrinsically Therapeutic Nanoparticles for Disease Treatment. Chem. Eng. J. 2022, 435, 134970. [Google Scholar] [CrossRef]
- Sadidi, H.; Hooshmand, S.; Ahmadabadi, A.; Hoseini, S.J.; Baino, F.; Vatanpour, M.; Kargozar, S. Cerium Oxide Nanoparticles (Nanoceria): Hopes in Soft Tissue Engineering. Molecules 2020, 25, 4559. [Google Scholar] [CrossRef]
- Yang, B.; Chen, Y.; Shi, J. Reactive Oxygen Species (ROS)-Based Nanomedicine. Chem. Rev. 2019, 119, 4881–4985. [Google Scholar] [CrossRef]
- Xu, C.; Qu, X. Cerium Oxide Nanoparticle: A Remarkably Versatile Rare Earth Nanomaterial for Biological Applications. NPG Asia Mater. 2014, 6, e90. [Google Scholar] [CrossRef] [Green Version]
- Niu, J.; Wang, K.; Kolattukudy, P.E. Cerium Oxide Nanoparticles Inhibits Oxidative Stress and Nuclear Factor-κB Activation in H9c2 Cardiomyocytes Exposed to Cigarette Smoke Extract. J. Pharmacol. Exp. Ther. 2011, 338, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Estevez, A.Y.; Pritchard, S.; Harper, K.; Aston, J.W.; Lynch, A.; Lucky, J.J.; Ludington, J.S.; Chatani, P.; Mosenthal, W.P.; Leiter, J.C.; et al. Neuroprotective Mechanisms of Cerium Oxide Nanoparticles in a Mouse Hippocampal Brain Slice Model of Ischemia. Free Radic. Biol. Med. 2011, 51, 1155–1163. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, Z.; Wang, T.; Ma, C.; Li, H.; Lei, H.; Yang, Y.; Wang, Y.; Pei, Z.; Liu, Z.; et al. Cerium Oxide Nanoparticles with Antioxidative Neurorestoration for Ischemic Stroke. Biomaterials 2022, 291, 121904. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.K.; Kim, T.; Choi, I.-Y.; Soh, M.; Kim, D.; Kim, Y.-J.; Jang, H.; Yang, H.-S.; Kim, J.Y.; Park, H.-K.; et al. Ceria Nanoparticles That Can Protect against Ischemic Stroke. Angew. Chem. Int. Ed. 2012, 51, 11039–11043. [Google Scholar] [CrossRef] [PubMed]
- Najafi, R.; Hosseini, A.; Ghaznavi, H.; Mehrzadi, S.; Sharifi, A.M. Neuroprotective Effect of Cerium Oxide Nanoparticles in a Rat Model of Experimental Diabetic Neuropathy. Brain Res. Bull. 2017, 131, 117–122. [Google Scholar] [CrossRef] [PubMed]
- Badia, A.; Duarri, A.; Salas, A.; Rosell, J.; Ramis, J.; Gusta, M.F.; Casals, E.; Zapata, M.A.; Puntes, V.; García-Arumí, J. Repeated Topical Administration of 3 Nm Cerium Oxide Nanoparticles Reverts Disease Atrophic Phenotype and Arrests Neovascular Degeneration in AMD Mouse Models. ACS Nano 2023, 17, 910–926. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Patil, S.; Seal, S.; McGinnis, J.F. Rare Earth Nanoparticles Prevent Retinal Degeneration Induced by Intracellular Peroxides. Nat. Nanotechnol. 2006, 1, 142–150. [Google Scholar] [CrossRef]
- Carvajal, S.; Perramón, M.; Oró, D.; Casals, E.; Fernández-Varo, G.; Casals, G.; Parra, M.; González de la Presa, B.; Ribera, J.; Pastor, Ó.; et al. Cerium Oxide Nanoparticles Display Antilipogenic Effect in Rats with Non-Alcoholic Fatty Liver Disease. Sci. Rep. 2019, 9, 12848. [Google Scholar] [CrossRef] [Green Version]
- Córdoba-Jover, B.; Arce-Cerezo, A.; Ribera, J.; Pauta, M.; Oró, D.; Casals, G.; Fernández-Varo, G.; Casals, E.; Puntes, V.; Jiménez, W.; et al. Cerium Oxide Nanoparticles Improve Liver Regeneration after Acetaminophen-Induced Liver Injury and Partial Hepatectomy in Rats. J. Nanobiotechnol. 2019, 17, 112. [Google Scholar] [CrossRef]
- Fernández-Varo, G.; Perramón, M.; Carvajal, S.; Oró, D.; Casals, E.; Boix, L.; Oller, L.; Macías-Muñoz, L.; Marfà, S.; Casals, G.; et al. Bespoken Nanoceria: An Effective Treatment in Experimental Hepatocellular Carcinoma. Hepatology 2020, 72, 1267–1282. [Google Scholar] [CrossRef] [Green Version]
- Rice, K.M.; Selvaraj, V.; Manne, N.; Arvapalli, R.; Hambuchen, M. Cerium Oxide Nanoparticle Attenuates Lipopolysaccharide (LPS) Induced Acute Kidney Injury (AKI) and Acute Lung Injury (ALI) in Male Sprague Dawley Rats. Nano Res. Appl. 2018, 4, 8. [Google Scholar] [CrossRef]
- Hashem, R.M.; Rashd, L.A.; Hashem, K.S.; Soliman, H.M. Cerium Oxide Nanoparticles Alleviate Oxidative Stress and Decreases Nrf-2/HO-1 in D-GALN/LPS Induced Hepatotoxicity. Biomed. Pharmacother. 2015, 73, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Cox, G.M.; Harrison, T.S.; McDade, H.C.; Taborda, C.P.; Heinrich, G.; Casadevall, A.; Perfect, J.R. Superoxide Dismutase Influences the Virulence of Cryptococcus Neoformans by Affecting Growth within Macrophages. Infect. Immun. 2003, 71, 173–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, D.D.; Yao, C.H.; Lue, S.J.; Yang, C.J.; Su, Y.H.; Huang, C.C.; Lai, J.Y. Amination-Mediated Nano Eye-Drops with Enhanced Corneal Permeability and Effective Burst Release for Acute Glaucoma Treatment. Chem. Eng. J. 2023, 451, 138620. [Google Scholar] [CrossRef]
- Luo, L.J.; Nguyen, D.D.; Lai, J.Y. Harnessing the Tunable Cavity of Nanoceria for Enhancing Y-27632-Mediated Alleviation of Ocular Hypertension. Theranostics 2021, 11, 5447–5463. [Google Scholar] [CrossRef]
- Colon, J.; Hsieh, N.; Ferguson, A.; Kupelian, P.; Seal, S.; Jenkins, D.W.; Baker, C.H. Cerium Oxide Nanoparticles Protect Gastrointestinal Epithelium from Radiation-Induced Damage by Reduction of Reactive Oxygen Species and Upregulation of Superoxide Dismutase 2. Nanomedicine 2010, 6, 698–705. [Google Scholar] [CrossRef]
- Xu, P.T.; Maidment, B.W.; Antonic, V.; Jackson, I.L.; Das, S.; Zodda, A.; Zhang, X.; Seal, S.; Vujaskovic, Z. Cerium Oxide Nanoparticles: A Potential Medical Countermeasure to Mitigate Radiation-Induced Lung Injury in CBA/J Mice. Radiat. Res. 2016, 185, 516–526. [Google Scholar] [CrossRef] [Green Version]
- D’Angelo, B.; Santucci, S.; Benedetti, E.; di Loreto, S.; Phani, R.A.; Falone, S.; Amicarelli, F.; Ceru, M.P.; Cimini, A. Cerium Oxide Nanoparticles Trigger Neuronal Survival in a Human Alzheimer Disease Model by Modulating BDNF Pathway. Curr. Nanosci. 2009, 5, 167–176. [Google Scholar] [CrossRef]
- Pinna, A.; Malfatti, L.; Galleri, G.; Manetti, R.; Cossu, S.; Rocchitta, G.; Migheli, R.; Serra, P.A.; Innocenzi, P. Ceria Nanoparticles for the Treatment of Parkinson-like Diseases Induced by Chronic Manganese Intoxication. RSC Adv. 2015, 5, 20432–20439. [Google Scholar] [CrossRef]
- Casals, E.; Gusta, M.F.; Piella, J.; Casals, G.; Jiménez, W.; Puntes, V. Intrinsic and Extrinsic Properties Affecting Innate Immune Responses to Nanoparticles: The Case of Cerium Oxide. Front. Immunol. 2017, 8, 970. [Google Scholar] [CrossRef] [Green Version]
- Mossman, B.T.; Churg, A. Mechanisms in the Pathogenesis of Asbestosis and Silicosis. Am. J. Respir. Crit. Care Med. 2012, 157, 1666–1680. [Google Scholar] [CrossRef] [Green Version]
- Longmire, M.; Choyke, P.L.; Kobayashi, H. Clearance Properties of Nano-Sized Particles and Molecules as Imaging Agents: Considerations and Caveats. Nanomedicine 2008, 3, 703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soo Choi, H.; Liu, W.; Misra, P.; Tanaka, E.; Zimmer, J.P.; Itty Ipe, B.; Bawendi, M.G.; Frangioni, J.V. Renal Clearance of Nanoparticles. Nat. Biotechnol. 2007, 25, 1165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comper, W.D.; Glasgow, E.F. Charge Selectivity in Kidney Ultrafiltration. Kidney Int. 1995, 47, 1242–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, H.; Le, N.; Kim, I.-S.; Kim, M.-K.; Pie, J.-E.; Drumm, D.; Paik, D.S.; Waldmann, T.A.; Paik, C.H.; Carrasquillo, J.A.; et al. The Pharmacokinetic Characteristics of Glycolated Humanized Anti-Tac Fabs Are Determined by Their Isoelectric Points. Cancer Res. 1999, 59, 422–430. [Google Scholar]
- Tsoi, K.M.; Macparland, S.A.; Ma, X.Z.; Spetzler, V.N.; Echeverri, J.; Ouyang, B.; Fadel, S.M.; Sykes, E.A.; Goldaracena, N.; Kaths, J.M.; et al. Mechanism of Hard-Nanomaterial Clearance by the Liver. Nat. Mater. 2016, 15, 1212–1221. [Google Scholar] [CrossRef]
- Poon, W.; Zhang, Y.N.; Ouyang, B.; Kingston, B.R.; Wu, J.L.; Wilhelm, S.; Chan, W.C. Elimination Pathways of Nanoparticles. ACS Nano 2019, 13, 5785–5798. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, M.F.; Rohrer, U.H.; Kündig, T.M.; Bürki, K.; Hengartner, H.; Zinkernagel, R.M. The Influence of Antigen Organization on B Cell Responsiveness. Science 1993, 262, 1448–1451. [Google Scholar] [CrossRef]
- Sonavane, G.; Tomoda, K.; Makino, K. Biodistribution of Colloidal Gold Nanoparticles after Intravenous Administration: Effect of Particle Size. Colloids Surf. B Biointerfaces 2008, 66, 274–280. [Google Scholar] [CrossRef]
- Barbero, F.; Russo, L.; Vitali, M.; Piella, J.; Salvo, I.; Borrajo, M.L.; Busquets-Fité, M.; Grandori, R.; Bastús, N.G.; Casals, E.; et al. Formation of the Protein Corona: The Interface between Nanoparticles and the Immune System. Semin. Immunol. 2017, 34, 52–60. [Google Scholar] [CrossRef]
- Piella, J.; Bastús, N.G.; Puntes, V. Size-Dependent Protein-Nanoparticle Interactions in Citrate-Stabilized Gold Nanoparticles: The Emergence of the Protein Corona. Bioconjug. Chem. 2017, 28, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Bachmann, M.F.; Jennings, G.T. Vaccine Delivery: A Matter of Size, Geometry, Kinetics and Molecular Patterns. Nat. Rev. Immunol. 2010, 10, 787–796. [Google Scholar] [CrossRef] [PubMed]
- Ernst, L.M.; Casals, E.; Italiani, P.; Boraschi, D.; Puntes, V. The Interactions between Nanoparticles and the Innate Immune System from a Nanotechnologist Perspective. Nanomaterials 2021, 11, 2991. [Google Scholar] [CrossRef] [PubMed]
- Veronese, F.M.; Pasut, G. PEGylation, Successful Approach to Drug Delivery. Drug Discov. Today 2005, 10, 1451–1458. [Google Scholar] [CrossRef]
- Milton Harris, J.; Chess, R.B. Effect of Pegylation on Pharmaceuticals. Nat. Rev. Drug Discov. 2003, 2, 214–221. [Google Scholar] [CrossRef]
- Casals, E.; Vázquez-Campos, S.; Bastús, N.G.; Puntes, V. Distribution and Potential Toxicity of Engineered Inorganic Nanoparticles and Carbon Nanostructures in Biological Systems. TrAC Trends Anal. Chem. 2008, 27, 672–683. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Wautier, J.L.; Wautier, M.P. Vascular Permeability in Diseases. Int. J. Mol. Sci. 2022, 23, 3645. [Google Scholar] [CrossRef]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef]
- Ngoune, R.; Peters, A.; von Elverfeldt, D.; Winkler, K.; Pütz, G. Accumulating Nanoparticles by EPR: A Route of No Return. J. Control. Release 2016, 238, 58–70. [Google Scholar] [CrossRef] [Green Version]
- Portioli, C.; Benati, D.; Pii, Y.; Bernardi, P.; Crucianelli, M.; Santucci, S.; Bentivoglio, M.; Passacantando, M. Short-Term Biodistribution of Cerium Oxide Nanoparticles in Mice: Focus on Brain Parenchyma. Nanosci. Nanotechnol. Lett. 2013, 5, 1174–1181. [Google Scholar] [CrossRef]
- Bao, Q.; Hu, P.; Xu, Y.; Cheng, T.; Wei, C.; Pan, L.; Shi, J. Simultaneous Blood–Brain Barrier Crossing and Protection for Stroke Treatment Based on Edaravone-Loaded Ceria Nanoparticles. ACS Nano 2018, 12, 6794–6805. [Google Scholar] [CrossRef]
- Subedi, L.; Venkatesan, R.; Kim, S.Y. Neuroprotective and Anti-Inflammatory Activities of Allyl Isothiocyanate through Attenuation of JNK/NF-ΚB/TNF-α Signaling. Int. J. Mol. Sci. 2017, 18, 1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzolini, J.; Weber, R.J.M.; Chen, H.S.; Khan, A.; Guggenheim, E.; Shaw, R.K.; Chipman, J.K.; Viant, M.R.; Rappoport, J.Z. Protein Corona Modulates Uptake and Toxicity of Nanoceria via Clathrin-Mediated Endocytosis. Biol. Bull. 2016, 231, 40–60. [Google Scholar] [CrossRef] [PubMed]
- Clària, J.; Jiménez, W. Experimental Models of Cirrhosis and Ascites. In Ascites and Renal Dysfunction in Liver Disease: Pathogenesis, Diagnosis, and Treatment, 2nd ed.; Wiley-Blackwell: Hoboken, NJ, USA, 2007; pp. 215–226. [Google Scholar] [CrossRef]
- Oró, D.; Yudina, T.; Fernández-Varo, G.; Casals, E.; Reichenbach, V.; Casals, G.; González de la Presa, B.; Sandalinas, S.; Carvajal, S.; Puntes, V.; et al. Cerium Oxide Nanoparticles Reduce Steatosis, Portal Hypertension and Display Anti-Inflammatory Properties in Rats with Liver Fibrosis. J. Hepatol. 2016, 64, 691–698. [Google Scholar] [CrossRef]
- Kuntz, E.; Kuntz, H.-D. Hepatology Textbook and Atlas, 3rd ed.; Springer Berlin Heidelberg: Graz, Austria, 2008. [Google Scholar]
- Pourbaix, M. Atlas of Electrochemical Equilibria in Aqueous Solutions, 1st ed.; Pergamon Press: Oxford, UK, 1966. [Google Scholar]
- Plakhova, T.V.; Romanchuk, A.Y.; Yakunin, S.N.; Dumas, T.; Demir, S.; Wang, S.; Minasian, S.G.; Shuh, D.K.; Tyliszczak, T.; Shiryaev, A.A.; et al. Solubility of Nanocrystalline Cerium Dioxide: Experimental Data and Thermodynamic Modeling. J. Phys. Chem. C 2016, 120, 22615–22626. [Google Scholar] [CrossRef] [Green Version]
- Auffan, M.; Rose, J.; Bottero, J.Y.; Lowry, G.V.; Jolivet, J.P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef]
- Muhammad, F.; Wang, A.; Qi, W.; Zhang, S.; Zhu, G. Intracellular Antioxidants Dissolve Man-Made Antioxidant Nanoparticles: Using Redox Vulnerability of Nanoceria to Develop a Responsive Drug Delivery System. ACS Appl. Mater. Interfaces 2014, 6, 19424–19433. [Google Scholar] [CrossRef]
- Galyamin, D.; Ernst, L.M.; Fitó-Parera, A.; Mira-Vidal, G.; Bastús, N.G.; Sabaté, N.; Puntes, V. Nanoceria Dissolution at Acidic PH by Breaking off the Catalytic Loop. Nanoscale 2022, 14, 14223–14230. [Google Scholar] [CrossRef]
- Mülhopt, S.; Diabaté, S.; Dilger, M.; Adelhelm, C.; Anderlohr, C.; Bergfeldt, T.; de la Torre, J.G.; Jiang, Y.; Valsami-Jones, E.; Langevin, D.; et al. Characterization of Nanoparticle Batch-To-Batch Variability. Nanomaterials 2018, 8, 311. [Google Scholar] [CrossRef] [Green Version]
- Casals, E.; Pfaller, T.; Duschl, A.; Oostingh, G.J.; Puntes, V.F. Hardening of the Nanoparticle–Protein Corona in Metal (Au, Ag) and Oxide (Fe3O4, CoO, and CeO2) Nanoparticles. Small 2011, 7, 3479–3486. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, M.; Vigliotti, C.; Mosca, T.; Cammarota, M.R.; Capone, D. Emerging Role of the Spleen in the Pharmacokinetics of Monoclonal Antibodies, Nanoparticles and Exosomes. Int. J. Mol. Sci. 2017, 18, 1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Demoy, M.; Andreux, J.P.; Weingarten, C.; Gouritin, B.; Guilloux, V.; Couvreur, P. In Vitro Evaluation of Nanoparticles Spleen Capture. Life Sci. 1999, 64, 1329–1337. [Google Scholar] [CrossRef] [PubMed]
- Park, K.; Park, J.; Lee, H.; Choi, J.; Yu, W.J.; Lee, J. Toxicity and Tissue Distribution of Cerium Oxide Nanoparticles in Rats by Two Different Routes: Single Intravenous Injection and Single Oral Administration. Arch. Pharm. Res. 2018, 41, 1108–1116. [Google Scholar] [CrossRef]
- Dubaj, T.; Kozics, K.; Sramkova, M.; Manova, A.; Bastús, N.G.; Moriones, O.H.; Kohl, Y.; Dusinska, M.; Runden-Pran, E.; Puntes, V.; et al. Pharmacokinetics of PEGylated Gold Nanoparticles: In Vitro-In Vivo Correlation. Nanomaterials 2022, 12, 511. [Google Scholar] [CrossRef]
- Ahmed, T.A. Pharmacokinetics of Drugs Following IV Bolus, IV Infusion, and Oral Administration. Basic Pharmacokinet. Concepts Some Clin. Appl. 2015, 10, 53–98. [Google Scholar] [CrossRef] [Green Version]
- Carlander, U.; Moto, T.P.; Desalegn, A.A.; Yokel, R.A.; Johanson, G. Physiologically Based Pharmacokinetic Modeling of Nanoceria Systemic Distribution in Rats Suggests Dose- and Route-Dependent Biokinetics. Int. J. Nanomed. 2018, 13, 2631–2646. [Google Scholar] [CrossRef] [Green Version]
- Kawagoe, M.; Ishikawa, K.; Wang, S.C.; Yoshikawa, K.; Arany, S.; Zhou, X.P.; Wang, J.S.; Ueno, Y.; Koizumi, Y.; Kameda, T.; et al. Acute Effects on the Lung and the Liver of Oral Administration of Cerium Chloride on Adult, Neonatal and Fetal Mice. J. Trace Elem. Med. Biol. 2008, 22, 59–65. [Google Scholar] [CrossRef]
- Yokel, R.A.; Hussain, S.; Garantziotis, S.; Demokritou, P.; Castranova, V.; Cassee, F.R. The Yin: An Adverse Health Perspective of Nanoceria: Uptake, Distribution, Accumulation, and Mechanisms of Its Toxicity. Environ. Sci. Nano 2014, 1, 406. [Google Scholar] [CrossRef] [Green Version]
- Yokel, R.A.; Au, T.C.; MacPhail, R.; Hardas, S.S.; Butterfield, D.A.; Sultana, R.; Goodman, M.; Tseng, M.T.; Dan, M.; Haghnazar, H.; et al. Distribution, Elimination, and Biopersistence to 90 Days of a Systemically Introduced 30 Nm Ceria-Engineered Nanomaterial in Rats. Toxicol. Sci. 2012, 127, 256–268. [Google Scholar] [CrossRef] [Green Version]
- Yokel, R.A.; Tseng, M.T.; Dan, M.; Unrine, J.M.; Graham, U.M.; Wu, P.; Grulke, E.A. Biodistribution and Biopersistence of Ceria Engineered Nanomaterials: Size Dependence. Nanomedicine 2013, 9, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Yin, H.; Li, J.; Zhang, L.; Hou, R.; Wang, S. Investigation of Rare Earth Elements in Urine and Drinking Water of Children in Mining Area. Medicine 2018, 97, e12717. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.Q. Coagulation Disorders Following an Accidental Ingestion of Cerium Dioxide Nanoparticles. Environ. Toxicol. Pharmacol. 2021, 82, 103560. [Google Scholar] [CrossRef] [PubMed]
- Funakoshi, T.; Furushima, K.; Shimada, H.; Kojima, S. Anticoagulant Action of Rare Earth Metals. Biochem. Int. 1992, 28, 113–119. [Google Scholar] [PubMed]
- Lee, J.; Jeong, J.S.; Kim, S.Y.; Lee, S.J.; Shin, Y.J.; Im, W.J.; Kim, S.H.; Park, K.; Jeong, E.J.; Nam, S.Y.; et al. Safety Assessment of Cerium Oxide Nanoparticles: Combined Repeated-Dose Toxicity with Reproductive/Developmental Toxicity Screening and Biodistribution in Rats. Nanotoxicology 2020, 14, 696–710. [Google Scholar] [CrossRef]
- GRAS Notices. Available online: https://www.cfsanappsexternal.fda.gov/scripts/fdcc/?set=GRASNotices&id=321&sort=GRN_No&order=DESC&startrow=1&type=column&search=Substance%C2%A4VARCHAR%C2%A4silica (accessed on 24 February 2023).







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ernst, L.M.; Mondragón, L.; Ramis, J.; Gustà, M.F.; Yudina, T.; Casals, E.; Bastús, N.G.; Fernández-Varo, G.; Casals, G.; Jiménez, W.; et al. Exploring the Long-Term Tissue Accumulation and Excretion of 3 nm Cerium Oxide Nanoparticles after Single Dose Administration. Antioxidants 2023, 12, 765. https://doi.org/10.3390/antiox12030765
Ernst LM, Mondragón L, Ramis J, Gustà MF, Yudina T, Casals E, Bastús NG, Fernández-Varo G, Casals G, Jiménez W, et al. Exploring the Long-Term Tissue Accumulation and Excretion of 3 nm Cerium Oxide Nanoparticles after Single Dose Administration. Antioxidants. 2023; 12(3):765. https://doi.org/10.3390/antiox12030765
Chicago/Turabian StyleErnst, Lena M., Laura Mondragón, Joana Ramis, Muriel F. Gustà, Tetyana Yudina, Eudald Casals, Neus G. Bastús, Guillermo Fernández-Varo, Gregori Casals, Wladimiro Jiménez, and et al. 2023. "Exploring the Long-Term Tissue Accumulation and Excretion of 3 nm Cerium Oxide Nanoparticles after Single Dose Administration" Antioxidants 12, no. 3: 765. https://doi.org/10.3390/antiox12030765