Antrodin C Isolated from Antrodia Cinnamomea Induced Apoptosis through ROS/AKT/ERK/P38 Signaling Pathway and Epigenetic Histone Acetylation of TNFα in Colorectal Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Antrodia cinnamomea Extracts and Analysis of Antrodin C
2.2. Cell Culture
2.3. Cell Growth and Cell-Cycle Distribution Analysis
2.4. Measurement of Apoptosis Assay and Reactive Oxygen Species
2.5. Protein Extraction and Immunoblot Analyses
2.6. Animal Study
2.7. Histochemistry and Immunohistochemistry Analysis
2.8. Chromatin Immunoprecipitation (ChIP) Analysis
2.9. Statistical Analysis
3. Results
3.1. Antrodin C Induces Cell Apoptosis of Human Colorectal Cancer Cells
3.2. Antrodin-C-Mediated In Vivo Growth Inhibition of Colorectal Cancer Cell Xenograft
3.3. Activation of the Extrinsic Cell Apoptosis Pathway in HCT-116 Cells by Antrodin C
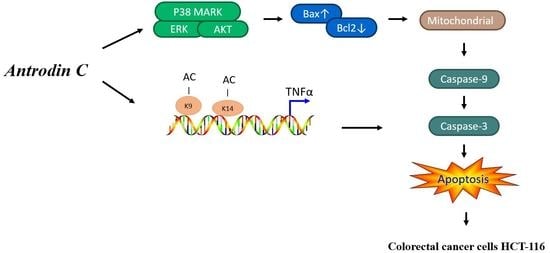
3.4. Antrodin C Increases the Expression of Tumor Necrosis Factor α via Reactive Oxygen Species and ERK/AKT/p38-Mediated Epigenetic Histone Acetylation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brenner, H.; Chen, C. The colorectal cancer epidemic: Challenges and opportunities for primary, secondary and tertiary prevention. Br. J. Cancer 2018, 119, 785–792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef] [Green Version]
- Li-Weber, M. Targeting apoptosis pathways in cancer by Chinese medicine. Cancer Lett. 2013, 332, 304–312. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Huang, C.H.; Chang, Y.Y.; Liu, C.W.; Kang, W.Y.; Lin, Y.L.; Chang, H.C.; Chen, Y.C. Fruiting body of Niuchangchih (Antrodia camphorata) protects livers against chronic alcohol consumption damage. J. Agric. Food Chem. 2010, 58, 3859–3866. [Google Scholar] [CrossRef]
- Wu, M.T.; Tzang, B.S.; Chang, Y.Y.; Chiu, C.H.; Kang, W.Y.; Huang, C.H.; Chen, Y.C. Effects of Antrodia camphorata on alcohol clearance and antifibrosis in livers of rats continuously fed alcohol. J. Agric. Food Chem. 2011, 59, 4248–4254. [Google Scholar] [CrossRef]
- Chou, M.C.; Chang, R.N.; Hung, Y.H.; Chen, Y.C.; Chiu, C.H. Antrodia camphorata ameliorates high-fat-diet induced hepatic steatosis via improving lipid metabolism and antioxidative status. J. Funct. Foods 2013, 5, 1317–1325. [Google Scholar] [CrossRef]
- Zhu, P.L.; Fu, X.Q.; Li, J.K.; Tse, A.K.; Guo, H.; Yin, C.L.; Chou, J.Y.; Wang, Y.P.; Liu, Y.X.; Chen, Y.J.; et al. Antrodia camphorata Mycelia Exert Anti-liver Cancer Effects and Inhibit STAT3 Signaling in vitro and in vivo. Front. Pharmacol. 2018, 9, 1449. [Google Scholar] [CrossRef]
- Chen, C.C.; Liu, Y.W.; Ker, Y.B.; Wu, Y.Y.; Lai, E.Y.; Chyau, C.C.; Hseu, T.H.; Peng, R.Y. Chemical characterization and anti-inflammatory effect of polysaccharides fractionated from submerge-cultured Antrodia camphorata mycelia. J. Agric. Food Chem. 2007, 55, 5007–5012. [Google Scholar] [CrossRef]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsiao, G.; Shen, M.Y.; Lin, K.H.; Lan, M.H.; Wu, L.Y.; Chou, D.S.; Lin, C.H.; Su, C.H.; Sheu, J.R. Antioxidative and hepatoprotective effects of Antrodia camphorata extract. J. Agric. Food Chem. 2003, 51, 3302–3308. [Google Scholar] [CrossRef] [PubMed]
- Fa, K.N.; Yang, C.M.; Chen, P.C.; Lee, Y.Y.; Chyau, C.C.; Hu, M.L. Anti-metastatic effects of antrodan, the Antrodia cinnamomea mycelia glycoprotein, in lung carcinoma cells. Int. J. Biol. Macromol. 2015, 74, 476–482. [Google Scholar] [CrossRef]
- Yang, H.; Bai, X.; Zhang, H.; Zhang, J.; Wu, Y.; Tang, C.; Liu, Y.; Yang, Y.; Liu, Z.; Jia, W.; et al. Antrodin C, an NADPH Dependent Metabolism, Encourages Crosstalk between Autophagy and Apoptosis in Lung Carcinoma Cells by Use of an AMPK Inhibition-Independent Blockade of the Akt/mTOR Pathway. Molecules 2019, 24, 993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, K.J.; Vani, M.G.; Chueh, P.J.; Mau, J.L.; Wang, S.Y. Antrodin C inhibits epithelial-to-mesenchymal transition and metastasis of breast cancer cells via suppression of Smad2/3 and beta-catenin signaling pathways. PLoS ONE 2015, 10, e01171112015. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.W.; Chen, C.C.; Wu, M.J.; Chen, Y.S.; Chen, C.C.; Sheu, S.J.; Lin, T.W.; Chou, S.H.; Lin, S.C.; Liu, C.J.; et al. Active Component of Antrodia cinnamomea Mycelia Targeting Head and Neck Cancer Initiating Cells through Exaggerated Autophagic Cell Death. Evid.-Based Complement. Altern. Med. 2013, 2013, 946451. [Google Scholar] [CrossRef] [Green Version]
- Loo, G. Redox-sensitive mechanisms of phytochemical-mediated inhibition of cancer cell proliferation (review). J. Nutr. Biochem. 2003, 14, 64–73. [Google Scholar] [CrossRef]
- Tung, S.Y.; Lee, K.C.; Lee, K.F.; Yang, Y.L.; Huang, W.S.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; Teng, C.C.; Shen, C.H.; et al. Apoptotic mechanisms of gastric cancer cells induced by isolated erinacine S through epigenetic histone H3 methylation of FasL and TRAIL. Food Funct. 2021, 12, 3455–3468. [Google Scholar] [CrossRef]
- Insinga, A.; Monestiroli, S.; Ronzoni, S.; Gelmetti, V.; Marchesi, F.; Viale, A.; Altucci, L.; Nervi, C.; Minucci, S.; Pelicci, P.G. Inhibitors of histone deacetylases induce tumor-selective apoptosis through activation of the death receptor pathway. Nat. Med. 2005, 11, 71–76. [Google Scholar] [CrossRef]
- Wu, M.D.; Cheng, M.J.; Wang, B.C.; Yech, Y.J.; Lai, J.T.; Kuo, Y.H.; Yuan, G.F.; Chen, I.S. Maleimide and maleic anhydride derivatives from the mycelia of Antrodia cinnamomea and their nitric oxide inhibitory activities in macrophages. J. Nat. Prod. 2008, 71, 1258–1261. [Google Scholar] [CrossRef] [PubMed]
- Tsay, H.J.; Liu, H.K.; Kuo, Y.H.; Chiu, C.S.; Liang, C.C.; Chung, C.W.; Chen, C.C.; Chen, Y.P.; Shiao, Y.J. EK100 and Antrodin C Improve Brain Amyloid Pathology in APP/PS1 Transgenic Mice by Promoting Microglial and Perivascular Clearance Pathways. Int. J. Mol. Sci. 2021, 22, 10413. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.Y.; Lee, K.C.; Tung, S.Y.; Huang, W.S.; Teng, C.C.; Lee, K.F.; Hsieh, M.C.; Kuo, H.C. 2D-DIGE-MS Proteomics Approaches for Identification of Gelsolin and Peroxiredoxin 4 with Lymph Node Metastasis in Colorectal Cancer. Cancers 2022, 14, 3189. [Google Scholar] [CrossRef] [PubMed]
- Teng, C.C.; Tung, S.Y.; Lee, K.C.; Lee, K.F.; Huang, W.S.; Shen, C.H.; Hsieh, M.C.; Huang, C.Y.; Sheen, J.M.; Kuo, H.C. Novel regulator role of CIL-102 in the epigenetic modification of TNFR1/TRAIL to induce cell apoptosis in human gastric cancer. Food Chem. Toxicol. 2021, 147, 111856. [Google Scholar] [CrossRef]
- Cheng, K.C.; Kuo, H.C.; Hsieh, M.C.; Huang, C.Y.; Teng, C.C.; Tung, S.Y.; Shen, C.H.; Lee, K.F.; Yang, Y.L.; Lee, K.C. Identification of Two Novel CIL-102 Upregulations of ERP29 and FUMH to Inhibit the Migration and Invasiveness of Colorectal Cancer Cells by Using the Proteomic Approach. Biomolecules 2021, 11, 1280. [Google Scholar] [CrossRef]
- Lu, C.C.; Huang, W.S.; Lee, K.F.; Lee, K.C.; Hsieh, M.C.; Huang, C.Y.; Lee, L.Y.; Lee, B.O.; Teng, C.C.; Shen, C.H.; et al. Inhibitory effect of Erinacines A on the growth of DLD-1 colorectal cancer cells is induced by generation of reactive oxygen species and activation of p70S6K and p21. J. Funct. Foods 2016, 21, 474–484. [Google Scholar] [CrossRef]
- Lee, K.C.; Kuo, H.C.; Shen, C.H.; Lu, C.C.; Huang, W.S.; Hsieh, M.C.; Huang, C.Y.; Kuo, Y.H.; Hsieh, Y.Y.; Teng, C.C.; et al. A proteomics approach to identifying novel protein targets involved in erinacine A-mediated inhibition of colorectal cancer cells’ aggressiveness. J. Cell. Mol. Med. 2017, 21, 588–599. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.C.; Lee, K.F.; Tung, S.Y.; Huang, W.S.; Lee, L.Y.; Chen, W.P.; Chen, C.C.; Teng, C.C.; Shen, C.H.; Hsieh, M.C.; et al. Induction Apoptosis of Erinacine A in Human Colorectal Cancer Cells Involving the Expression of TNFR, Fas, and Fas Ligand via the JNK/p300/p50 Signaling Pathway With Histone Acetylation. Front. Pharmacol. 2019, 10, 1174. [Google Scholar] [CrossRef]
- Yue, P.Y.; Wong, Y.Y.; Wong, K.Y.; Tsoi, Y.K.; Leung, K.S. Current evidence for the hepatoprotective activities of the medicinal mushroom Antrodia cinnamomea. Chin. Med. 2013, 8, 21. [Google Scholar] [CrossRef] [Green Version]
- Nebbioso, A.; Clarke, N.; Voltz, E.; Germain, E.; Ambrosino, C.; Bontempo, P.; Alvarez, R.; Schiavone, E.M.; Ferrara, F.; Bresciani, F.; et al. Tumor-selective action of HDAC inhibitors involves TRAIL induction in acute myeloid leukemia cells. Nat. Med. 2005, 11, 77–84. [Google Scholar] [CrossRef]
- Elmallah, M.I.Y.; Micheau, O. Epigenetic Regulation of TRAIL Signaling: Implication for Cancer Therapy. Cancers 2019, 11, 850. [Google Scholar] [CrossRef] [Green Version]
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Quang, T.H.; Kim, D.C.; Van Kiem, P.; Van Minh, C.; Nhiem, N.X.; Tai, B.H.; Yen, P.H.; Thi Thanh Ngan, N.; Kim, H.J.; Oh, H. Macrolide and phenolic metabolites from the marine-derived fungus Paraconiothyrium sp. VK-13 with anti-inflammatory activity. J. Antibiot. 2018, 71, 826–830. [Google Scholar] [CrossRef]
- Ranjan, A.; Ramachandran, S.; Gupta, N.; Kaushik, I.; Wright, S.; Srivastava, S.; Das, H.; Srivastava, S.; Prasad, S.; Srivastava, S.K. Role of Phytochemicals in Cancer Prevention. Int. J. Mol. Sci. 2019, 20, 4981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patra, S.; Pradhan, B.; Nayak, R.; Behera, C.; Panda, K.C.; Das, S.; Jena, M.; Bhutia, S.K. Apoptosis and autophagy modulating dietary phytochemicals in cancer therapeutics: Current evidences and future perspectives. Phytother. Res. 2021, 35, 4194–4214. [Google Scholar] [CrossRef]
- Wang, J.; Yi, J. Cancer cell killing via ROS: To increase or decrease, that is the question. Cancer Biol. Ther. 2008, 7, 1875–1884. [Google Scholar] [CrossRef] [PubMed]
- Mosieniak, G.; Adamowicz, M.; Alster, O.; Jaskowiak, H.; Szczepankiewicz, A.A.; Wilczynski, G.M.; Ciechomska, I.A.; Sikora, E. Curcumin induces permanent growth arrest of human colon cancer cells: Link between senescence and autophagy. Mech. Ageing Dev. 2012, 133, 444–455. [Google Scholar] [CrossRef]
- Randhawa, H.; Kibble, K.; Zeng, H.; Moyer, M.P.; Reindl, K.M. Activation of ERK signaling and induction of colon cancer cell death by piperlongumine. Toxicol. Vitr. 2013, 27, 1626–1633. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Gao, S.; Yang, Y.; Zhao, X.; Fan, Y.; Ma, W.; Yang, D.; Yang, A.; Yu, Y. Curcumin induced autophagy anticancer effects on human lung adenocarcinoma cell line A549. Oncol. Lett. 2017, 14, 2775–2782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moosavi, M.A.; Haghi, A.; Rahmati, M.; Taniguchi, H.; Mocan, A.; Echeverria, J.; Gupta, V.K.; Tzvetkov, N.T.; Atanasov, A.G. Phytochemicals as potent modulators of autophagy for cancer therapy. Cancer Lett. 2018, 424, 46–69. [Google Scholar] [CrossRef]
- Miyo, M.; Yamamoto, H.; Konno, M.; Colvin, H.; Nishida, N.; Koseki, J.; Kawamoto, K.; Ogawa, H.; Hamabe, A.; Uemura, M.; et al. Tumour-suppressive function of SIRT4 in human colorectal cancer. Br. J. Cancer 2015, 113, 492–499. [Google Scholar] [CrossRef] [Green Version]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.C.; Patchva, S.; Aggarwal, B.B. Therapeutic roles of curcumin: Lessons learned from clinical trials. AAPS J. 2013, 15, 195–218. [Google Scholar] [CrossRef] [Green Version]
- Turk, M.A.; Murphy, C.; McCaffrey, J.; Murray, K. Predictors of adverse gambling behaviours amongst elite athletes. Sci. Rep. 2023, 13, 823. [Google Scholar] [CrossRef] [PubMed]
- Liebl, M.C.; Hofmann, T.G. The Role of p53 Signaling in Colorectal Cancer. Cancers 2021, 13, 2125. [Google Scholar] [CrossRef]
- Otto, T.; Sicinski, P. Cell cycle proteins as promising targets in cancer therapy. Nat. Rev. Cancer 2017, 17, 93–115. [Google Scholar] [CrossRef] [Green Version]
- Zhu, J.Y.; Lavrik, I.N.; Mahlknecht, U.; Giaisi, M.; Proksch, P.; Krammer, P.H.; Li-Weber, M. The traditional Chinese herbal compound rocaglamide preferentially induces apoptosis in leukemia cells by modulation of mitogen-activated protein kinase activities. Int. J. Cancer 2007, 121, 1839–1846. [Google Scholar] [CrossRef] [PubMed]
- Li-Weber, M. New therapeutic aspects of flavones: The anticancer properties of Scutellaria and its main active constituents Wogonin, Baicalein and Baicalin. Cancer Treat. Rev. 2009, 35, 57–68. [Google Scholar] [CrossRef]
- Huang, W.S.; Kuo, Y.H.; Kuo, H.C.; Hsieh, M.C.; Huang, C.Y.; Lee, K.C.; Lee, K.F.; Shen, C.H.; Tung, S.Y.; Teng, C.C. CIL-102-Induced Cell Cycle Arrest and Apoptosis in Colorectal Cancer Cells via Upregulation of p21 and GADD45. PLoS ONE 2017, 12, e0168989. [Google Scholar] [CrossRef] [Green Version]
- Kuo, H.C.; Kuo, Y.R.; Lee, K.F.; Hsieh, M.C.; Huang, C.Y.; Hsieh, Y.Y.; Lee, K.C.; Kuo, H.L.; Lee, L.Y.; Chen, W.P.; et al. A Comparative Proteomic Analysis of Erinacine A’s Inhibition of Gastric Cancer Cell Viability and Invasiveness. Cell. Physiol. Biochem. 2017, 43, 195–208. [Google Scholar] [CrossRef]
- Teng, C.C.; Kuo, H.C.; Sze, C.I. Quantitative proteomic analysis of the inhibitory effects of CIL-102 on viability and invasiveness in human glioma cells. Toxicol. Appl. Pharmacol. 2013, 272, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Senthil, K.K.J.; Gokila, V.M.; Wang, S.Y. Activation of Nrf2-mediated anti-oxidant genes by antrodin C prevents hyperglycemia-induced senescence and apoptosis in human endothelial cells. Oncotarget 2017, 8, 96568–96587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eckschlager, T.; Plch, J.; Stiborova, M.; Hrabeta, J. Histone Deacetylase Inhibitors as Anticancer Drugs. Int. J. Mol. Sci. 2017, 18, 1414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouyahya, A.; Mechchate, H.; Oumeslakht, L.; Zeouk, I.; Aboulaghras, S.; Balahbib, A.; Zengin, G.; Kamal, M.A.; Gallo, M.; Montesano, D.; et al. The Role of Epigenetic Modifications in Human Cancers and the Use of Natural Compounds as Epidrugs: Mechanistic Pathways and Pharmacodynamic Actions. Biomolecules 2022, 12, 367. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Tian, Y.; Zhu, W.G. The Roles of Histone Deacetylases and Their Inhibitors in Cancer Therapy. Front. Cell Dev. Biol. 2020, 8, 576946. [Google Scholar] [CrossRef]
- Pelaez, I.M.; Kalogeropoulou, M.; Ferraro, A.; Voulgari, A.; Pankotai, T.; Boros, I.; Pintzas, A. Oncogenic RAS alters the global and gene-specific histone modification pattern during epithelial-mesenchymal transition in colorectal carcinoma cells. Int. J. Biochem. Cell Biol. 2010, 42, 911–920. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salvador, L.M.; Park, Y.; Cottom, J.; Maizels, E.T.; Jones, J.C.; Schillace, R.V.; Carr, D.W.; Cheung, P.; Allis, C.D.; Jameson, J.L.; et al. Follicle-stimulating hormone stimulates protein kinase A-mediated histone H3 phosphorylation and acetylation leading to select gene activation in ovarian granulosa cells. J. Biol. Chem. 2001, 276, 40146–40155. [Google Scholar] [CrossRef] [Green Version]
- Covarrubias, A.J.; Aksoylar, H.I.; Yu, J.; Snyder, N.W.; Worth, A.J.; Iyer, S.S.; Wang, J.; Ben-Sahra, I.; Byles, V.; Polynne-Stapornkul, T.; et al. Akt-mTORC1 signaling regulates Acly to integrate metabolic input to control of macrophage activation. eLife 2016, 5, e11612. [Google Scholar] [CrossRef]








| Apoptosis (%) | |
|---|---|
| Control | 0 |
| ADC 50 μM | 10 ± 3 |
| ADC plus NAC | 3 ± 1 |
| ADC plus PD | 10 ± 2 |
| ADC plus Wort | 6 ± 2 |
| ADC plus SB | 5 ± 2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-Y.; Lee, K.-C.; Cheng, K.-C.; Lee, K.-F.; Yang, Y.-L.; Chu, H.-T.; Lin, T.-W.; Chen, C.-C.; Hsieh, M.-C.; Huang, C.-Y.; et al. Antrodin C Isolated from Antrodia Cinnamomea Induced Apoptosis through ROS/AKT/ERK/P38 Signaling Pathway and Epigenetic Histone Acetylation of TNFα in Colorectal Cancer Cells. Antioxidants 2023, 12, 764. https://doi.org/10.3390/antiox12030764
Hsieh Y-Y, Lee K-C, Cheng K-C, Lee K-F, Yang Y-L, Chu H-T, Lin T-W, Chen C-C, Hsieh M-C, Huang C-Y, et al. Antrodin C Isolated from Antrodia Cinnamomea Induced Apoptosis through ROS/AKT/ERK/P38 Signaling Pathway and Epigenetic Histone Acetylation of TNFα in Colorectal Cancer Cells. Antioxidants. 2023; 12(3):764. https://doi.org/10.3390/antiox12030764
Chicago/Turabian StyleHsieh, Yung-Yu, Ko-Chao Lee, Kung-Chuan Cheng, Kam-Fai Lee, Ya-Ling Yang, Hsin-Tung Chu, Ting-Wei Lin, Chin-Chu Chen, Meng-Chiao Hsieh, Cheng-Yi Huang, and et al. 2023. "Antrodin C Isolated from Antrodia Cinnamomea Induced Apoptosis through ROS/AKT/ERK/P38 Signaling Pathway and Epigenetic Histone Acetylation of TNFα in Colorectal Cancer Cells" Antioxidants 12, no. 3: 764. https://doi.org/10.3390/antiox12030764