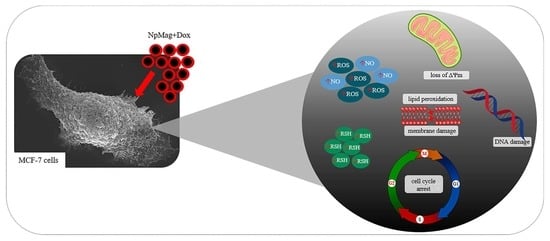
Doxorubicin-Loaded Iron Oxide Nanoparticles Induce Oxidative Stress and Cell Cycle Arrest in Breast Cancer Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nanoparticles Synthesis
2.2. Doxorubicin Loading
2.3. Nanoparticles Characterization
2.4. MCF-7 Cells Treatment
2.5. Determination the Generation of Total Reactive Oxygen Species (ROS)
2.6. Detection of Nitric Oxide
2.7. Reduced Thiols Assay
2.8. DNA Fragmentation
2.9. Determination of Lipid Peroxidation
2.10. Assessment of Cell Membrane Integrity
2.11. Assessment of Mitochondrial Membrane Potential (ΔΨm)
2.12. Cell Cycle Analysis
2.13. Wound Healing Assay
2.14. Clonogenic Assay
2.15. Evaluation of Magnetic-Field-Mediated Targeted Drug Delivery
2.16. Statistical Analysis
3. Results
3.1. Nanoparticle Characterization
3.2. In Vitro Biological Assays
3.2.1. NpMag+Dox Increases the Reactive Oxygen and Nitrogen Species in MCF-7 Cells
3.2.2. NpMag+Dox Decreases the Levels of Reduced Thiols in MCF-7 Cells
3.2.3. NpMag+Dox Induces DNA Damage, Increases Lipid Peroxidation, Induces Membrane Integrity Loss, and Decreases ΔΨm in MCF-7 Cells
3.2.4. NpMag+Dox Increases G2 Cell Cycle Arrest in MCF7 Cells
3.2.5. NpMag+Dox Inhibits MCF7 Cells Migration
3.2.6. NpMag+Dox Inhibits MCF7 Cells Colonies Formation
3.2.7. NpMag+Dox Inhibits MCF-7 Cells Grow in the Region of Magnetic Field
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Long, N.V.; Yang, Y.; Teranishi, T.; Thi, C.M.; Cao, Y.; Nogami, M. Biomedical Applications of Advanced Multifunctional Magnetic Nanoparticles. J. Nanosci. Nanotechnol. 2015, 15, 10091–10107. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted Drug Delivery with Polymers and Magnetic Nanoparticles: Covalent and Noncovalent Approaches, Release Control, and Clinical Studies. Chem. Rev. 2016, 116, 5338–5431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.-H.; von Maltzahn, G.; Ruoslahti, E.; Bhatia, S.N.; Sailor, M.J. Micellar Hybrid Nanoparticles for Simultaneous Magnetofluorescent Imaging and Drug Delivery. Angew. Chem. 2008, 120, 7394–7398. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Z.; Huang, D.; Yin, Z.; Chi, X.; Wang, X.; Gao, J. Magnetite Nanoparticles as Smart Carriers to Manipulate the Cytotoxicity of Anticancer Drugs: Magnetic Control and PH-Responsive Release. J. Mater. Chem. 2012, 22, 15717–15725. [Google Scholar] [CrossRef]
- Gupta, A.K.; Gupta, M. Synthesis and Surface Engineering of Iron Oxide Nanoparticles for Biomedical Applications. Biomaterials 2005, 26, 3995–4021. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, T.; Gao, J. Biocompatible Iron Oxide Nanoparticles for Targeted Cancer Gene Therapy: A Review. Nanomaterials 2022, 12, 3323. [Google Scholar] [CrossRef]
- Stanicki, D.; Vangijzegem, T.; Ternad, I.; Laurent, S. An Update on the Applications and Characteristics of Magnetic Iron Oxide Nanoparticles for Drug Delivery. Expert Opin. Drug Deliv. 2022, 19, 321–335. [Google Scholar] [CrossRef]
- Avasthi, A.; Caro, C.; Pozo-Torres, E.; Leal, M.P.; García-Martín, M.L. Magnetic Nanoparticles as MRI Contrast Agents. Top. Curr. Chem. 2020, 378, 49–50. [Google Scholar] [CrossRef]
- Laurent, S.; Saei, A.A.; Behzadi, S.; Panahifar, A.; Mahmoudi, M. Superparamagnetic Iron Oxide Nanoparticles for Delivery of Therapeutic Agents: Opportunities and Challenges. Expert Opin. Drug Deliv. 2014, 11, 1449–1470. [Google Scholar] [CrossRef]
- González-Gómez, M.A.; Belderbos, S.; Yañez-Vilar, S.; Piñeiro, Y.; Cleeren, F.; Bormans, G.; Deroose, C.M.; Gsell, W.; Himmelreich, U.; Rivas, J. Development of Superparamagnetic Nanoparticles Coated with Polyacrylic Acid and Aluminum Hydroxide as an Efficient Contrast Agent for Multimodal Imaging. Nanomaterials 2019, 9, 1626. [Google Scholar] [CrossRef]
- Mushtaq, A.; Zhao, R.; Luo, D.; Dempsey, E.; Wang, X.; Iqbal, M.Z.; Kong, X. Magnetic Hydroxyapatite Nanocomposites: The Advances from Synthesis to Biomedical Applications. Mater. Des. 2021, 197, 109269. [Google Scholar] [CrossRef]
- Mushtaq, A.; Tang, Z.; Hou, Y.; Zhu, Z.; Tian, C.; Wu, Y.; Lu, Y.; Iqbal, M.Z.; Kong, X. Biocompatible Magnetic Hydroxyapatite Fe3O4-HAp Nanocomposites for T1-Magnetic Resonance Imaging Guided Photothermal Therapy of Breast Cancer. Mater. Today Commun. 2022, 31, 103734. [Google Scholar] [CrossRef]
- Mushtaq, A.; Zhang, H.; Cui, M.; Lin, X.; Huang, S.; Tang, Z.; Hou, Y.; Zubair Iqbal, M.; Kong, X. ROS-Responsive Chlorin E6 and Silk Fibroin Loaded Ultrathin Magnetic Hydroxyapatite Nanorods for T1-Magnetic Resonance Imaging Guided Photodynamic Therapy in Vitro. Colloids Surfaces A Physicochem. Eng. Asp. 2023, 656, 130513. [Google Scholar] [CrossRef]
- Aroui, S.; Brahim, S.; Waard, M.D.; Kenani, A. Cytotoxicity, Intracellular Distribution and Uptake of Doxorubicin and Doxorubicin Coupled to Cell-Penetrating Peptides in Different Cell Lines: A Comparative Study. Biochem. Biophys. Res. Commun. 2010, 391, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Yuan, A.; Wu, J.; Song, C.; Tang, X.; Qiao, Q.; Zhao, L.; Hu, Y. A Novel Self-Assembly Albumin Nanocarrier for Reducing Doxorubicin-Mediated Cardiotoxicity. J. Pharm. Sci. 2013, 101, 2271–2280. [Google Scholar] [CrossRef]
- Liang, P.-C.; Chen, Y.-C.; Chiang, C.-F.; Mo, L.-R.; Wei, S.-Y.; Hsieh, W.-Y.; Lin, W.-L. Doxorubicin-Modified Magnetic Nanoparticles as a Drug Delivery System for Magnetic Resonance Imaging-Monitoring Magnet-Enhancing Tumor Chemotherapy. Int. J. Nanomed. 2016, 11, 2021. [Google Scholar] [CrossRef] [Green Version]
- Zou, Y.; Liu, P.; Liu, C.-H.; Zhi, X.-T. Doxorubicin-Loaded Mesoporous Magnetic Nanoparticles to Induce Apoptosis in Breast Cancer Cells. Biomed. Pharm. 2015, 69, 355–360. [Google Scholar] [CrossRef]
- Hernandes, E.P.; Bini, R.D.; Endo, K.M.; de Oliveira Junior, V.A.; de Almeida, I.V.; Dias, G.S.; dos Santos, I.A.; de Oliveira, P.N.; Vicentini, V.E.P.; Cotica, L.F. Doxorubicin-Loaded Magnetic Nanoparticles: Enhancement of Doxorubicin’s Effect on Breast Cancer Cells (MCF-7). Magnetochemistry 2022, 8, 114. [Google Scholar] [CrossRef]
- Kayal, S.; Ramanujan, R.V. Doxorubicin Loaded PVA Coated Iron Oxide Nanoparticles for Targeted Drug Delivery. Mater. Sci. Eng. C 2010, 30, 484–490. [Google Scholar] [CrossRef]
- Royall, J.A.; Ischiropoulos, H. Evaluation of 2′,7′-Dichlorofluorescin and Dihydrorhodamine 123 as Fluorescent Probes for Intracellular H2O2 in Cultured Endothelial Cells. Arch. Biochem. Biophys. 1993, 302, 348–355. [Google Scholar] [CrossRef]
- Chris, D.S.; Laurent, T.C.M. Mast Cells; Hughes, M.R., McNagny, K.M., Eds.; Methods in Molecular Biology; Springer: New York, NY, USA, 2015; Volume 1220, ISBN 978-1-4939-1567-5. [Google Scholar]
- Ellman, G.L. Tissue Sufhydril Groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Kyrylkova, K.; Kyryachenko, S.; Leid, M.; Kioussi, C. Detection of Apoptosis by TUNEL Assay. Methods Mol. Biol. 2012, 887, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Okimoto, Y.; Watanabe, A.; Niki, E.; Yamashita, T.; Noguchi, N. A Novel Fluorescent Probe Diphenyl-1-Pyrenylphosphine to Follow Lipid Peroxidation in Cell Membranes. FEBS Lett. 2000, 474, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Kasibhatla, S.; Amarante-Mendes, G.P.; Finucane, D.; Brunner, T.; Bossy-Wetzel, E.; Green, D.R. Propidium Iodide (PI) Uptake Assay to Detect Apoptosis. Cold Spring Harb. Protoc. 2006, 2006, pdb-prot4495. [Google Scholar] [CrossRef] [PubMed]
- Baracca, A.; Sgarbi, G.; Solaini, G.; Lenaz, G. Rhodamine 123 as a Probe of Mitochondrial Membrane Potential: Evaluation of Proton Flux through F0 during ATP Synthesis. Biochim. Biophys. Acta Bioenerg. 2003, 1606, 137–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pozarowski, P.; Darzynkiewicz, Z. Analysis of Cell Cycle by Flow Cytometry. Methods Mol. Biol. 2004, 281, 301–311. [Google Scholar] [CrossRef]
- Rodriguez, L.G.; Wu, X.; Guan, J.L. Wound-Healing Assay. Methods Mol. Biol. 2005, 294, 23–29. [Google Scholar] [CrossRef]
- Franken, N.A.P.; Rodermond, H.M.; Stap, J.; Haveman, J.; van Bree, C. Clonogenic Assay of Cells in Vitro. Nat. Protoc. 2006, 1, 2315–2319. [Google Scholar] [CrossRef]
- Ding, W.; Guo, L. Immobilized Transferrin Fe3O4@SiO2 Nanoparticle with High Doxorubicin Loading for Dual-Targeted Tumor Drug Delivery. Int. J. Nanomed. 2013, 8, 4631–4639. [Google Scholar] [CrossRef] [Green Version]
- Bansal, R.; Singh, R.; Kaur, K. Quantitative Analysis of Doxorubicin Hydrochloride and Arterolane Maleate by Mid IR Spectroscopy Using Transmission and Reflectance Modes. BMC Chem. 2021, 15, 27. [Google Scholar] [CrossRef]
- Rana, S.; Gallo, A.; Srivastava, R.S.; Misra, R.D.K. On the Suitability of Nanocrystalline Ferrites as a Magnetic Carrier for Drug Delivery: Functionalization, Conjugation and Drug Release Kinetics. Acta Biomater. 2007, 3, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Saepudin, E.; Fadhilah, H.R.; Khalil, M. The Influence of Carboxylate Moieties for Efficient Loading and PH-Controlled Release of Doxorubicin in Fe3O4 Magnetic Nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2020, 602, 125137. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS and Zeta Potential—What They Are and What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.; Barick, K.C.; Bahadur, D. Development of Citrate-Stabilized Fe3O4 Nanoparticles: Conjugation and Release of Doxorubicin for Therapeutic Applications. J. Magn. Magn. Mater. 2011, 323, 237–243. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Jiang, W.; Luo, K.; Song, H.; Lan, F.; Wu, Y.; Gu, Z. Superparamagnetic Iron Oxide Nanoparticles as MRI Contrast Agents for Non-Invasive Stem Cell Labeling and Tracking. Theranostics 2013, 3, 595–615. [Google Scholar] [CrossRef]
- Circu, M.L.; Tee, T.A. Reactive Oxygen Species, Cellular Redox Systems and Apoptosis. Bone 2011, 23, 749–762. [Google Scholar] [CrossRef] [Green Version]
- Ignarro, L.J. Biosynthesis and Metabolism of Endothelium-Derived Nitric Oxide. Annu. Rev. Pharmacol. Toxicol. 1990, 30, 535–560. [Google Scholar] [CrossRef]
- Soum, E.; Drapier, J.C. Nitric Oxide and Peroxynitrite Promote Complete Disruption of the [4Fe-4S] Cluster of Recombinant Human Iron Regulatory Protein 1. J. Biol. Inorg. Chem. 2003, 8, 226–232. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free Radicals and Antioxidants in Normal Physiological Functions and Human Disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Li, Y.; Yang, J.; Sun, X. Reactive Oxygen Species-Based Nanomaterials for Cancer Therapy. Front. Chem. 2021, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Lungu, I.I.; Nistorescu, S.; Badea, M.A.; Petre, A.M.; Udrea, A.M.; Banici, A.M.; Fleacă, C.; Andronescu, E.; Dinischiotu, A.; Dumitrache, F.; et al. Doxorubicin-Conjugated Iron Oxide Nanoparticles Synthesized by Laser Pyrolysis: In Vitro Study on Human Breast Cancer Cells. Polymers. 2020, 12, 2799. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Fu, A.; Wu, R.; Wei, N.; Song, K.; Lim, S.; Luo, K.Q. High Expression of G6PD Increases Doxorubicin Resistance in Triple Negative Breast Cancer Cells by Maintaining GSH Level. Int. J. Biol. Sci. 2022, 18, 1120–1133. [Google Scholar] [CrossRef]
- Popescu, R.C.; Straticiuc, M.; Mustăciosu, C.; Temelie, M.; Trușcă, R.; Vasile, B.; Ștefan; Boldeiu, A.; Mirea, D.; Andrei, R.F.; et al. Enhanced Internalization of Nanoparticles Following Ionizing Radiation Leads to Mitotic Catastrophe in MG-63 Human Osteosarcoma Cells. Int. J. Mol. Sci. 2020, 21, 7220. [Google Scholar] [CrossRef]
- Stark, G.R.; Taylor, W.R. Analyzing the G2/M Checkpoint; Springer: Berlin/Heidelberg, Germany, 2004; Volume 280, pp. 51–82. [Google Scholar]
- Ye, P.; Ye, Y.; Chen, X.; Zou, H.; Zhou, Y.; Zhao, X.; Chang, Z.; Han, B.; Kong, X. Ultrasmall Fe3O4 Nanoparticles Induce S-Phase Arrest and Inhibit Cancer Cells Proliferation. Nanotechnol. Rev. 2020, 9, 61–69. [Google Scholar] [CrossRef] [Green Version]
- Mohan, P.; Rapoport, N. Doxorubicin as a Molecular Nanotheranostic Agent: Effect of Doxorubicin Encapsulation in Micelles or Nanoemulsions on the Ultrasound-Mediated Intracellular Delivery and Nuclear Trafficking. Mol. Pharm. 2010, 7, 1959–1973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomankova, K.; Polakova, K.; Pizova, K.; Binder, S.; Havrdova, M.; Kolarova, M.; Kriegova, E.; Zapletalova, J.; Malina, L.; Horakova, J.; et al. In Vitro Cytotoxicity Analysis of Doxorubicin-Loaded/Superparamagnetic Iron Oxide Colloidal Nanoassemblies on MCF7 and NIH3T3 Cell Lines. Int. J. Nanomed. 2015, 10, 949–961. [Google Scholar] [CrossRef] [Green Version]
- Martin, T.; Ye, L.; AJ, S.; Lane, J.; Jiang, W. Cancer Invasion And Metastasis: Molecular and Cellular Perspective. In Metastatic Cancer Clinical Biological Perspectives; Landes Bioscience: Austin, TX, USA, 2013; pp. 135–168. ISBN 978-1-58706-659-7. [Google Scholar]
- Unnikrishnan, B.S.; Sen, A.; Preethi, G.U.; Joseph, M.M.; Maya, S.; Shiji, R.; Anusree, K.S.; Sreelekha, T.T. Folic Acid-Appended Galactoxyloglucan-Capped Iron Oxide Nanoparticles as a Biocompatible Nanotheranostic Agent for Tumor-Targeted Delivery of Doxorubicin. Int. J. Biol. Macromol. 2021, 168, 130–142. [Google Scholar] [CrossRef]
- Akhtar, N.; Mohammed, H.A.; Yusuf, M.; Al-Subaiyel, A.; Sulaiman, G.M.; Khan, R.A. SPIONs Conjugate Supported Anticancer Drug Doxorubicin’s Delivery: Current Status, Challenges, and Prospects. Nanomaterials 2022, 12, 3686. [Google Scholar] [CrossRef]
- Kovrigina, E.; Chubarov, A.; Dmitrienko, E. High Drug Capacity Doxorubicin-Loaded Iron Oxide Nanocomposites for Cancer Therapy. Magnetochemistry 2022, 8, 54. [Google Scholar] [CrossRef]
- Halder, J.; Pradhan, D.; Biswasroy, P.; Rai, V.K.; Kar, B.; Ghosh, G.; Rath, G. Trends in Iron Oxide Nanoparticles: A Nano-Platform for Theranostic Application in Breast Cancer. J. Drug Target. 2022, 30, 1055–1075. [Google Scholar] [CrossRef] [PubMed]









| Nanoparticle | Hydrodynamic Radius by DLS (nm) | Polydispersity Index (PDI) | Zeta Potential (mV) |
|---|---|---|---|
| NpMag (Fe3O4) | 125.7 ± 30.9 | 0.17 ± 0.10 | −9.6 ± 0.5 |
| NpMag+Dox | 193.2 ± 34 | 0.20 ± 0.03 | −0.7 ± 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hernandes, E.P.; Lazarin-Bidóia, D.; Bini, R.D.; Nakamura, C.V.; Cótica, L.F.; de Oliveira Silva Lautenschlager, S. Doxorubicin-Loaded Iron Oxide Nanoparticles Induce Oxidative Stress and Cell Cycle Arrest in Breast Cancer Cells. Antioxidants 2023, 12, 237. https://doi.org/10.3390/antiox12020237
Hernandes EP, Lazarin-Bidóia D, Bini RD, Nakamura CV, Cótica LF, de Oliveira Silva Lautenschlager S. Doxorubicin-Loaded Iron Oxide Nanoparticles Induce Oxidative Stress and Cell Cycle Arrest in Breast Cancer Cells. Antioxidants. 2023; 12(2):237. https://doi.org/10.3390/antiox12020237
Chicago/Turabian StyleHernandes, Elisa Parcero, Danielle Lazarin-Bidóia, Raquel Dosciatti Bini, Celso Vataru Nakamura, Luiz Fernando Cótica, and Sueli de Oliveira Silva Lautenschlager. 2023. "Doxorubicin-Loaded Iron Oxide Nanoparticles Induce Oxidative Stress and Cell Cycle Arrest in Breast Cancer Cells" Antioxidants 12, no. 2: 237. https://doi.org/10.3390/antiox12020237