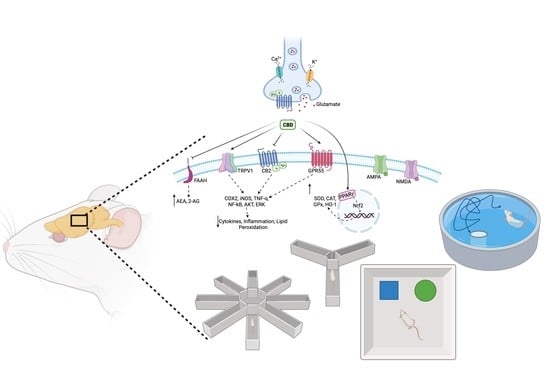
Cannabidiol: Bridge between Antioxidant Effect, Cellular Protection, and Cognitive and Physical Performance
Abstract
:1. Introduction
- Type I, with high content of Δ9-THC;
- Type II, containing different ratios of both CBD and Δ9-THC (predominantly CBD);
- Type III, with high content of CBD, and low content in Δ9-THC;
2. Cannabidiol: Pharmacological Targets
3. Link between Cannabidiol and Oxidative Stress
4. Cannabidiol Involvement in Neurodegeneration and Cellular Protection
5. Cannabidiol and Cognitive Performance Related to Oxidative Stress
6. Cannabidiol and Physical Performance
7. Cannabidiol and Autophagy
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Moldes, A.B.; Vecino, X.; Cruz, J.M. Nutraceuticals and Food Additives. In Current Developments in Biotechnology and Bioengineering; Pandey, A., Sanromán, M.Á., Du, G., Soccol, C.R., Dussap, C.-G., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 143–164. ISBN 9780444636669. [Google Scholar] [CrossRef]
- Williamson, E.M.; Liu, X.; Izzo, A.A. Trends in use, pharmacology, and clinical applications of emerging herbal nutraceuticals. Br. J. Pharmacol. 2020, 177, 1227–1240. [Google Scholar] [CrossRef]
- Sachdeva, V.; Roy, A.; Bharadvaja, N. Current Prospects of Nutraceuticals: A Review. Curr. Pharm. Biotechnol. 2020, 21, 884–896. [Google Scholar] [CrossRef]
- Current FDA Regulation of Hemp/CBD in Foods and Dietary Supplements. Available online: https://www.fdli.org/wp-content/uploads/2022/03/140-240-Preparing-for-a-world.pdf (accessed on 5 November 2022).
- Zuardi, A.W. History of cannabis as a medicine: A review. Braz. J. Psychiatry 2006, 28, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Hillard, C.J.; Weinlander, K.M.; Stuhr, K.L. Contributions of endocannabinoid signaling to psychiatric disorders in humans: Genetic and biochemical evidence. Neuroscience 2012, 204, 207–229. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R.; Parker, L.A. The endocannabinoid system and the brain. Annu. Rev. Psychol. 2013, 64, 21–47. [Google Scholar] [CrossRef]
- Mechoulam, R.; Shani, A.; Edery, H.; Grunfeld, Y. Chemical basis of hashish activity. Science 1970, 169, 611–612. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Guy, G.W. A tale of two cannabinoids: The therapeutic rationale for combining tetrahydrocannabinol and cannabidiol. Med. Hypotheses 2006, 66, 234–246. [Google Scholar] [CrossRef]
- Andre, C.M.; Hausman, J.F.; Guerriero, G. Cannabis sativa: The Plant of the Thousand and One Molecules. Front. Plant Sci. 2016, 7, 19. [Google Scholar] [CrossRef]
- Brighenti, V.; Pellati, F.; Steinbach, M.; Maran, D.; Benvenuti, S. Development of a new extraction technique and HPLC method for the analysis of non-psychoactive cannabinoids in fibre-type Cannabis sativa L. (hemp). J. Pharm. Biomed. Anal. 2017, 143, 228–236. [Google Scholar] [CrossRef]
- Pellati, F.; Brighenti, V.; Sperlea, J.; Marchetti, L.; Bertelli, D.; Benvenuti, S. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp). Molecules 2018, 23, 2639. [Google Scholar] [CrossRef]
- Scherma, M.; Masia, P.; Satta, V.; Fratta, W.; Fadda, P.; Tanda, G. Brain activity of anandamide: A rewarding bliss? Acta Pharmacol. Sin. 2019, 40, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Di Marzo, V.; Fontana, A.; Cadas, H.; Schinelli, S.; Cimino, G.; Schwartz, J.C.; Piomelli, D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature 1994, 372, 686–691. [Google Scholar] [CrossRef]
- Marrs, W.R.; Blankman, J.L.; Horne, E.A.; Thomazeau, A.; Lin, Y.H.; Coy, J.; Bodor, A.L.; Muccioli, G.G.; Hu, S.S.; Woodruff, G.; et al. The serine hydrolase ABHD6 controls the accumulation and efficacy of 2-AG at cannabinoid receptors. Nat. Neurosci. 2010, 13, 951–957. [Google Scholar] [CrossRef]
- Cravatt, B.F.; Demarest, K.; Patricelli, M.P.; Bracey, M.H.; Giang, D.K.; Martin, B.R.; Lichtman, A.H. Supersensitivity to anandamide and enhanced endogenous cannabinoid signaling in mice lacking fatty acid amide hydrolase. Proc. Natl. Acad. Sci. USA 2001, 98, 9371–9376. [Google Scholar] [CrossRef] [PubMed]
- Blankman, J.L.; Simon, G.M.; Cravatt, B.F. A comprehensive profile of brain enzymes that hydrolyze the endocannabinoid 2-arachidonoylglycerol. Chem. Biol. 2007, 14, 1347–1356. [Google Scholar] [CrossRef]
- Kohnz, R.A.; Nomura, D.K. Chemical approaches to therapeutically target the metabolism and signaling of the endocannabinoid 2-AG and eicosanoids. Chem. Soc. Rev. 2014, 43, 6859–6869. [Google Scholar] [CrossRef] [PubMed]
- Bodor, A.L.; Katona, I.; Nyíri, G.; Mackie, K.; Ledent, C.; Hájos, N.; Freund, T.F. Endocannabinoid signaling in rat somatosensory cortex: Laminar differences and involvement of specific interneuron types. J. Neurosci. 2005, 25, 6845–6856. [Google Scholar] [CrossRef]
- Hu, S.S.; Mackie, K. Distribution of the Endocannabinoid System in the Central Nervous System. In Endocannabinoids; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2015; Volume 231, pp. 59–93. [Google Scholar] [CrossRef]
- Lu, H.C.; Mackie, K. Review of the Endocannabinoid System. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 607–615. [Google Scholar] [CrossRef]
- Walsh, K.B.; Holmes, A.E. Pharmacology of Minor Cannabinoids at the Cannabinoid CB1 Receptor: Isomer- and Ligand-Dependent Antagonism by Tetrahydrocannabivarin. Receptors 2022, 1, 3–12. [Google Scholar] [CrossRef]
- Franco-Vadillo, A.; Toledo-Blass, M.; Rivera-Herrera, Z.; Guevara-Balcazar, G.; Orihuela-Rodriguez, O.; Morales-Carmona, J.A.; Kormanovski-Kovzova, A.; Lopez-Sanchez, P.; Rubio-Gayosso, I.; Castillo-Hernandez, M.D.C. Cannabidiol-mediated RISK PI3K/AKT and MAPK/ERK pathways decreasing reperfusion myocardial damage. Pharmacol. Res. Perspect. 2021, 9, e00784. [Google Scholar] [CrossRef]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-mediated control of synaptic transmission. Physiol. Rev. 2009, 9, 309–380. [Google Scholar] [CrossRef] [PubMed]
- Galiègue, S.; Mary, S.; Marchand, J.; Dussossoy, D.; Carrière, D.; Carayon, P.; Bouaboula, M.; Shire, D.; Le Fur, G.; Casellas, P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur. J. Biochem. 1995, 232, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Cabral, G.A.; Ferreira, G.A.; Jamerson, M.J. Endocannabinoids and the Immune System in Health and Disease. In Endocannabinoids; Handbook of Experimental Pharmacology; Springer: Cham, Switzerland, 2015; Volume 231, pp. 185–211. [Google Scholar] [CrossRef]
- Ofek, O.; Karsak, M.; Leclerc, N.; Fogel, M.; Frenkel, B.; Wright, K.; Tam, J.; Attar-Namdar, M.; Kram, V.; Shohami, E.; et al. Peripheral cannabinoid receptor, CB2, regulates bone mass. Proc. Natl. Acad. Sci. USA 2006, 103, 696–701. [Google Scholar] [CrossRef]
- Juan-Picó, P.; Fuentes, E.; Bermúdez-Silva, F.J.; Javier Díaz-Molina, F.; Ripoll, C.; Rodríguez de Fonseca, F.; Nadal, A. Cannabinoid receptors regulate Ca(2+) signals and insulin secretion in pancreatic beta-cell. Cell Calcium 2006, 39, 155–162. [Google Scholar] [CrossRef]
- Atwood, B.K.; Mackie, K. CB2: A cannabinoid receptor with an identity crisis. Br. J. Pharmacol. 2010, 160, 467–479. [Google Scholar] [CrossRef]
- Appendino, G.; Chianese, G.; Taglialatela-Scafati, O. Cannabinoids: Occurrence and medicinal chemistry. Curr. Med. Chem. 2011, 18, 1085–1099. [Google Scholar] [CrossRef]
- Micale, V.; Di Marzo, V.; Sulcova, A.; Wotjak, C.T.; Drago, F. Endocannabinoid system and mood disorders: Priming a target for new therapies. Pharmacol. Ther. 2013, 138, 18–37. [Google Scholar] [CrossRef] [PubMed]
- Kucerova, J.; Tabiova, K.; Drago, F.; Micale, V. Therapeutic potential of cannabinoids in schizophrenia. Recent Pat. CNS Drug Discov. 2014, 9, 13–25. [Google Scholar] [CrossRef]
- Micale, V.; Tabiova, K.; Kucerova, J.; Drago, F. Role of the endocannabinoid system in depression: From preclinical to clinical evidence. In Cannabinoid Modulation of Emotion, Memory, and Motivation; Campolongo, P., Fattore, L., Eds.; Springer: New York, NY, USA, 2015; pp. 97–129. [Google Scholar] [CrossRef]
- Micale, V.; Drago, F. Endocannabinoid system, stress and HPA axis. Eur. J. Pharmacol. 2018, 834, 230–239. [Google Scholar] [CrossRef]
- Stark, T.; Di Martino, S.; Drago, F.; Wotjak, C.T.; Micale, V. Phytocannabinoids and schizophrenia: Focus on adolescence as a critical window of enhanced vulnerability and opportunity for treatment. Pharmacol. Res. 2021, 174, 105938. [Google Scholar] [CrossRef]
- Castillo, P.E.; Younts, T.J.; Chávez, A.E.; Hashimotodani, Y. Endocannabinoid signaling and synaptic function. Neuron 2012, 76, 70–81. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ruiz, J.; Moreno-Martet, M.; Rodríguez-Cueto, C.; Palomo-Garo, C.; Gómez-Cañas, M.; Valdeolivas, S.; Guaza, C.; Romero, J.; Guzmán, M.; Mechoulam, R.; et al. Prospects for cannabinoid therapies in basal ganglia disorders. Br. J. Pharmacol. 2011, 163, 1365–1378. [Google Scholar] [CrossRef] [PubMed]
- Alexander, S.P. Therapeutic potential of cannabis-related drugs. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 64, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Campos, A.C.; Fogaça, M.V.; Sonego, A.B.; Guimarães, F.S. Cannabidiol, neuroprotection and neuropsychiatric disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef]
- Pereira, S.R.; Hackett, B.; O’Driscoll, D.N.; Sun, M.C.; Downer, E.J. Cannabidiol modulation of oxidative stress and signalling. Neuronal Signal. 2021, 5, NS20200080. [Google Scholar] [CrossRef] [PubMed]
- Pisanti, S.; Malfitano, A.M.; Ciaglia, E.; Lamberti, A.; Ranieri, R.; Cuomo, G.; Abate, M.; Faggiana, G.; Proto, M.C.; Fiore, D.; et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017, 175, 133–150. [Google Scholar] [CrossRef]
- Chung, H.; Fierro, A.; Pessoa-Mahana, C.D. Cannabidiol binding and negative allosteric modulation at the cannabinoid type 1 receptor in the presence of delta-9-tetrahydrocannabinol: An In Silico study. PLoS ONE 2019, 14, e0220025. [Google Scholar] [CrossRef]
- Laprairie, R.B.; Bagher, A.M.; Kelly, M.E.; Denovan-Wright, E.M. Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor. Br. J. Pharmacol. 2015, 172, 4790–4805. [Google Scholar] [CrossRef]
- Tham, M.; Yilmaz, O.; Alaverdashvili, M.; Kelly, M.E.M.; Denovan-Wright, E.M.; Laprairie, R.B. Allosteric and orthosteric pharmacology of cannabidiol and cannabidiol-dimethylheptyl at the type 1 and type 2 cannabinoid receptors. Br. J. Pharmacol. 2019, 176, 1455–1469. [Google Scholar] [CrossRef]
- Galaj, E.; Bi, G.H.; Yang, H.J.; Xi, Z.X. Cannabidiol attenuates the rewarding effects of cocaine in rats by CB2, 5-HT1A and TRPV1 receptor mechanisms. Neuropharmacology 2020, 167, 107740. [Google Scholar] [CrossRef]
- Vilela, L.R.; Lima, I.V.; Kunsch, É.B.; Pinto, H.P.P.; de Miranda, A.S.; Vieira, É.L.M.; de Oliveira, A.C.P.; Moraes, M.F.D.; Teixeira, A.L.; Moreira, F.A. Anticonvulsant effect of cannabidiol in the pentylenetetrazole model: Pharmacological mechanisms, electroencephalographic profile, and brain cytokine levels. Epilepsy Behav. 2017, 75, 29–35. [Google Scholar] [CrossRef] [PubMed]
- Milligan, C.J.; Anderson, L.L.; Bowen, M.T.; Banister, S.D.; McGregor, I.S.; Arnold, J.C.; Petrou, S.A. nutraceutical product, extracted from Cannabis sativa, modulates voltage-gated sodium channel function. J. Cannabis Res. 2022, 4, 30. [Google Scholar] [CrossRef] [PubMed]
- De Petrocellis, L.; Ligresti, A.; Moriello, A.S.; Allarà, M.; Bisogno, T.; Petrosino, S.; Stott, C.G.; Di Marzo, V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br. J. Pharmacol. 2011, 163, 1479–1494. [Google Scholar] [CrossRef]
- Nabissi, M.; Morelli, M.B.; Santoni, M.; Santoni, G. Triggering of the TRPV2 channel by cannabidiol sensitizes glioblastoma cells to cytotoxic chemotherapeutic agents. Carcinogenesis 2013, 34, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Nabissi, M.; Morelli, M.B.; Amantini, C.; Liberati, S.; Santoni, M.; Ricci-Vitiani, L.; Pallini, R.; Santoni, G. Cannabidiol stimulates Aml-1a-dependent glial differentiation and inhibits glioma stem-like cells proliferation by inducing autophagy in a TRPV2-dependent manner. Int. J. Cancer 2015, 137, 1855–1869. [Google Scholar] [CrossRef]
- Zhong, H.; Gutkin, D.W.; Han, B.; Ma, Y.; Keskinov, A.A.; Shurin, M.R.; Shurin, G.V. Origin and pharmacological modulation of tumor-associated regulatory dendritic cells. Int. J. Cancer 2014, 134, 2633–2645. [Google Scholar] [CrossRef] [Green Version]
- Lazarini-Lopes, W.; Do Val-da Silva, R.A.; da Silva-Júnior, R.M.P.; Leite, J.P.; Garcia-Cairasco, N. The anticonvulsant effects of cannabidiol in experimental models of epileptic seizures: From behavior and mechanisms to clinical insights. Neurosci. Biobehav. Rev. 2020, 111, 166–182. [Google Scholar] [CrossRef] [PubMed]
- Etemad, L.; Karimi, G.; Alavi, M.S.; Roohbakhsh, A. Pharmacological effects of cannabidiol by transient receptor potential channels. Life Sci. 2022, 300, 120582. [Google Scholar] [CrossRef] [PubMed]
- Soares, V.P.; Campos, A.C. Evidences for the Anti-panic Actions of Cannabidiol. Curr. Neuropharmacol. 2017, 15, 291–299. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-Karpowicz, I.; Skrzydlewska, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2019, 9, 21. [Google Scholar] [CrossRef]
- Oláh, A.; Szekanecz, Z.; Bíró, T. Targeting Cannabinoid Signaling in the Immune System: "High"-ly Exciting Questions, Possibilities, and Challenges. Front. Immunol. 2017, 8, 1487. [Google Scholar] [CrossRef] [PubMed]
- Zuardi, A.W.; Cosme, R.A.; Graeff, F.G.; Guimarães, F.S. Effects of ipsapirone and cannabidiol on human experimental anxiety. J. Psychopharmacol. 1993, 7, 82–88. [Google Scholar] [CrossRef] [PubMed]
- Crippa, J.A.; Derenusson, G.N.; Ferrari, T.B.; Wichert-Ana, L.; Duran, F.L.; Martin-Santos, R.; Simões, M.V.; Bhattacharyya, S.; Fusar-Poli, P.; Atakan, Z.; et al. Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: A preliminary report. J. Psychopharmacol. 2011, 25, 121–130. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Rodrigues, N.P.; Silva, A.L.; Bernardo, S.A.; Hallak, J.E.C.; Guimarães, F.S.; Crippa, J.A.S. Inverted U-Shaped Dose-Response Curve of the Anxiolytic Effect of Cannabidiol during Public Speaking in Real Life. Front. Pharmacol. 2017, 8, 259. [Google Scholar] [CrossRef] [PubMed]
- Linares, I.M.; Zuardi, A.W.; Pereira, L.C.; Queiroz, R.H.; Mechoulam, R.; Guimarães, F.S.; Crippa, J.A. Cannabidiol presents an inverted U-shaped dose-response curve in a simulated public speaking test. Braz. J. Psychiatry 2019, 41, 9–14. [Google Scholar] [CrossRef]
- Appiah-Kusi, E.; Petros, N.; Wilson, R.; Colizzi, M.; Bossong, M.G.; Valmaggia, L.; Mondelli, V.; McGuire, P.; Bhattacharyya, S. Effects of short-term cannabidiol treatment on response to social stress in subjects at clinical high risk of developing psychosis. Psychopharmacology 2020, 237, 1121–1130. [Google Scholar] [CrossRef] [Green Version]
- Llorente-Berzal, A.; Terzian, A.L.B.; di Marzo, V.; Micale, V.; Viveros, M.P.; Wotjak, C.T. 2-AG promotes the expression of conditioned fear via cannabinoid receptor type 1 on GABAergic neurons. Psychopharmacology 2015, 232, 2811–2825. [Google Scholar] [CrossRef]
- Terzian, A.L.; Micale, V.; Wotjak, C.T. Cannabinoid receptor type 1 receptors on GABAergic vs. glutamatergic neurons differentially gate sex-dependent social interest in mice. Eur. J. Neurosci. 2014, 40, 2293–2298. [Google Scholar] [CrossRef]
- Terzian, A.L.; Drago, F.; Wotjak, C.T.; Micale, V. The Dopamine and Cannabinoid Interaction in the Modulation of Emotions and Cognition: Assessing the Role of Cannabinoid CB1 Receptor in Neurons Expressing Dopamine D1 Receptors. Front. Behav. Neurosci. 2011, 5, 49. [Google Scholar] [CrossRef]
- Micale, V.; Stepan, J.; Jurik, A.; Pamplona, F.A.; Marsch, R.; Drago, F.; Eder, M.; Wotjak, C.T. Extinction of avoidance behavior by safety learning depends on endocannabinoid signaling in the hippocampus. J. Psychiatr. Res. 2017, 90, 46–59. [Google Scholar] [CrossRef]
- Hallak, J.E.; Machado-de-Sousa, J.P.; Crippa, J.A.; Sanches, R.F.; Trzesniak, C.; Chaves, C.; Bernardo, S.A.; Regalo, S.C.; Zuardi, A.W. Performance of schizophrenic patients in the Stroop Color Word Test and electrodermal responsiveness after acute administration of cannabidiol (CBD). Braz. J. Psychiatry 2010, 32, 56–61. [Google Scholar] [CrossRef]
- Leweke, F.M.; Piomelli, D.; Pahlisch, F.; Muhl, D.; Gerth, C.W.; Hoyer, C.; Klosterkötter, J.; Hellmich, M.; Koethe, D. Cannabidiol enhances anandamide signaling and alleviates psychotic symptoms of schizophrenia. Transl. Psychiatry 2012, 2, e94. [Google Scholar] [CrossRef] [PubMed]
- Arkell, T.R.; Lintzeris, N.; Kevin, R.C.; Ramaekers, J.G.; Vandrey, R.; Irwin, C.; Haber, P.S.; McGregor, I.S. Cannabidiol (CBD) content in vaporized cannabis does not prevent tetrahydrocannabinol (THC)-induced impairment of driving and cognition. Psychopharmacology 2019, 236, 2713–2724. [Google Scholar] [CrossRef] [PubMed]
- Stark, T.; Ruda-Kucerova, J.; Iannotti, F.A.; D’Addario, C.; Di Marco, R.; Pekarik, V.; Drazanova, E.; Piscitelli, F.; Bari, M.; Babinska, Z.; et al. Peripubertal cannabidiol treatment rescues behavioral and neurochemical abnormalities in the MAM model of schizophrenia. Neuropharmacology 2019, 146, 212–221. [Google Scholar] [CrossRef] [PubMed]
- Di Bartolomeo, M.; Stark, T.; Maurel, O.M.; Iannotti, F.A.; Kuchar, M.; Ruda-Kucerova, J.; Piscitelli, F.; Laudani, S.; Pekarik, V.; Salomone, S.; et al. Crosstalk between the transcriptional regulation of dopamine D2 and cannabinoid CB1 receptors in schizophrenia: Analyses in patients and in perinatal Δ9-tetrahydrocannabinol-exposed rats. Pharmacol. Res. 2021, 164, 105357. [Google Scholar] [CrossRef] [PubMed]
- Brancato, A.; Castelli, V.; Lavanco, G.; Tringali, G.; Micale, V.; Kuchar, M.; D’Amico, C.; Pizzolanti, G.; Feo, S.; Cannizzaro, C. Binge-like Alcohol Exposure in Adolescence: Behavioural, Neuroendocrine and Molecular Evidence of Abnormal Neuroplasticity… and Return. Biomedicines 2021, 9, 1161. [Google Scholar] [CrossRef]
- Seeman, P. Cannabidiol is a partial agonist at dopamine D2High receptors, predicting its antipsychotic clinical dose. Transl. Psychiatry 2016, 6, e920. [Google Scholar] [CrossRef]
- Stark, T.; Di Bartolomeo, M.; Di Marco, R.; Drazanova, E.; Platania, C.B.M.; Iannotti, F.A.; Ruda-Kucerova, J.; D’Addario, C.; Kratka, L.; Pekarik, V.; et al. Altered dopamine D3 receptor gene expression in MAM model of schizophrenia is reversed by peripubertal cannabidiol treatment. Biochem. Pharmacol. 2020, 177, 114004. [Google Scholar] [CrossRef]
- Micale, V.; Di Bartolomeo, M.; Di Martino, S.; Stark, T.; Dell’Osso, B.; Drago, F.; D’Addario, C. Are the epigenetic changes predictive of therapeutic efficacy for psychiatric disorders? A translational approach towards novel drug targets. Pharmacol. Ther. 2023, 241, 108279. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S. Cannabidiol in Dravet Syndrome Study Group. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. GWPCARE3 Study Group. Effect of Cannabidiol on Drop Seizures in the Lennox-Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef]
- Hess, E.J.; Moody, K.A.; Geffrey, A.L.; Pollack, S.F.; Skirvin, L.A.; Bruno, P.L.; Paolini, J.L.; Thiele, E.A. Cannabidiol as a new treatment for drug-resistant epilepsy in tuberous sclerosis complex. Epilepsia 2016, 57, 1617–1624. [Google Scholar] [CrossRef]
- Zuardi, A.W.; Crippa, J.A.; Hallak, J.E.; Pinto, J.P.; Chagas, M.H.; Rodrigues, G.G.; Dursun, S.M.; Tumas, V. Cannabidiol for the treatment of psychosis in Parkinson’s disease. J. Psychopharmacol. 2009, 23, 979–983. [Google Scholar] [CrossRef]
- Chagas, M.H.; Zuardi, A.W.; Tumas, V.; Pena-Pereira, M.A.; Sobreira, E.T.; Bergamaschi, M.M.; dos Santos, A.C.; Teixeira, A.L.; Hallak, J.E.; Crippa, J.A. Effects of cannabidiol in the treatment of patients with Parkinson’s disease: An exploratory double-blind trial. J. Psychopharmacol. 2014, 28, 1088–1098. [Google Scholar] [CrossRef] [PubMed]
- Guo, K.; Mou, X.; Huang, J.; Xiong, N.; Li, H. Trans-caryophyllene suppresses hypoxia-induced neuroinflammatory responses by inhibiting NF-κB activation in microglia. J. Mol. Neurosci. 2014, 54, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Watt, G.; Karl, T. In vivo Evidence for Therapeutic Properties of Cannabidiol (CBD) for Alzheimer’s Disease. Front. Pharmacol. 2017, 8, 20. [Google Scholar] [CrossRef] [Green Version]
- Scuderi, C.; Steardo, L.; Esposito, G. Cannabidiol promotes amyloid precursor protein ubiquitination and reduction of beta amyloid expression in SHSY5YAPP+ cells through PPARγ involvement. Phytother. Res. 2014, 28, 1007–1013. [Google Scholar] [CrossRef]
- Hampson, A.J.; Grimaldi, M.; Axelrod, J.; Wink, D. Cannabidiol and (-)Delta9-tetrahydrocannabinol are neuroprotective antioxidants. Proc. Natl. Acad. Sci. USA 1998, 95, 8268–8273. [Google Scholar] [CrossRef]
- Borges, R.S.; Batista, J., Jr.; Viana, R.B.; Baetas, A.C.; Orestes, E.; Andrade, M.A.; Honório, K.M.; da Silva, A.B. Understanding the molecular aspects of tetrahydrocannabinol and cannabidiol as antioxidants. Molecules 2013, 18, 12663–12674. [Google Scholar] [CrossRef]
- Borges, R.S.; da Silva, A.B.F. Chapter e12—Cannabidiol as an Antioxidant. In Handbook of Cannabis and Related Pathologies; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. e122–e130. ISBN 9780128007563. [Google Scholar] [CrossRef]
- Hamelink, C.; Hampson, A.; Wink, D.A.; Eiden, L.E.; Eskay, R.L. Comparison of cannabidiol, antioxidants, and diuretics in reversing binge ethanol-induced neurotoxicity. J. Pharmacol. Exp. Ther. 2005, 314, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Wheal, A.J.; Jadoon, K.; Randall, M.D.; O’Sullivan, S.E. In Vivo Cannabidiol Treatment Improves Endothelium-Dependent Vasorelaxation in Mesenteric Arteries of Zucker Diabetic Fatty Rats. Front. Pharmacol. 2017, 8, 248. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Trovato, A.E.; Comelli, F.; Giagnoni, G.; Colleoni, M. The non-psychoactive cannabis constituent cannabidiol is an orally effective therapeutic agent in rat chronic inflammatory and neuropathic pain. Eur. J. Pharmacol. 2007, 556, 75–83. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; García, C.; Sagredo, O.; Gómez-Ruiz, M.; de Lago, E. The endocannabinoid system as a target for the treatment of neuronal damage. Expert Opin. Ther. Targets 2010, 14, 387–404. [Google Scholar] [CrossRef]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Gao, F.; Coppola, G.; Geschwind, D.; Vogel, Z. Microarray and pathway analysis reveal distinct mechanisms underlying cannabinoid-mediated modulation of LPS-induced activation of BV-2 microglial cells. PLoS ONE 2013, 8, e61462. [Google Scholar] [CrossRef]
- Vomund, S.; Schäfer, A.; Parnham, M.J.; Brüne, B.; von Knethen, A. Nrf2, the Master Regulator of Anti-Oxidative Responses. Int. J. Mol. Sci. 2017, 18, 2772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Xuan, Y.; Zhu, B.; Wang, X.; Tian, X.; Zhao, L.; Wang, Y.; Jiang, X.; Wen, N. Protective Effects of Cannabidiol on Chemotherapy-Induced Oral Mucositis via the Nrf2/Keap1/ARE Signaling Pathways. Oxid. Med. Cell. Longev. 2022, 2022, 4619760. [Google Scholar] [CrossRef] [PubMed]
- Fouad, A.A.; Albuali, W.H.; Al-Mulhim, A.S.; Jresat, I. Cardioprotective effect of cannabidiol in rats exposed to doxorubicin toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 347–357. [Google Scholar] [CrossRef]
- Iffland, K.; Grotenhermen, F. An Update on Safety and Side Effects of Cannabidiol: A Review of Clinical Data and Relevant Animal Studies. Cannabis Cannabinoid Res. 2017, 2, 139–154. [Google Scholar] [CrossRef]
- Jîtcă, G.; Fogarasi, E.; Ősz, B.E.; Vari, C.E.; Tero-Vescan, A.; Miklos, A.; Bătrînu, M.G.; Rusz, C.M.; Croitoru, M.D.; Dogaru, M.T. A Simple HPLC/DAD Method Validation for the Quantification of Malondialdehyde in Rodent’s Brain. Molecules 2021, 26, 5066. [Google Scholar] [CrossRef] [PubMed]
- Jîtcă, G.; Fogarasi, E.; Ősz, B.E.; Vari, C.E.; Fülöp, I.; Croitoru, M.D.; Rusz, C.M.; Dogaru, M.T. Profiling the Concentration of Reduced and Oxidized Glutathione in Rat Brain Using HPLC/DAD Chromatographic System. Molecules 2021, 26, 6590. [Google Scholar] [CrossRef]
- El-Remessy, A.B.; Al-Shabrawey, M.; Khalifa, Y.; Tsai, N.T.; Caldwell, R.B.; Liou, G.I. Neuroprotective and blood-retinal barrier-preserving effects of cannabidiol in experimental diabetes. Am. J. Pathol. 2006, 168, 235–244. [Google Scholar] [CrossRef]
- Gianazza, E.; Crawford, J.; Miller, I. Detecting oxidative post-translational modifications in proteins. Amino Acids 2007, 33, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Miklos, A.P.; Rusz, C.M.; Bătrînu, M.G.; Vari, C.E. Positive Aspects of Oxidative Stress at Different Levels of the Human Body: A Review. Antioxidants 2022, 11, 572. [Google Scholar] [CrossRef] [PubMed]
- di Giacomo, V.; Chiavaroli, A.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Leone, S.; Brunetti, L.; Menghini, L.; Recinella, L.; et al. Neuroprotective and Neuromodulatory Effects Induced by Cannabidiol and Cannabigerol in Rat Hypo-E22 cells and Isolated Hypothalamus. Antioxidants 2020, 9, 71. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Hu, F.; Wu, J.; Zhang, S. Cannabidiol attenuates OGD/R-induced damage by enhancing mitochondrial bioenergetics and modulating glucose metabolism via pentose-phosphate pathway in hippocampal neurons. Redox Biol. 2017, 11, 577–585. [Google Scholar] [CrossRef]
- Valvassori, S.S.; Elias, G.; de Souza, B.; Petronilho, F.; Dal-Pizzol, F.; Kapczinski, F.; Trzesniak, C.; Tumas, V.; Dursun, S.; Chagas, M.H.; et al. Effects of cannabidiol on amphetamine-induced oxidative stress generation in an animal model of mania. J. Psychopharmacol. 2011, 25, 274–280. [Google Scholar] [CrossRef]
- Jîtcă, G.; Ősz, B.E.; Tero-Vescan, A.; Vari, C.E. Psychoactive Drugs-From Chemical Structure to Oxidative Stress Related to Dopaminergic Neurotransmission. A Review. Antioxidants 2021, 10, 381. [Google Scholar] [CrossRef]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Chronic cannabidiol treatment improves social and object recognition in double transgenic APPswe/PS1∆E9 mice. Psychopharmacology 2014, 231, 3009–3017. [Google Scholar] [CrossRef]
- Han, K.H.; Lim, S.; Ryu, J.; Lee, C.W.; Kim, Y.; Kang, J.H.; Kang, S.S.; Ahn, Y.K.; Park, C.S.; Kim, J.J. CB1 and CB2 cannabinoid receptors differentially regulate the production of reactive oxygen species by macrophages. Cardiovasc. Res. 2009, 84, 378–386. [Google Scholar] [CrossRef]
- Muller, C.; Morales, P.; Reggio, P.H. Cannabinoid Ligands Targeting TRP Channels. Front. Mol. Neurosci. 2019, 11, 487. [Google Scholar] [CrossRef]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef]
- Ogawa, N.; Kurokawa, T.; Fujiwara, K.; Polat, O.K.; Badr, H.; Takahashi, N.; Mori, Y. Functional and Structural Divergence in Human TRPV1 Channel Subunits by Oxidative Cysteine Modification. J. Biol. Chem. 2016, 291, 4197–4210. [Google Scholar] [CrossRef]
- Hellenthal, K.E.M.; Brabenec, L.; Gross, E.R.; Wagner, N.M. TRP Channels as Sensors of Aldehyde and Oxidative Stress. Biomolecules 2021, 11, 1401. [Google Scholar] [CrossRef] [PubMed]
- Minke, B. TRP channels and Ca2+ signaling. Cell Calcium 2006, 40, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Bujak, J.K.; Kosmala, D.; Szopa, I.M.; Majchrzak, K.; Bednarczyk, P. Inflammation, Cancer and Immunity-Implication of TRPV1 Channel. Front. Oncol. 2019, 9, 1087. [Google Scholar] [CrossRef]
- Vallée, A.; Lecarpentier, Y.; Guillevin, R.; Vallée, J.N. Effects of cannabidiol interactions with Wnt/β-catenin pathway and PPARγ on oxidative stress and neuroinflammation in Alzheimer’s disease. Acta Biochim. Biophys. Sin. 2017, 49, 853–866. [Google Scholar] [CrossRef]
- Paunkov, A.; Chartoumpekis, D.V.; Ziros, P.G.; Sykiotis, G.P. A Bibliometric Review of the Keap1/Nrf2 Pathway and its Related Antioxidant Compounds. Antioxidants 2019, 8, 353. [Google Scholar] [CrossRef]
- Cho, H.Y.; Gladwell, W.; Wang, X.; Chorley, B.; Bell, D.; Reddy, S.P.; Kleeberger, S.R. Nrf2-regulated PPAR{gamma} expression is critical to protection against acute lung injury in mice. Am. J. Respir. Crit. Care Med. 2010, 182, 170–182. [Google Scholar] [CrossRef]
- Lee, C. Collaborative Power of Nrf2 and PPARγActivators against Metabolic and Drug-Induced Oxidative Injury. Oxid. Med. Cell. Longev. 2017, 2017, 1378175. [Google Scholar] [CrossRef]
- O’Sullivan, S.E. An update on PPAR activation by cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef] [PubMed]
- Sylantyev, S.; Jensen, T.P.; Ross, R.A.; Rusakov, D.A. Cannabinoid- and lysophosphatidylinositol-sensitive receptor GPR55 boosts neurotransmitter release at central synapses. Proc. Natl. Acad. Sci. USA 2013, 110, 5193–5198. [Google Scholar] [CrossRef]
- Booz, G.W. Cannabidiol as an emergent therapeutic strategy for lessening the impact of inflammation on oxidative stress. Free Radic. Biol. Med. 2011, 51, 1054–1061. [Google Scholar] [CrossRef]
- Azouzi, S.; Santuz, H.; Morandat, S.; Pereira, C.; Côté, F.; Hermine, O.; El Kirat, K.; Colin, Y.; Le Van Kim, C.; Etchebest, C.; et al. Antioxidant and Membrane Binding Properties of Serotonin Protect Lipids from Oxidation. Biophys. J. 2017, 112, 1863–1873. [Google Scholar] [CrossRef]
- Mecha, M.; Feliú, A.; Iñigo, P.M.; Mestre, L.; Carrillo-Salinas, F.J.; Guaza, C. Cannabidiol provides long-lasting protection against the deleterious effects of inflammation in a viral model of multiple sclerosis: A role for A2A receptors. Neurobiol. Dis. 2013, 59, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Casares, L.; García, V.; Garrido-Rodríguez, M.; Millán, E.; Collado, J.A.; García-Martín, A.; Peñarando, J.; Calzado, M.A.; de la Vega, L.; Muñoz, E. Cannabidiol induces antioxidant pathways in keratinocytes by targeting BACH1. Redox Biol. 2020, 28, 101321. [Google Scholar] [CrossRef] [PubMed]
- Bublitz, K.; Böckmann, S.; Peters, K.; Hinz, B. Cannabinoid-Induced Autophagy and Heme Oxygenase-1 Determine the Fate of Adipose Tissue-Derived Mesenchymal Stem Cells under Stressful Conditions. Cells 2020, 9, 2298. [Google Scholar] [CrossRef] [PubMed]
- Duvigneau, J.C.; Trovato, A.; Müllebner, A.; Miller, I.; Krewenka, C.; Krenn, K.; Zich, W.; Moldzio, R. Cannabidiol Protects Dopaminergic Neurons in Mesencephalic Cultures against the Complex I Inhibitor Rotenone Via Modulation of Heme Oxygenase Activity and Bilirubin. Antioxidants 2020, 9, 135. [Google Scholar] [CrossRef]
- Pan, H.; Mukhopadhyay, P.; Rajesh, M.; Patel, V.; Mukhopadhyay, B.; Gao, B.; Haskó, G.; Pacher, P. Cannabidiol attenuates cisplatin-induced nephrotoxicity by decreasing oxidative/nitrosative stress, inflammation, and cell death. J. Pharmacol. Exp. Ther. 2009, 328, 708–714. [Google Scholar] [CrossRef]
- Wang, Y.; Mukhopadhyay, P.; Cao, Z.; Wang, H.; Feng, D.; Haskó, G.; Mechoulam, R.; Gao, B.; Pacher, P. Cannabidiol attenuates alcohol-induced liver steatosis, metabolic dysregulation, inflammation and neutrophil-mediated injury. Sci. Rep. 2017, 7, 12064. [Google Scholar] [CrossRef]
- Dos-Santos-Pereira, M.; Guimarães, F.S.; Del-Bel, E.; Raisman-Vozari, R.; Michel, P.P. Cannabidiol prevents LPS-induced microglial inflammation by inhibiting ROS/NF-κB-dependent signaling and glucose consumption. Glia 2020, 68, 561–573. [Google Scholar] [CrossRef]
- Massi, P.; Vaccani, A.; Bianchessi, S.; Costa, B.; Macchi, P.; Parolaro, D. The non-psychoactive cannabidiol triggers caspase activation and oxidative stress in human glioma cells. Cell. Mol. Life Sci. 2006, 63, 2057–2066. [Google Scholar] [CrossRef] [PubMed]
- di Giacomo, V.; Chiavaroli, A.; Recinella, L.; Orlando, G.; Cataldi, A.; Rapino, M.; Di Valerio, V.; Ronci, M.; Leone, S.; Brunetti, L.; et al. Antioxidant and Neuroprotective Effects Induced by Cannabidiol and Cannabigerol in Rat CTX-TNA2 Astrocytes and Isolated Cortexes. Int. J. Mol. Sci. 2020, 21, 3575. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Xing, J.Z.; Krukoff, T.L. Neuroprotective effects of R,R-tetrahydrochrysene against glutamate-induced cell death through anti-excitotoxic and antioxidant actions involving estrogen receptor-dependent and -independent pathways. Neuroscience 2009, 162, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Khaksar, S.; Bigdeli, M.; Samiee, A.; Shirazi-Zand, Z. Antioxidant and anti-apoptotic effects of cannabidiol in model of ischemic stroke in rats. Brain Res. Bull. 2022, 180, 118–130. [Google Scholar] [CrossRef]
- Lastres-Becker, I.; Molina-Holgado, F.; Ramos, J.A.; Mechoulam, R.; Fernández-Ruiz, J. Cannabinoids provide neuroprotection against 6-hydroxydopamine toxicity in vivo and in vitro: Relevance to Parkinson’s disease. Neurobiol. Dis. 2005, 19, 96–107. [Google Scholar] [CrossRef]
- García-Arencibia, M.; González, S.; de Lago, E.; Ramos, J.A.; Mechoulam, R.; Fernández-Ruiz, J. Evaluation of the neuroprotective effect of cannabinoids in a rat model of Parkinson’s disease: Importance of antioxidant and cannabinoid receptor-independent properties. Brain Res. 2007, 1134, 162–170. [Google Scholar] [CrossRef]
- Vivekanantham, S.; Shah, S.; Dewji, R.; Dewji, A.; Khatri, C.; Ologunde, R. Neuroinflammation in Parkinson’s disease: Role in neurodegeneration and tissue repair. Int. J. Neurosci. 2015, 125, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Aso, E.; Ferrer, I. CB2 Cannabinoid Receptor as Potential Target against Alzheimer’s Disease. Front. Neurosci. 2016, 10, 243. [Google Scholar] [CrossRef]
- Concannon, R.M.; Okine, B.N.; Finn, D.P.; Dowd, E. Upregulation of the cannabinoid CB2 receptor in environmental and viral inflammation-driven rat models of Parkinson’s disease. Exp. Neurol. 2016, 283, 204–212. [Google Scholar] [CrossRef]
- Scuderi, C.; Filippis, D.D.; Iuvone, T.; Blasio, A.; Steardo, A.; Esposito, G. Cannabidiol in medicine: A review of its therapeutic potential in CNS disorders. Phytother. Res. 2009, 23, 597–602. [Google Scholar] [CrossRef]
- Esposito, G.; De Filippis, D.; Maiuri, M.C.; De Stefano, D.; Carnuccio, R.; Iuvone, T. Cannabidiol inhibits inducible nitric oxide synthase protein expression and nitric oxide production in beta-amyloid stimulated PC12 neurons through p38 MAP kinase and NF-kappaB involvement. Neurosci. Lett. 2006, 399, 91–95. [Google Scholar] [CrossRef]
- Esposito, G.; De Filippis, D.; Carnuccio, R.; Izzo, A.A.; Iuvone, T. The marijuana component cannabidiol inhibits beta-amyloid-induced tau protein hyperphosphorylation through Wnt/beta-catenin pathway rescue in PC12 cells. J. Mol. Med. 2006, 84, 253–258. [Google Scholar] [CrossRef]
- Sagredo, O.; Ramos, J.A.; Decio, A.; Mechoulam, R.; Fernández-Ruiz, J. Cannabidiol reduced the striatal atrophy caused 3-nitropropionic acid in vivo by mechanisms independent of the activation of cannabinoid, vanilloid TRPV1 and adenosine A2A receptors. Eur. J. Neurosci. 2007, 26, 843–851. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as novel therapeutic agents in CNS disorders. Pharmacol. Ther. 2012, 133, 79–97. [Google Scholar] [CrossRef]
- Fernández-Ruiz, J.; Sagredo, O.; Pazos, M.R.; García, C.; Pertwee, R.; Mechoulam, R.; Martínez-Orgado, J. Cannabidiol for neurodegenerative disorders: Important new clinical applications for this phytocannabinoid? Br. J. Clin. Pharmacol. 2013, 75, 323–333. [Google Scholar] [CrossRef]
- Vallée, A.; Vallée, J.N.; Lecarpentier, Y. Potential role of cannabidiol in Parkinson’s disease by targeting the WNT/β-catenin pathway, oxidative stress and inflammation. Aging 2021, 13, 10796–10813. [Google Scholar] [CrossRef]
- Hughes, B.; Herron, C.E. Cannabidiol Reverses Deficits in Hippocampal LTP in a Model of Alzheimer’s Disease. Neurochem. Res. 2019, 44, 703–713. [Google Scholar] [CrossRef]
- Cheng, D.; Spiro, A.S.; Jenner, A.M.; Garner, B.; Karl, T. Long-term cannabidiol treatment prevents the development of social recognition memory deficits in Alzheimer’s disease transgenic mice. J. Alzheimer’s Dis. 2014, 42, 1383–1396. [Google Scholar] [CrossRef] [PubMed]
- Libro, R.; Diomede, F.; Scionti, D.; Piattelli, A.; Grassi, G.; Pollastro, F.; Bramanti, P.; Mazzon, E.; Trubiani, O. Cannabidiol Modulates the Expression of Alzheimer’s Disease-Related Genes in Mesenchymal Stem Cells. Int. J. Mol. Sci. 2016, 18, 26. [Google Scholar] [CrossRef] [PubMed]
- Esposito, G.; Scuderi, C.; Savani, C.; Steardo, L., Jr.; De Filippis, D.; Cottone, P.; Iuvone, T.; Cuomo, V.; Steardo, L. Cannabidiol in vivo blunts beta-amyloid induced neuroinflammation by suppressing IL-1beta and iNOS expression. Br. J. Pharmacol. 2007, 151, 1272–1279. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Drake, J.; Pocernich, C.; Castegna, A. Evidence of oxidative damage in Alzheimer’s disease brain: Central role for amyloid β-peptide. Trends Mol. Med. 2001, 7, 548–554. [Google Scholar] [CrossRef]
- Turner, B.J.; Li, Q.X.; Laughton, K.M.; Masters, C.L.; Lopes, E.C.; Atkin, J.D.; Cheema, S.S. Brain beta-amyloid accumulation in transgenic mice expressing mutant superoxide dismutase 1. Neurochem. Res. 2004, 29, 2281–2286. [Google Scholar] [CrossRef] [PubMed]
- Haas, J.; Storch-Hagenlocher, B.; Biessmann, A.; Wildemann, B. Inducible nitric oxide synthase and argininosuccinate synthetase: Co-induction in brain tissue of patients with Alzheimer’s dementia and following stimulation with β-amyloid 1–42 in vitro. Neurosci. Lett. 2002, 322, 121–125. [Google Scholar] [CrossRef] [PubMed]
- Iuvone, T.; Esposito, G.; Esposito, R.; Santamaria, R.; Di Rosa, M.; Izzo, A.A. Neuroprotective effect of cannabidiol, a non-psychoactive component from Cannabis sativa, on beta-amyloid-induced toxicity in PC12 cells. J. Neurochem. 2004, 89, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Rees, D.D.; Schulz, R.; Palmer, R.M. Development and mechanism of a specific supersensitivity to nitrovasodilators after inhibition of vascular nitric oxide synthesis in vivo. Proc. Natl. Acad. Sci. USA 1991, 88, 2166–2170. [Google Scholar] [CrossRef] [Green Version]
- Esposito, G.; Scuderi, C.; Valenza, M.; Togna, G.I.; Latina, V.; De Filippis, D.; Cipriano, M.; Carratù, M.R.; Iuvone, T.; Steardo, L. Cannabidiol reduces Aβ-induced neuroinflammation and promotes hippocampal neurogenesis through PPARγ involvement. PLoS ONE 2011, 6, e28668. [Google Scholar] [CrossRef] [PubMed]
- Bedse, G.; Romano, A.; Cianci, S.; Lavecchia, A.M.; Lorenzo, P.; Elphick, M.R.; Laferla, F.M.; Vendemiale, G.; Grillo, C.; Altieri, F.; et al. Altered expression of the CB1 cannabinoid receptor in the triple transgenic mouse model of Alzheimer’s disease. J. Alzheimer’s Dis. 2014, 40, 701–712. [Google Scholar] [CrossRef]
- Gallelli, C.A.; Calcagnini, S.; Romano, A.; Koczwara, J.B.; de Ceglia, M.; Dante, D.; Villani, R.; Giudetti, A.M.; Cassano, T.; Gaetani, S. Modulation of the Oxidative Stress and Lipid Peroxidation by Endocannabinoids and Their Lipid Analogues. Antioxidants 2018, 7, 93. [Google Scholar] [CrossRef]
- Cassano, T.; Villani, R.; Pace, L.; Carbone, A.; Bukke, V.N.; Orkisz, S.; Avolio, C.; Serviddio, G. From Cannabis sativa to Cannabidiol: Promising Therapeutic Candidate for the Treatment of Neurodegenerative Diseases. Front. Pharmacol. 2020, 11, 124. [Google Scholar] [CrossRef]
- Ahmadi, S.; Zhu, S.; Sharma, R.; Wu, B.; Soong, R.; Dutta Majumdar, R.; Wilson, D.J.; Simpson, A.J.; Kraatz, H.B. Aggregation of Microtubule Binding Repeats of Tau Protein is Promoted by Cu2. ACS Omega 2019, 4, 5356–5366. [Google Scholar] [CrossRef]
- Santos, N.A.; Martins, N.M.; Sisti, F.M.; Fernandes, L.S.; Ferreira, R.S.; Queiroz, R.H.; Santos, A.C. The neuroprotection of cannabidiol against MPP⁺-induced toxicity in PC12 cells involves trkA receptors, upregulation of axonal and synaptic proteins, neuritogenesis, and might be relevant to Parkinson’s disease. Toxicol. In Vitro 2015, 30, 231–240. [Google Scholar] [CrossRef]
- Ahmadi, S.; Zhu, S.; Sharma, R.; Wilson, D.J.; Kraatz, H.B. Interaction of metal ions with tau protein. The case for a metal-mediated tau aggregation. J. Inorg. Biochem. 2019, 194, 44–51. [Google Scholar] [CrossRef]
- Wei, B.; McGuffey, J.E.; Blount, B.C.; Wang, L. Sensitive Quantification of Cannabinoids in Milk by Alkaline Saponification-Solid Phase Extraction Combined with Isotope Dilution UPLC-MS/MS. ACS Omega 2016, 1, 1307–1313. [Google Scholar] [CrossRef] [PubMed]
- Magen, I.; Avraham, Y.; Ackerman, Z.; Vorobiev, L.; Mechoulam, R.; Berry, E.M. Cannabidiol ameliorates cognitive and motor impairments in bile-duct ligated mice via 5-HT1A receptor activation. Br. J. Pharmacol. 2010, 159, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Blázquez, C.; Chiarlone, A.; Bellocchio, L.; Resel, E.; Pruunsild, P.; García-Rincón, D.; Sendtner, M.; Timmusk, T.; Lutz, B.; Galve-Roperh, I.; et al. The CB₁ cannabinoid receptor signals striatal neuroprotection via a PI3K/Akt/mTORC1/BDNF pathway. Cell Death Differ. 2015, 22, 1618–1629. [Google Scholar] [CrossRef] [PubMed]
- Valdeolivas, S.; Satta, V.; Pertwee, R.G.; Fernández-Ruiz, J.; Sagredo, O. Sativex-like combination of phytocannabinoids is neuroprotective in malonate-lesioned rats, an inflammatory model of Huntington’s disease: Role of CB1 and CB2 receptors. ACS Chem. Neurosci. 2012, 3, 400–406. [Google Scholar] [CrossRef]
- Calkins, M.J.; Jakel, R.J.; Johnson, D.A.; Chan, K.; Kan, Y.W.; Johnson, J.A. Protection from mitochondrial complex II inhibition in vitro and in vivo by Nrf2-mediated transcription. Proc. Natl. Acad. Sci. USA 2005, 102, 244–249. [Google Scholar] [CrossRef]
- Calkins, M.J.; Townsend, J.A.; Johnson, D.A.; Johnson, J.A. Cystamine protects from 3-nitropropionic acid lesioning via induction of nf-e2 related factor 2 mediated transcription. Exp. Neurol. 2010, 224, 307–317. [Google Scholar] [CrossRef]
- Sagredo, O.; Pazos, M.R.; Satta, V.; Ramos, J.A.; Pertwee, R.G.; Fernández-Ruiz, J. Neuroprotective effects of phytocannabinoid-based medicines in experimental models of Huntington’s disease. J. Neurosci. Res. 2011, 89, 1509–1518. [Google Scholar] [CrossRef]
- Borovac Štefanović, L.; Kalinić, D.; Mimica, N.; Beer Ljubić, B.; Aladrović, J.; Mandelsamen Perica, M.; Curić, M.; Grošić, P.F.; Delaš, I. Oxidative status and the severity of clinical symptoms in patients with post-traumatic stress disorder. Ann. Clin. Biochem. 2015, 52, 95–104. [Google Scholar] [CrossRef]
- Fidelman, S.; Mizrachi Zer-Aviv, T.; Lange, R.; Hillard, C.J.; Akirav, I. Chronic treatment with URB597 ameliorates post-stress symptoms in a rat model of PTSD. Eur. Neuropsychopharmacol. 2018, 28, 630–642. [Google Scholar] [CrossRef] [PubMed]
- Orsolini, L.; Chiappini, S.; Volpe, U.; Berardis, D.; Latini, R.; Papanti, G.D.; Corkery, A.J.M. Use of Medicinal Cannabis and Synthetic Cannabinoids in Post-Traumatic Stress Disorder (PTSD): A Systematic Review. Medicina 2019, 55, 525. [Google Scholar] [CrossRef]
- de Munter, J.; Pavlov, D.; Gorlova, A.; Sicker, M.; Proshin, A.; Kalueff, A.V.; Svistunov, A.; Kiselev, D.; Nedorubov, A.; Morozov, S.; et al. Increased Oxidative Stress in the Prefrontal Cortex as a Shared Feature of Depressive- and PTSD-Like Syndromes: Effects of a Standardized Herbal Antioxidant. Front. Nutr. 2021, 8, 661455. [Google Scholar] [CrossRef] [PubMed]
- Sales, A.J.; Fogaça, M.V.; Sartim, A.G.; Pereira, V.S.; Wegener, G.; Guimarães, F.S.; Joca, S.R.L. Cannabidiol Induces Rapid and Sustained Antidepressant-Like Effects Through Increased BDNF Signaling and Synaptogenesis in the Prefrontal Cortex. Mol. Neurobiol. 2019, 56, 1070–1081. [Google Scholar] [CrossRef]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: Role of 5-HT1A receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef] [Green Version]
- Bakas, T.; van Nieuwenhuijzen, P.S.; Devenish, S.O.; McGregor, I.S.; Arnold, J.C.; Chebib, M. The direct actions of cannabidiol and 2-arachidonoyl glycerol at GABAA receptors. Pharmacol. Res. 2017, 119, 358–370. [Google Scholar] [CrossRef]
- Surkin, P.N.; Gallino, S.L.; Luce, V.; Correa, F.; Fernandez-Solari, J.; De Laurentiis, A. Pharmacological augmentation of endocannabinoid signaling reduces the neuroendocrine response to stress. Psychoneuroendocrinology 2018, 87, 131–140. [Google Scholar] [CrossRef]
- Mansouri, A.; Demeilliers, C.; Amsellem, S.; Pessayre, D.; Fromenty, B. Acute ethanol administration oxidatively damages and depletes mitochondrial dna in mouse liver, brain, heart, and skeletal muscles: Protective effects of antioxidants. J. Pharmacol. Exp. Ther. 2001, 298, 737–743. [Google Scholar]
- Hao, E.; Mukhopadhyay, P.; Cao, Z.; Erdélyi, K.; Holovac, E.; Liaudet, L.; Lee, W.S.; Haskó, G.; Mechoulam, R.; Pacher, P. Cannabidiol Protects against Doxorubicin-Induced Cardiomyopathy by Modulating Mitochondrial Function and Biogenesis. Mol. Med. 2015, 21, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Weng, Q.Y.; Fisher, D.E. UV signaling pathways within the skin. J. Investig. Dermatol. 2014, 134, 2080–2085. [Google Scholar] [CrossRef]
- Atalay, S.; Gęgotek, A.; Wroński, A.; Domigues, P.; Skrzydlewska, E. Therapeutic application of cannabidiol on UVA and UVB irradiated rat skin. A proteomic study. J. Pharm. Biomed. Anal. 2021, 192, 113656. [Google Scholar] [CrossRef]
- Holmgren, A.; Lu, J. Thioredoxin and thioredoxin reductase: Current research with special reference to human disease. Biochem. Biophys. Res. Commun. 2010, 396, 120–124. [Google Scholar] [CrossRef]
- Łuczaj, W.; Domingues, M.D.R.; Domingues, P.; Skrzydlewska, E. Changes in Lipid Profile of Keratinocytes from Rat Skin Exposed to Chronic UVA or UVB Radiation and Topical Application of Cannabidiol. Antioxidants 2020, 9, 1178. [Google Scholar] [CrossRef] [PubMed]
- Petrovici, A.R.; Simionescu, N.; Sandu, A.I.; Paraschiv, V.; Silion, M.; Pinteala, M. New Insights on Hemp Oil Enriched in Cannabidiol: Decarboxylation, Antioxidant Properties and In Vitro Anticancer Effect. Antioxidants 2021, 10, 738. [Google Scholar] [CrossRef]
- Biernacki, M.; Jastrząb, A.; Skrzydlewska, E. Changes in Hepatic Phospholipid Metabolism in Rats under UV Irradiation and Topically Treated with Cannabidiol. Antioxidants 2021, 10, 1157. [Google Scholar] [CrossRef]
- Olivas-Aguirre, M.; Torres-López, L.; Valle-Reyes, J.S.; Hernández-Cruz, A.; Pottosin, I.; Dobrovinskaya, O. Cannabidiol directly targets mitochondria and disturbs calcium homeostasis in acute lymphoblastic leukemia. Cell Death Dis. 2019, 10, 779. [Google Scholar] [CrossRef] [Green Version]
- Kozela, E.; Pietr, M.; Juknat, A.; Rimmerman, N.; Levy, R.; Vogel, Z. Cannabinoids Delta(9)-tetrahydrocannabinol and cannabidiol differentially inhibit the lipopolysaccharide-activated NF-kappaB and interferon-beta/STAT proinflammatory pathways in BV-2 microglial cells. J. Biol. Chem. 2010, 285, 1616–1626. [Google Scholar] [CrossRef]
- Liou, G.I.; Auchampach, J.A.; Hillard, C.J.; Zhu, G.; Yousufzai, B.; Mian, S.; Khan, S.; Khalifa, Y. Mediation of cannabidiol anti-inflammation in the retina by equilibrative nucleoside transporter and A2A adenosine receptor. Investig. Ophthalmol. Vis. Sci. 2008, 49, 5526–5531. [Google Scholar] [CrossRef] [PubMed]
- Costa, B.; Colleoni, M.; Conti, S.; Parolaro, D.; Franke, C.; Trovato, A.E.; Giagnoni, G. Oral anti-inflammatory activity of cannabidiol, a non-psychoactive constituent of cannabis, in acute carrageenan-induced inflammation in the rat paw. Naunyn Schmiedebergs Arch. Pharmacol. 2004, 369, 294–299. [Google Scholar] [CrossRef]
- Oh, J.Y.; Giles, N.; Landar, A.; Darley-Usmar, V. Accumulation of 15-deoxy-delta(12,14)-prostaglandin J2 adduct formation with Keap1 over time: Effects on potency for intracellular antioxidant defence induction. Biochem. J. 2008, 411, 297–306. [Google Scholar] [CrossRef] [PubMed]
- Gilroy, D.W. Eicosanoids and the endogenous control of acute inflammatory resolution. Int. J. Biochem. Cell Biol. 2010, 42, 524–528. [Google Scholar] [CrossRef]
- Jastrząb, A.; Gęgotek, A.; Skrzydlewska, E. Cannabidiol Regulates the Expression of Keratinocyte Proteins Involved in the Inflammation Process through Transcriptional Regulation. Cells 2019, 8, 827. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo, M.C.; Zhang, D.D. The emerging role of the Nrf2-Keap1 signaling pathway in cancer. Genes Dev. 2013, 27, 2179–2191. [Google Scholar] [CrossRef] [PubMed]
- Pi, J.; Diwan, B.A.; Sun, Y.; Liu, J.; Qu, W.; He, Y.; Styblo, M.; Waalkes, M.P. Arsenic-induced malignant transformation of human keratinocytes: Involvement of Nrf2. Free Radic. Biol. Med. 2008, 45, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Gęgotek, A.; Rybałtowska-Kawałko, P.; Skrzydlewska, E. Rutin as a Mediator of Lipid Metabolism and Cellular Signaling Pathways Interactions in Fibroblasts Altered by UVA and UVB Radiation. Oxid. Med. Cell Longev. 2017, 2017, 4721352. [Google Scholar] [CrossRef] [PubMed]
- Juknat, A.; Pietr, M.; Kozela, E.; Rimmerman, N.; Levy, R.; Coppola, G.; Geschwind, D.; Vogel, Z. Differential transcriptional profiles mediated by exposure to the cannabinoids cannabidiol and Δ9-tetrahydrocannabinol in BV-2 microglial cells. Br. J. Pharmacol. 2012, 165, 2512–2528. [Google Scholar] [CrossRef] [Green Version]
- Chianese, G.; Sirignano, C.; Benetti, E.; Marzaroli, V.; Collado, J.A.; de la Vega, L.; Appendino, G.; Muñoz, E.; Taglialatela-Scafati, O. A Nrf-2 Stimulatory Hydroxylated Cannabidiol Derivative from Hemp (Cannabis sativa). J. Nat. Prod. 2022, 85, 1089–1097. [Google Scholar] [CrossRef]
- Zhang, X.; Guo, J.; Wei, X.; Niu, C.; Jia, M.; Li, Q.; Meng, D. Bach1: Function, Regulation, and Involvement in Disease. Oxid. Med. Cell Longev. 2018, 2018, 1347969. [Google Scholar] [CrossRef]
- Zhornitsky, S.; Potvin, S. Cannabidiol in humans-the quest for therapeutic targets. Pharmaceuticals 2012, 5, 529–552. [Google Scholar] [CrossRef]
- Pazos, M.R.; Cinquina, V.; Gómez, A.; Layunta, R.; Santos, M.; Fernández-Ruiz, J.; Martínez-Orgado, J. Cannabidiol administration after hypoxia-ischemia to newborn rats reduces long-term brain injury and restores neurobehavioral function. Neuropharmacology 2012, 63, 776–783. [Google Scholar] [CrossRef]
- Osborne, A.L.; Solowij, N.; Weston-Green, K. A systematic review of the effect of cannabidiol on cognitive function: Relevance to schizophrenia. Neurosci. Biobehav. Rev. 2017, 72, 310–324. [Google Scholar] [CrossRef]
- Martin, R.C.; Gaston, T.E.; Thompson, M.; Ampah, S.B.; Cutter, G.; Bebin, E.M.; Szaflarski, J.P. Cognitive functioning following long-term cannabidiol use in adults with treatment-resistant epilepsy. Epilepsy Behav. 2019, 97, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Leweke, F.M.; Rohleder, C.; Gerth, C.W.; Hellmich, M.; Pukrop, R.; Koethe, D. Cannabidiol and Amisulpride Improve Cognition in Acute Schizophrenia in an Explorative, Double-Blind, Active-Controlled, Randomized Clinical Trial. Front. Pharmacol. 2021, 12, 614811. [Google Scholar] [CrossRef]
- Morgan, C.J.; Schafer, G.; Freeman, T.P.; Curran, H.V. Impact of cannabidiol on the acute memory and psychotomimetic effects of smoked cannabis: Naturalistic study: Naturalistic study [corrected]. Br. J. Psychiatry 2010, 197, 285–290. [Google Scholar] [CrossRef]
- McGuire, J.L.; DePasquale, E.A.K.; Watanabe, M.; Anwar, F.; Ngwenya, L.B.; Atluri, G.; Romick-Rosendale, L.E.; McCullumsmith, R.E.; Evanson, N.K. Chronic Dysregulation of Cortical and Subcortical Metabolism After Experimental Traumatic Brain Injury. Mol. Neurobiol. 2019, 56, 2908–2921. [Google Scholar] [CrossRef]
- Tasker, J.G.; Chen, C.; Fisher, M.O.; Fu, X.; Rainville, J.R.; Weiss, G.L. Endocannabinoid Regulation of Neuroendocrine Systems. Int. Rev. Neurobiol. 2015, 125, 163–201. [Google Scholar] [CrossRef] [PubMed]
- Ueberall, M.A.; Essner, U.; Mueller-Schwefe, G.H. Effectiveness and tolerability of THC:CBD oromucosal spray as add-on measure in patients with severe chronic pain: Analysis of 12-week open-label real-world data provided by the German Pain e-Registry. J. Pain Res. 2019, 12, 1577–1604. [Google Scholar] [CrossRef]
- Sarris, J.; Sinclair, J.; Karamacoska, D.; Davidson, M.; Firth, J. Medicinal cannabis for psychiatric disorders: A clinically-focused systematic review. BMC Psychiatry 2020, 20, 24. [Google Scholar] [CrossRef]
- Osborne, A.L.; Solowij, N.; Babic, I.; Huang, X.F.; Weston-Green, K. Improved Social Interaction, Recognition and Working Memory with Cannabidiol Treatment in a Prenatal Infection (poly I:C) Rat Model. Neuropsychopharmacology 2017, 42, 1447–1457. [Google Scholar] [CrossRef] [PubMed]
- Demirakca, T.; Sartorius, A.; Ende, G.; Meyer, N.; Welzel, H.; Skopp, G.; Mann, K.; Hermann, D. Diminished gray matter in the hippocampus of cannabis users: Possible protective effects of cannabidiol. Drug Alcohol Depend. 2011, 114, 242–245. [Google Scholar] [CrossRef]
- Friedman, L.K.; Peng, H.; Zeman, R.J. Cannabidiol reduces lesion volume and restores vestibulomotor and cognitive function following moderately severe traumatic brain injury. Exp. Neurol. 2021, 346, 113844. [Google Scholar] [CrossRef] [PubMed]
- Fagherazzi, E.V.; Garcia, V.A.; Maurmann, N.; Bervanger, T.; Halmenschlager, L.H.; Busato, S.B.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; Schröder, N. Memory-rescuing effects of cannabidiol in an animal model of cognitive impairment relevant to neurodegenerative disorders. Psychopharmacology 2012, 219, 1133–1140. [Google Scholar] [CrossRef] [PubMed]
- Cassol, O.J., Jr.; Comim, C.M.; Silva, B.R.; Hermani, F.V.; Constantino, L.S.; Felisberto, F.; Petronilho, F.; Hallak, J.E.; De Martinis, B.S.; Zuardi, A.W.; et al. Treatment with cannabidiol reverses oxidative stress parameters, cognitive impairment and mortality in rats submitted to sepsis by cecal ligation and puncture. Brain Res. 2010, 1348, 128–138. [Google Scholar] [CrossRef]
- Halliwell, B. Oxidative stress and neurodegeneration: Where are we now? J. Neurochem. 2006, 97, 1634–1658. [Google Scholar] [CrossRef] [PubMed]
- Tuon, L.; Comim, C.M.; Petronilho, F.; Barichello, T.; Izquierdo, I.; Quevedo, J.; Dal-Pizzol, F. Time-dependent behavioral recovery after sepsis in rats. Intensive Care Med. 2008, 34, 1724–1731. [Google Scholar] [CrossRef]
- Boggs, D.L.; Surti, T.; Gupta, A.; Gupta, S.; Niciu, M.; Pittman, B.; Schnakenberg Martin, A.M.; Thurnauer, H.; Davies, A.; D’Souza, D.C.; et al. The effects of cannabidiol (CBD) on cognition and symptoms in outpatients with chronic schizophrenia a randomized placebo controlled trial. Psychopharmacology 2018, 235, 1923–1932. [Google Scholar] [CrossRef]
- Blaes, S.L.; Orsini, C.A.; Holik, H.M.; Stubbs, T.D.; Ferguson, S.N.; Heshmati, S.C.; Bruner, M.M.; Wall, S.C.; Febo, M.; Bruijnzeel, A.W.; et al. Enhancing effects of acute exposure to cannabis smoke on working memory performance. Neurobiol. Learn. Mem. 2019, 157, 151–162. [Google Scholar] [CrossRef]
- McLaughlin, R.J.; Hill, M.N.; Gorzalka, B.B. A critical role for prefrontocortical endocannabinoid signaling in the regulation of stress and emotional behavior. Neurosci. Biobehav. Rev. 2014, 42, 116–131. [Google Scholar] [CrossRef]
- Khodadadi, H.; Salles, É.L.; Jarrahi, A.; Costigliola, V.; Khan, M.B.; Yu, J.C.; Morgan, J.C.; Hess, D.C.; Vaibhav, K.; Dhandapani, K.M.; et al. Cannabidiol Ameliorates Cognitive Function via Regulation of IL-33 and TREM2 Upregulation in a Murine Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2021, 80, 973–977. [Google Scholar] [CrossRef]
- Peres, F.F.; Levin, R.; Suiama, M.A.; Diana, M.C.; Gouvêa, D.A.; Almeida, V.; Santos, C.M.; Lungato, L.; Zuardi, A.W.; Hallak, J.E.; et al. Cannabidiol Prevents Motor and Cognitive Impairments Induced by Reserpine in Rats. Front. Pharmacol. 2016, 7, 343. [Google Scholar] [CrossRef]
- Razavi, Y.; Shabani, R.; Mehdizadeh, M.; Haghparast, A. Neuroprotective effect of chronic administration of cannabidiol during the abstinence period on methamphetamine-induced impairment of recognition memory in the rats. Behav. Pharmacol. 2020, 31, 385–396. [Google Scholar] [CrossRef] [PubMed]
- Renard, J.; Loureiro, M.; Rosen, L.G.; Zunder, J.; de Oliveira, C.; Schmid, S.; Rushlow, W.J.; Laviolette, S.R. Cannabidiol Counteracts Amphetamine-Induced Neuronal and Behavioral Sensitization of the Mesolimbic Dopamine Pathway through a Novel mTOR/p70S6 Kinase Signaling Pathway. J. Neurosci. 2016, 36, 5160–5169. [Google Scholar] [CrossRef]
- Schiavon, A.P.; Soares, L.M.; Bonato, J.M.; Milani, H.; Guimarães, F.S.; Weffort de Oliveira, R.M. Protective effects of cannabidiol against hippocampal cell death and cognitive impairment induced by bilateral common carotid artery occlusion in mice. Neurotox. Res. 2014, 26, 307–316. [Google Scholar] [CrossRef]
- Barichello, T.; Ceretta, R.A.; Generoso, J.S.; Moreira, A.P.; Simões, L.R.; Comim, C.M.; Quevedo, J.; Vilela, M.C.; Zuardi, A.W.; Crippa, J.A.; et al. Cannabidiol reduces host immune response and prevents cognitive impairments in Wistar rats submitted to pneumococcal meningitis. Eur. J. Pharmacol. 2012, 697, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Martín-Moreno, A.M.; Reigada, D.; Ramírez, B.G.; Mechoulam, R.; Innamorato, N.; Cuadrado, A.; de Ceballos, M.L. Cannabidiol and other cannabinoids reduce microglial activation in vitro and in vivo: Relevance to Alzheimer’s disease. Mol. Pharmacol. 2011, 79, 964–973. [Google Scholar] [CrossRef] [PubMed]
- García-Baos, A.; Puig-Reyne, X.; García-Algar, Ó.; Valverde, O. Cannabidiol attenuates cognitive deficits and neuroinflammation induced by early alcohol exposure in a mice model. Biomed. Pharmacother. 2021, 141, 111813. [Google Scholar] [CrossRef]
- Mori, M.A.; Meyer, E.; Soares, L.M.; Milani, H.; Guimarães, F.S.; de Oliveira, R.M.W. Cannabidiol reduces neuroinflammation and promotes neuroplasticity and functional recovery after brain ischemia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 75, 94–105. [Google Scholar] [CrossRef] [PubMed]
- Mori, M.A.; Meyer, E.; da Silva, F.F.; Milani, H.; Guimarães, F.S.; Oliveira, R.M.W. Differential contribution of CB1, CB2, 5-HT1A, and PPAR-γ receptors to cannabidiol effects on ischemia-induced emotional and cognitive impairments. Eur. J. Neurosci. 2021, 53, 1738–1751. [Google Scholar] [CrossRef]
- Magen, I.; Avraham, Y.; Ackerman, Z.; Vorobiev, L.; Mechoulam, R.; Berry, E.M. Cannabidiol ameliorates cognitive and motor impairments in mice with bile duct ligation. J. Hepatol. 2009, 51, 528–534. [Google Scholar] [CrossRef]
- Moreira, F.A.; Aguiar, D.C.; Terzian, A.L.; Guimarães, F.S.; Wotjak, C.T. Cannabinoid type 1 receptors and transient receptor potential vanilloid type 1 channels in fear and anxiety-two sides of one coin? Neuroscience 2012, 204, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Kozela, E.; Krawczyk, M.; Kos, T.; Juknat, A.; Vogel, Z.; Popik, P. Cannabidiol Improves Cognitive Impairment and Reverses Cortical Transcriptional Changes Induced by Ketamine, in Schizophrenia-Like Model in Rats. Mol. Neurobiol. 2020, 57, 1733–1747. [Google Scholar] [CrossRef] [PubMed]
- Corrê, M.D.S.; de Freitas, B.S.; Machado, G.D.B.; Pires, V.N.; Bromberg, E.; Hallak, J.E.C.; Zuardi, A.W.; Crippa, J.A.S.; Schröder, N. Cannabidiol reverses memory impairments and activates components of the Akt/GSK3β pathway in an experimental model of estrogen depletion. Behav. Brain Res. 2022, 417, 113555. [Google Scholar] [CrossRef] [PubMed]
- de Paula Faria, D.; Estessi de Souza, L.; Duran, F.L.S.; Buchpiguel, C.A.; Britto, L.R.; Crippa, J.A.S.; Filho, G.B.; Real, C.C. Cannabidiol Treatment Improves Glucose Metabolism and Memory in Streptozotocin-Induced Alzheimer’s Disease Rat Model: A Proof-of-Concept Study. Int. J. Mol. Sci. 2022, 23, 1076. [Google Scholar] [CrossRef] [PubMed]
- Kruk-Slomka, M.; Biala, G. Cannabidiol Attenuates MK-801-Induced Cognitive Symptoms of Schizophrenia in the Passive Avoidance Test in Mice. Molecules 2021, 26, 5977. [Google Scholar] [CrossRef]
- The Prohibited List. Available online: https://www.wada-ama.org/en/prohibited-list#search-anchor (accessed on 5 November 2022).
- Bergamaschi, M.M.; Crippa, J.A. Why should Cannabis be Considered Doping in Sports? Front. Psychiatry 2013, 4, 32. [Google Scholar] [CrossRef]
- Kennedy, M.C. Cannabis: Exercise performance and sport. A systematic review. J. Sci. Med. Sport 2017, 20, 825–829. [Google Scholar] [CrossRef]
- Ware, M.A.; Jensen, D.; Barrette, A.; Vernec, A.; Derman, W. Cannabis and the Health and Performance of the Elite Athlete. Clin J. Sport Med. 2018, 28, 480–484. [Google Scholar] [CrossRef]
- WADA. Summary of Major Modifications and Explanatory Notes. 2020 Prohibited List; S8 Cannabinoids; World Anti-Doping Agency: Montreal, QC, Canada, 2019; Available online: https://www.wada-ama.org/sites/default/files/wada_2020_english_summary_of_modifications_.pdf (accessed on 5 November 2022).
- Lachenmeier, D.W.; Diel, P. A Warning against the Negligent Use of Cannabidiol in Professional and Amateur Athletes. Sports 2019, 7, 251. [Google Scholar] [CrossRef]
- Hindocha, C.; Freeman, T.P.; Schafer, G.; Gardener, C.; Das, R.K.; Morgan, C.J.; Curran, H.V. Acute effects of delta-9-tetrahydrocannabinol, cannabidiol and their combination on facial emotion recognition: A randomised, double-blind, placebo-controlled study in cannabis users. Eur. Neuropsychopharmacol. 2015, 25, 325–334. [Google Scholar] [CrossRef]
- Masataka, N. Anxiolytic Effects of Repeated Cannabidiol Treatment in Teenagers with Social Anxiety Disorders. Front. Psychol. 2019, 10, 2466. [Google Scholar] [CrossRef]
- Isenmann, E.; Blume, F.; Bizjak, D.A.; Hundsdörfer, V.; Pagano, S.; Schibrowski, S.; Simon, W.; Schmandra, L.; Diel, P. Comparison of Pro-Regenerative Effects of Carbohydrates and Protein Administrated by Shake and Non-Macro-Nutrient Matched Food Items on the Skeletal Muscle after Acute Endurance Exercise. Nutrients 2019, 11, 744. [Google Scholar] [CrossRef] [PubMed]
- Lieberman, H.R.; Marriott, B.P.; Williams, C.; Judelson, D.A.; Glickman, E.L.; Geiselman, P.J.; Dotson, L.; Mahoney, C.R. Patterns of dietary supplement use among college students. Clin. Nutr. 2015, 34, 976–985. [Google Scholar] [CrossRef]
- Tavares, F.; Smith, T.B.; Driller, M. Fatigue and Recovery in Rugby: A Review. Sports Med. 2017, 47, 1515–1530. [Google Scholar] [CrossRef] [PubMed]
- Wilson, L.J.; Cockburn, E.; Paice, K.; Sinclair, S.; Faki, T.; Hills, F.A.; Gondek, M.B.; Wood, A.; Dimitriou, L. Recovery following a marathon: A comparison of cold water immersion, whole body cryotherapy and a placebo control. Eur. J. Appl. Physiol. 2018, 118, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Isenmann, E.; Veit, S.; Starke, L.; Flenker, U.; Diel, P. Effects of Cannabidiol Supplementation on Skeletal Muscle Regeneration after Intensive Resistance Training. Nutrients 2021, 13, 3028. [Google Scholar] [CrossRef] [PubMed]
- Philpott, H.T.; O’Brien, M.; McDougall, J.J. Attenuation of early phase inflammation by cannabidiol prevents pain and nerve damage in rat osteoarthritis. Pain 2017, 158, 2442–2451. [Google Scholar] [CrossRef]
- Iannotti, F.A.; Pagano, E.; Moriello, A.S.; Alvino, F.G.; Sorrentino, N.C.; D’Orsi, L.; Gazzerro, E.; Capasso, R.; De Leonibus, E.; De Petrocellis, L.; et al. Effects of non-euphoric plant cannabinoids on muscle quality and performance of dystrophic mdx mice. Br. J. Pharmacol. 2019, 176, 1568–1584. [Google Scholar] [CrossRef]
- Langer, H.T.; Mossakowski, A.A.; Pathak, S.; Mascal, M.; Baar, K. Cannabidiol Does Not Impair Anabolic Signaling Following Eccentric Contractions in Rats. Int. J. Sport Nutr. Exerc. Metab. 2021, 31, 93–100. [Google Scholar] [CrossRef]
- Cochrane-Snyman, K.C.; Cruz, C.; Morales, J.; Coles, M. The Effects of Cannabidiol Oil on Noninvasive Measures of Muscle Damage in Men. Med. Sci. Sports Exerc. 2021, 53, 1460–1472. [Google Scholar] [CrossRef]
- Hatchett, A.; Armstrong, K.; Hughes, B.; Parr, B.B. The influence cannabidiol on delayed onset of muscle soreness. Int. J. Phys. Educ. Sports Health 2020, 7, 89–94. [Google Scholar]
- Gamelin, F.X.; Cuvelier, G.; Mendes, A.; Aucouturier, J.; Berthoin, S.; Di Marzo, V.; Heyman, E. Cannabidiol in sport: Ergogenic or else? Pharmacol. Res. 2020, 156, 104764. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- McCartney, D.; Benson, M.J.; Desbrow, B.; Irwin, C.; Suraev, A.; McGregor, I.S. Cannabidiol and Sports Performance: A Narrative Review of Relevant Evidence and Recommendations for Future Research. Sports Med. Open 2020, 6, 27. [Google Scholar] [CrossRef]
- Shrivastava, A.; Kuzontkoski, P.M.; Groopman, J.E.; Prasad, A. Cannabidiol induces programmed cell death in breast cancer cells by coordinating the cross-talk between apoptosis and autophagy. Mol. Cancer Ther. 2011, 10, 1161–1172. [Google Scholar] [CrossRef] [PubMed]
- Ohsumi, Y. Historical landmarks of autophagy research. Cell Res. 2014, 24, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Lee, X.C.; Werner, E.; Falasca, M. Molecular Mechanism of Autophagy and Its Regulation by Cannabinoids in Cancer. Cancers 2021, 13, 1211. [Google Scholar] [CrossRef]
- Wang, W.; Wang, X.; Fujioka, H.; Hoppel, C.; Whone, A.L.; Caldwell, M.A.; Cullen, P.J.; Liu, J.; Zhu, X. Parkinson’s disease-associated mutant VPS35 causes mitochondrial dysfunction by recycling DLP1 complexes. Nat. Med. 2016, 2, 54–63. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.; Li, J.; Yao, Z.; Liu, J. Cannabidiol Induces Autophagy to Protects Neural Cells from Mitochondrial Dysfunction by Upregulating SIRT1 to Inhibits NF-κB and NOTCH Pathways. Front. Cell. Neurosci. 2021, 15, 654340. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.I.; Kim, H.J.; Park, J.S.; Kim, I.J.; Bae, E.H.; Ma, S.K.; Kim, S.W. PGC-1α attenuates hydrogen peroxide-induced apoptotic cell death by upregulating Nrf-2 via GSK3β inactivation mediated by activated p38 in HK-2 Cells. Sci. Rep. 2017, 7, 4319. [Google Scholar] [CrossRef]
- Moors, T.E.; Hoozemans, J.J.; Ingrassia, A.; Beccari, T.; Parnetti, L.; Chartier-Harlin, M.C.; van de Berg, W.D. Therapeutic potential of autophagy-enhancing agents in Parkinson’s disease. Mol. Neurodegener. 2017, 12, 11. [Google Scholar] [CrossRef]
- Vrechi, T.A.M.; Leão, A.H.F.F.; Morais, I.B.M.; Abílio, V.C.; Zuardi, A.W.; Hallak, J.E.C.; Crippa, J.A.; Bincoletto, C.; Ureshino, R.P.; Smaili, S.S.; et al. Cannabidiol induces autophagy via ERK1/2 activation in neural cells. Sci. Rep. 2021, 11, 5434. [Google Scholar] [CrossRef]
- Pandelides, Z.; Thornton, C.; Faruque, A.S.; Whitehead, A.P.; Willett, K.L.; Ashpole, N.M. Developmental exposure to cannabidiol (CBD) alters longevity and health span of zebrafish (Danio rerio). Geroscience 2020, 42, 785–800. [Google Scholar] [CrossRef] [PubMed]
- Land, M.H.; Toth, M.L.; MacNair, L.; Vanapalli, S.A.; Lefever, T.W.; Peters, E.N.; Bonn-Miller, M.O. Effect of Cannabidiol on the Long-Term Toxicity and Lifespan in the Preclinical Model Caenorhabditis elegans. Cannabis Cannabinoid Res. 2021, 6, 522–527. [Google Scholar] [CrossRef]
- Wang, Z.; Zheng, P.; Xie, Y.; Chen, X.; Solowij, N.; Green, K.; Chew, Y.L.; Huang, X.F. Cannabidiol regulates CB1-pSTAT3 signaling for neurite outgrowth, prolongs lifespan, and improves health span in Caenorhabditis elegans of Aβ pathology models. FASEB J. 2021, 35, e21537. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zheng, P.; Chen, X.; Xie, Y.; Weston-Green, K.; Solowij, N.; Chew, Y.L.; Huang, X.F. Cannabidiol induces autophagy and improves neuronal health associated with SIRT1 mediated longevity. Geroscience 2022, 44, 1505–1524. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.H.; Chen, Y.C.; Jiang, H.C.; Chen, C.K.; Pan, C.L. Neuronal aging: Learning from C. elegans. J. Mol. Signal. 2013, 8, 14. [Google Scholar] [CrossRef]
- Chan, J.Z.; Duncan, R.E. Regulatory Effects of Cannabidiol on Mitochondrial Functions: A Review. Cells 2021, 10, 1251. [Google Scholar] [CrossRef]
- da Silva, V.K.; de Freitas, B.S.; da Silva Dornelles, A.; Nery, L.R.; Falavigna, L.; Ferreira, R.D.; Bogo, M.R.; Hallak, J.E.; Zuardi, A.W.; Crippa, J.A.; et al. Cannabidiol normalizes caspase 3, synaptophysin, and mitochondrial fission protein DNM1L expression levels in rats with brain iron overload: Implications for neuroprotection. Mol. Neurobiol. 2014, 49, 222–233. [Google Scholar] [CrossRef]
- Castillo, A.; Tolón, M.R.; Fernández-Ruiz, J.; Romero, J.; Martinez-Orgado, J. The neuroprotective effect of cannabidiol in an in vitro model of newborn hypoxic-ischemic brain damage in mice is mediated by CB(2) and adenosine receptors. Neurobiol. Dis. 2010, 37, 434–440. [Google Scholar] [CrossRef]


| Animal Model | Cognitive Impairment Model | Task | Treatment (Dose) | Duration | Observations | Ref. |
|---|---|---|---|---|---|---|
| Sabra female mice (8 weeks) | Hepatic encephalopathy (bile duct ligation) | Eight-arm maze test | CBD i.p. (5 mg/kg) | 21 days | Restored cognitive function (p = 0.001). CBD had no effect on sham animals. | [160] |
| Male and female Wistar rats (7–10 days old) | Hypoxia-ischemia (left carotid artery electrocoagulation) | Novel object recognition test (NOR) | CBD s.c. (1 mg/kg) | 3 days | CBD-treated rats test performance was similar to that of sham animals (p < 0.05). | [196] |
| Adult male and female Sprague–Dawley and Wistar rats | Controlled cortical contusion injury | T-maze test Novel object recognition test (NOR) | CBD i.p. (40 mg/kg, 20 mg/kg) | 15 days | CBD treatment increased the discrimination index compared to the traumatic brain injury group, being able to discriminate the novel object. | [207] |
| Male Wistar rats (3 weeks) | Iron neonatal treatment | Inhibitory avoidance task Novel object recognition test (NOR) | CBD i.p. (2.5, 5, 10 mg/kg) | 14 days |
The 10 mg/kg dose of CBD was able to reverse iron-induced memory deficits, recognition index of this group being significantly higher when compared to the iron treated group (p < 0.0001). | [208] |
| Male Wistar rats (3–4 months) | Sepsis induction-cecal ligation and perforation | Inhibitory avoidance test | CBD i.p. (2.5, 5, 10 mg/kg) | 9 days | CBD in all doses reverted the memory alteration (p = 0.001). | [209] |
| Male and female Long Evans rats | - | Working memory test | Cannabis cigarettes (5.6% THC, 0% CBD, 0.4% CBN) | - | In male rats, cannabis smoke exposure did not affect choice accuracy. In female rats, however, exposure to cannabis smoke significantly enhanced choice accuracy. The effect on working memory accuracy, cannabis smoke increased the number of trials completed in females (p = 0.01) but not in males (p = 0.48) and decreased locomotor activity in both sexes (males: p = 0.04; females; p = 0.03). It should be noted that the performance-enhancing effect after exposure to cannabis smoke was evident only in female rats, whose initial performance was significantly lower than in males. | [213] |
| 5xFAD male mice (9–12 months) | Transgenic mice | Novel object recognition test (NOR) | CBD i.p. (10 mg/kg) | 2 weeks (one dose every other day) | CBD treatment improved cognitive function as measured by NOR (Discrimination Index increased to 0.5 ± 0.9 from −0.2 ± 0.8, p ≤ 0.04). | [215] |
| Male Wistar rats (3 months) | Motor and cognitive impairments induced by reserpine | Plus maze discriminative avoidance task | CBD i.p (0.5 and 5 mg/kg) | 4 days | The time spent in aversive enclosed arm is lower than the time spent in the non-aversive enclosed arm for CBD 0.5 (p < 0.05), but not for CBD 5. | [216] |
| Male Wistar rats | Methamphetamine chronic exposure (10 days, twice/day) | The Y-maze (YM) test Novel object recognition test (NOR) | CBD ICV microinjection (32 and 162 nmol) | 10 days | Both doses of CBD significantly improved spatial memory. The higher dose of CBD was more effective, p < 0.001). A high dose of CBD(160 nmol) could improve long-term memory p < 0.025). | [217] |
| Male and female offspring C57BL/6J | Alcohol exposure | The Y-maze (YM) test Novel object recognition test (NOR) | CBD i.p. (20 mg/kg) | 10 days | CBD-treated group showed a significantly higher preference for the novel arm as compared to alcohol-exposed group (p < 0.05), revealing that CBD treatment hampers the detrimental effect on reference memory caused by alcohol. CBD does not affect the recognition memory (NOR), but CBD treatment impedes the deleterious effects of alcohol on object location memory. | [222] |
| Male C57BL/6J mice (2–3 months) | Bilateral common carotid artery occlusion (BCCAO) | The Y-maze (YM) test | CBD i.p. (10 mg/kg) | 0.5, 3, 24, 48 h after the surgery | CBD increased in the % of the time in the novel arm of the YM when compared to BCCAO animals treated only with vehicle p < 0.01. | [224] |
| Male Sprague–Dawley rats | Schizophrenia-like cognitive deficits induced by repeated ketamine administration | Novel object recognition test (NOR) | CBD i.p. (7.5 mg/kg) | 6 days | Ketamine-induced cognitive deficits were restored by CBD (p < 0.001). | [227] |
| Adult female Wistar rats | Estrogen depletion (surgery) | Inhibitory avoidance | CBD i.p. (10 mg/kg) | 14 days | Ovariectomized rats treated with CBD had a higher latency to step down (p = 0.001). | [228] |
| Male Wistar rats (3 weeks) | ICV injection of streptozotocin | Novel object recognition test (NOR) | CBD i.p. (20 mg/kg) | 7 days | CBD-treated group showed a better performance both on short- and long-term memory (p < 0.0001). | [229] |
| Swiss male mice (4 weeks) | MK-801 injection | Passive avoidance test | CBD i.p. (1, 5, 30 mg/kg) | Acute experiment | CBD treatment in dose of 30 mg/kg appeared to have a better performance (p < 0.001). | [230] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jîtcă, G.; Ősz, B.E.; Vari, C.E.; Rusz, C.-M.; Tero-Vescan, A.; Pușcaș, A. Cannabidiol: Bridge between Antioxidant Effect, Cellular Protection, and Cognitive and Physical Performance. Antioxidants 2023, 12, 485. https://doi.org/10.3390/antiox12020485
Jîtcă G, Ősz BE, Vari CE, Rusz C-M, Tero-Vescan A, Pușcaș A. Cannabidiol: Bridge between Antioxidant Effect, Cellular Protection, and Cognitive and Physical Performance. Antioxidants. 2023; 12(2):485. https://doi.org/10.3390/antiox12020485
Chicago/Turabian StyleJîtcă, George, Bianca E. Ősz, Camil E. Vari, Carmen-Maria Rusz, Amelia Tero-Vescan, and Amalia Pușcaș. 2023. "Cannabidiol: Bridge between Antioxidant Effect, Cellular Protection, and Cognitive and Physical Performance" Antioxidants 12, no. 2: 485. https://doi.org/10.3390/antiox12020485