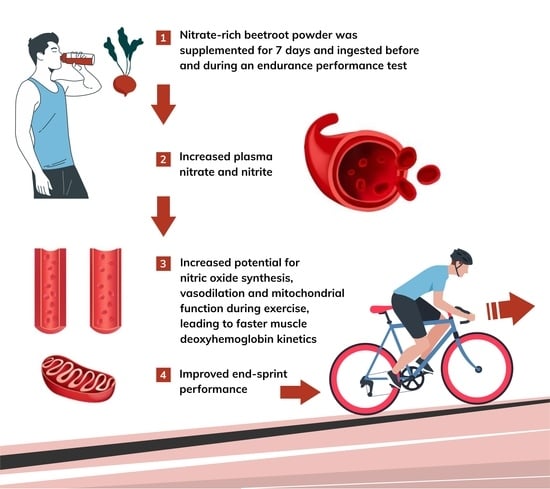
Dietary Nitrate Supplementation Enhances Performance and Speeds Muscle Deoxyhaemoglobin Kinetics during an End-Sprint after Prolonged Moderate-Intensity Exercise
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participant Characteristics
2.2. Experimental Design
2.3. Incremental Test
2.4. Familiarisation Test
2.5. Supplementation Procedure
2.6. Experimental Tests
2.7. Measurements
2.7.1. Sprint Performance
2.7.2. Muscle Oxygenation
2.7.3. Ratings of Perceived Exertion and Heart Rate
2.7.4. Plasma [NO3−] and [NO2−]
2.8. Data Analysis Procedures
[NO3−] and [NO3−] Determination
2.9. Statistical Analysis
3. Results
3.1. Plasma [NO3−] and [NO2−]
3.2. Sprint Performance
3.3. Muscle Oxygenation
3.4. Ratings of Perceived Exertion and Heart Rate
4. Discussion
4.1. Effect of BR Supplementation on Plasma [NO3−] and [NO2−]
4.2. Effect of BR Supplementation on Maximal End-Sprint Performance
4.3. Effect of BR Supplementation on Muscle Oxygenation Variables
4.4. Experimental Considerations and Implications
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Senefeld, J.W.; Wiggins, C.C.; Regimbal, R.J.; Dominelli, P.B.; Baker, S.E.; Joyner, M.J. Ergogenic Effect of Nitrate Supplementation: A Systematic Review and Meta-analysis. Med. Sci. Sports Exerc. 2000, 52, 2250–2261. [Google Scholar] [CrossRef] [PubMed]
- Jones, A.M.; Vanhatalo, A.; Seals, D.R.; Rossman, M.J.; Piknova, B.; Jonvik, K.L. Dietary Nitrate and Nitric Oxide Metabolism: Mouth, Circulation, Skeletal Muscle, and Exercise Performance. Med. Sci. Sports Exerc. 2001, 53, 280–294. [Google Scholar] [CrossRef] [PubMed]
- Spiegelhalder, B.; Eisenbrand, G.; Preussmann, R. Influence of dietary nitrate on nitrite content of human saliva: Possible relevance to in vivo formation of N-nitroso compounds. Food Cosmet. Toxicol. 1976, 14, 545–548. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, J.O.; Govoni, M. Inorganic nitrate is a possible source for systemic generation of nitric oxide. Free Radic. Biol. Med. 2004, 37, 395–400. [Google Scholar] [CrossRef]
- Bryan, N.S.; Burleigh, M.C.; Easton, C. The oral microbiome, nitric oxide and exercise performance. Nitric. Oxide 2022, 125–126, 23–30. [Google Scholar] [CrossRef]
- Lundberg, J.O.; Weitzberg, E.; Cole, J.A.; Benjamin, N. Nitrate, bacteria and human health. Nat. Rev. Microbiol. 2004, 2, 593–602. [Google Scholar] [CrossRef]
- Abu-Alghayth, M.; Vanhatalo, A.; Wylie, L.J.; McDonagh, S.T.; Thompson, C.; Kadach, S.; Kerr, P.; Smallwood, M.J.; Jones, A.M.; Winyard, P.G. S-nitrosothiols, and other products of nitrate metabolism, are increased in multiple human blood compartments following ingestion of beetroot juice. Redox Biol. 2001, 43, 101974. [Google Scholar] [CrossRef]
- Piknova, B.; Schechter, A.N.; Park, J.W.; Vanhatalo, A.; Jones, A.M. Skeletal Muscle Nitrate as a Regulator of Systemic Nitric Oxide Homeostasis. Exerc. Sport Sci. Rev. 2022, 50, 2–13. [Google Scholar] [CrossRef]
- Wylie, L.J.; Park, J.W.; Vanhatalo, A.; Kadach, S.; Black, M.I.; Stoyanov, Z.; Schechter, A.N.; Jones, A.M.; Piknova, B. Human skeletal muscle nitrate store: Influence of dietary nitrate supplementation and exercise. J. Physiol. 2019, 597, 5565–5576. [Google Scholar] [CrossRef] [Green Version]
- Moon, Y.; Balke, J.E.; Madorma, D.; Siegel, M.P.; Knowels, G.; Brouckaert, P.; Buys, E.S.; Marcinek, D.J.; Percival, J.M. Nitric Oxide Regulates Skeletal Muscle Fatigue, Fiber Type, Microtubule Organization, and Mitochondrial ATP Synthesis Efficiency Through cGMP-Dependent Mechanisms. Antioxid Redox Signal. 2017, 26, 966–985. [Google Scholar] [CrossRef]
- Stamler, J.S.; Meissner, G. Physiology of nitric oxide in skeletal muscle. Physiol. Rev. 2001, 81, 209–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, S.J.; Fulford, J.; Vanhatalo, A.; Winyard, P.G.; Blackwell, J.R.; DiMenna, F.J.; Wilkerson, D.P.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. J. Appl. Physiol. 2010, 109, 135–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bailey, S.J.; Winyard, P.; Vanhatalo, A.; Blackwell, J.R.; Dimenna, F.J.; Wilkerson, D.P.; Tarr, J.; Benjamin, N.; Jones, A.M. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. J. Appl. Physiol. 2009, 107, 1144–1155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkerson, D.P.; Hayward, G.M.; Bailey, S.J.; Vanhatalo, A.; Blackwell, J.R.; Jones, A.M. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. Eur. J. Appl. Physiol. 2012, 112, 4127–4134. [Google Scholar] [CrossRef] [PubMed]
- Tan, R.; Wylie, L.J.; Thompson, C.; Blackwell, J.R.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M. Beetroot juice ingestion during prolonged moderate-intensity exercise attenuates progressive rise in O2 uptake. J. Appl. Physiol. 2018, 124, 1254–1263. [Google Scholar] [CrossRef] [Green Version]
- Chidnok, W.; Fulford, J.; Bailey, S.J.; Dimenna, F.J.; Skiba, P.F.; Vanhatalo, A.; Jones, A.M. Muscle metabolic determinants of exercise tolerance following exhaustion: Relationship to the “critical power”. J. Appl. Physiol. 2013, 115, 243–250. [Google Scholar] [CrossRef] [Green Version]
- Kadach, S.; Piknova, B.; Black, M.I.; Park, J.W.; Wylie, L.J.; Stoyanov, Z.; Thomas, S.M.; McMahon, N.F.; Vanhatalo, A.; Schechter, A.N.; et al. Time course of human skeletal muscle nitrate and nitrite concentration changes following dietary nitrate ingestion. Nitric. Oxide 2022, 121, 1–10. [Google Scholar] [CrossRef]
- Thiel, C.; Foster, C.; Banzer, W.; De Koning, J. Pacing in Olympic track races: Competitive tactics versus best performance strategy. J. Sports Sci. 2010, 30, 1107–1115. [Google Scholar] [CrossRef] [Green Version]
- Coggan, A.R.; Leibowitz, J.L.; Kadkhodayan, A.; Thomas, D.P.; Ramamurthy, S.; Spearie, C.A.; Waller, S.; Farmer, M.; Peterson, L.R. Effect of acute dietary nitrate intake on maximal knee extensor speed and power in healthy men and women. Nitric. Oxide 2015, 48, 16–21. [Google Scholar] [CrossRef] [Green Version]
- Haider, G.; Folland, J.P. Nitrate supplementation enhances the contractile properties of human skeletal muscle. Med. Sci. Sports Exerc. 2014, 46, 2234–2243. [Google Scholar] [CrossRef]
- Whitfield, J.; Gamu, D.; Heigenhauser, G.J.F.; Van Loon, L.J.C.; Spriet, L.L.; Tupling, A.R.; Holloway, G.P. Beetroot Juice Increases Human Muscle Force without Changing Ca2+-Handling Proteins. Med. Sci. Sports Exerc. 2017, 49, 2016–2024. [Google Scholar] [CrossRef] [PubMed]
- Tillin, N.A.; Moudy, S.; Nourse, K.M.; Tyler, C.J. Nitrate Supplement Benefits Contractile Forces in Fatigued but Not Unfatigued Muscle. Med. Sci. Sports Exerc. 2018, 50, 2122–2131. [Google Scholar] [CrossRef] [PubMed]
- Jodra, P.; Domínguez, R.; Sánchez-Oliver, A.J.; Veiga-Herreros, P.; Bailey, S.J. Effect of Beetroot Juice Supplementation on Mood, Perceived Exertion, and Performance During a 30-Second Wingate Test. Int. J. Sports Physiol. Perform. 2020, 15, 243–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jonvik, K.L.; Nyakayiru, J.; Van Dijk, J.W.; Maase, K.; Ballak, S.B.; Senden, J.M.G.; Van Loon, L.J.C.; Verdijk, L.B. Repeated-sprint performance and plasma responses following beetroot juice supplementation do not differ between recreational, competitive and elite sprint athletes. Eur. J. Sport Sci. 2018, 18, 524–533. [Google Scholar] [CrossRef]
- Rimer, E.G.; Peterson, L.R.; Coggan, A.R.; Martin, J.C. Increase in Maximal Cycling Power with Acute Dietary Nitrate Supplementation. Int. J. Sports Physiol. Perform. 2016, 11, 715–720. [Google Scholar] [CrossRef] [Green Version]
- Wylie, L.J.; Bailey, S.J.; Kelly, J.; Blackwell, J.R.; Vanhatalo, A.; Jones, A.M. Influence of beetroot juice supplementation on intermittent exercise performance. Eur. J. Appl. Physiol. 2016, 116, 415–425. [Google Scholar] [CrossRef] [Green Version]
- Gollnick, P.D.; Piehl, K.; Saltin, B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J. Physiol. 1974, 241, 45–57. [Google Scholar] [CrossRef]
- Krustrup, P.; Söderlund, K.; Mohr, M.; Bangsbo, J. The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch. 2004, 447, 855–866. [Google Scholar] [CrossRef]
- Krustrup, P.; Söderlund, K.; Relu, M.U.; Ferguson, R.A.; Bangsbo, J. Heterogeneous recruitment of quadriceps muscle portions and fibre types during moderate intensity knee-extensor exercise: Effect of thigh occlusion. Scand. J. Med. Sci. Sports 2019, 19, 576–584. [Google Scholar] [CrossRef]
- Vøllestad, N.K.; Blom, P.C. Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol. Scand. 1985, 125, 395–405. [Google Scholar] [CrossRef]
- Harkema, S.J.; Adams, G.R.; Meyer, R.A. Acidosis has no effect on the ATP cost of contraction in cat fast- and slow-twitch skeletal muscles. Am. J. Physiol. 1997, 272, 485–490. [Google Scholar] [CrossRef] [PubMed]
- McDonough, P.; Behnke, B.J.; Padilla, D.J.; Musch, T.I.; Poole, D.C. Control of microvascular oxygen pressures in rat muscles comprised of different fibre types. J. Physiol. 2005, 563, 903–913. [Google Scholar] [CrossRef] [PubMed]
- Castello, P.R.; David, P.S.; McClure, T.; Crook, Z.; Poyton, R.O. Mitochondrial cytochrome oxidase produces nitric oxide under hypoxic conditions: Implications for oxygen sensing and hypoxic signaling in eukaryotes. Cell Metab. 2006, 3, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Modin, A.; Björne, H.; Herulf, M.; Alving, K.; Weitzberg, E.; Lundberg, J.O. Nitrite-derived nitric oxide: A possible mediator of ‘acidic-metabolic’ vasodilation. Acta Physiol. Scand. 2001, 171, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Piknova, B.; Park, J.W.; Lam, K.K.J.; Schechter, A.N. Nitrate as a source of nitrite and nitric oxide during exercise hyperemia in rat skeletal muscle. Nitric. Oxide 2016, 255–256, 54–61. [Google Scholar] [CrossRef] [Green Version]
- Breese, B.C.; McNarry, M.A.; Marwood, S.; Blackwell, J.R.; Bailey, S.J.; Jones, A.M. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 305, 1441–1450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Govoni, M.; Jansson, E.A.; Weitzberg, E.; Lundberg, J.O. The increase in plasma nitrite after a dietary nitrate load is markedly attenuated by an antibacterial mouthwash. Nitric. Oxide 2008, 19, 333–337. [Google Scholar] [CrossRef]
- Wylie, L.J.; Kelly, J.; Bailey, S.J.; Blackwell, J.R.; Skiba, P.F.; Winyard, P.G.; Jeukendrup, A.E.; Vanhatalo, A.; Jones, A.M. Beetroot juice and exercise: Pharmacodynamic and dose-response relationships. J. Appl. Physiol. 2013, 115, 325–336. [Google Scholar] [CrossRef] [Green Version]
- de Aguiar, R.A.; Turnes, T.; Borszcz, F.K.; Raimundo, J.A.G.; Caputo, F. Near-infrared spectroscopy-derived muscle O2 kinetics after moderate running exercise in healthy males: Reliability and associations with parameters of aerobic fitness. Exp. Physiol. 2022, 107, 476–488. [Google Scholar] [CrossRef]
- Cocksedge, S.P.; Breese, B.C.; Morgan, P.T.; Nogueira, L.; Thompson, C.; Wylie, L.J.; Jones, A.M.; Bailey, S.J. Influence of muscle oxygenation and nitrate-rich beetroot juice supplementation on O2 uptake kinetics and exercise tolerance. Nitric. Oxide 2020, 99, 25–33. [Google Scholar] [CrossRef]
- Kelly, J.; Vanhatalo, A.; Bailey, S.J.; Wylie, L.J.; Tucker, C.; List, S.; Winyard, P.G.; Jones, A.M. Dietary nitrate supplementation: Effects on plasma nitrite and pulmonary O2 uptake dynamics during exercise in hypoxia and normoxia. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2014, 307, 920–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wylie, L.J.; Mohr, M.; Krustrup, P.; Jackman, S.R.; Ermιdis, G.; Kelly, J.; Black, M.I.; Bailey, S.J.; Vanhatalo, A.; Jones, A.M. Dietary nitrate supplementation improves team sport-specific intense intermittent exercise performance. Eur. J. Appl. Physiol. 2013, 113, 1673–1684. [Google Scholar] [CrossRef] [PubMed]
- Richardson, R.S.; Noyszewski, E.A.; Kendrick, K.F.; Leigh, J.S.; Wagner, P.D. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J. Clin. Investig. 1995, 96, 1916–1926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iannetta, D.; Zhang, J.; Murias, J.M.; Aboodarda, S.J. Neuromuscular and perceptual mechanisms of fatigue accompanying task failure in response to moderate-, heavy-, severe-, and extreme-intensity cycling. J. Appl. Physiol. 2022, 133, 323–334. [Google Scholar] [CrossRef] [PubMed]
- Koga, S.; Kano, Y.; Barstow, T.J.; Ferreira, L.F.; Ohmae, E.; Sudo, M.; Poole, D.C. Kinetics of muscle deoxygenation and microvascular PO2 during contractions in rat: Comparison of optical spectroscopy and phosphorescence-quenching techniques. J. Appl. Physiol. 2012, 112, 26–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krustrup, P.; Jones, A.M.; Wilkerson, D.P.; Calbet, J.A.; Bangsbo, J. Muscular and pulmonary O2 uptake kinetics during moderate- and high-intensity sub-maximal knee-extensor exercise in humans. J. Physiol. 2009, 587, 1843–1856. [Google Scholar] [CrossRef] [PubMed]
- Brock, K.; Antonellis, P.; Black, M.I.; DiMenna, F.J.; Vanhatalo, A.; Jones, A.M.; Bailey, S.J. Improvement of Oxygen-Uptake Kinetics and Cycling Performance with Combined Prior Exercise and Fast Start. Int. J. Sports Physiol. Perform. 2018, 13, 305–312. [Google Scholar] [CrossRef] [Green Version]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef] [Green Version]
- Casado, A.; Hanley, B.; Jiménez-Reyes, P.; Renfree, A. Pacing profiles and tactical behaviors of elite runners. J. Sport Health Sci. 2021, 10, 537–549. [Google Scholar] [CrossRef]
- Cermak, N.M.; Res, P.; Stinkens, R.; Lundberg, J.O.; Gibala, M.J.; van Loon, L.J. No improvement in endurance performance after a single dose of beetroot juice. Int. J. Sport Nutr. Exerc. Metab. 2012, 22, 470–478. [Google Scholar] [CrossRef]
- Robinson, G.P.; Killer, S.C.; Stoyanov, Z.; Stephens, H.; Read, L.; James, L.J.; Bailey, S.J. Influence of Dietary Nitrate Supplementation on High-Intensity Intermittent Running Performance at Different Doses of Normobaric Hypoxia in Endurance-Trained Males. Int. J. Sport Nutr. Exerc. Metab. 2001, 31, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bailey, S.J.; Gandra, P.G.; Jones, A.M.; Hogan, M.C.; Nogueira, L. Incubation with sodium nitrite attenuates fatigue development in intact single mouse fibres at physiological PO2. J. Physiol. 2019, 597, 5429–5443. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.; Schiffer, T.A.; Ivarsson, N.; Cheng, A.J.; Bruton, J.D.; Lundberg, J.O.; Weitzberg, E.; Westerblad, H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. J. Physiol. 2012, 590, 3575–3583. [Google Scholar] [CrossRef] [PubMed]



| Placebo | Beetroot | |
|---|---|---|
| Plasma [NO3−] | ||
| Resting baseline (μM) | 59 ± 10 | 300 ± 73 * |
| Post exercise (μM) | 73 ± 15 | 562 ± 89 *# |
| Plasma [NO2−] | ||
| Resting baseline (μM) | 144 ± 44 | 280 ± 58 * |
| Post exercise (μM) | 135 ± 70 | 228 ± 63 * |
| Placebo | Beetroot | |
|---|---|---|
| Time to peak power output (s) | 5.8 ± 1.5 | 6.2 ± 0.9 |
| Mean power output 0–60 s (W) | 472 ± 73 | 495 ± 67 |
| Mean power output 0–30 s (W) | 578 ± 132 | 600 ± 116 |
| Mean power output 30–60 s (W) | 365 ± 41 | 390 ± 38 * |
| Placebo | Beetroot | |
|---|---|---|
| Moderate-intensity exercise | ||
| Baseline (%) | 66 ± 3 | 69 ± 5 |
| 20 min (%) | 64 ± 4 | 66 ± 7 |
| 40 min (%) | 65 ± 5 | 66 ± 7 |
| 60 min (%) | 65 ± 4 | 65 ± 7 |
| 80 min (%) | 65 ± 4 | 64 ± 7 |
| 100 min (%) | 65 ± 5 | 65 ± 7 |
| End-sprint | ||
| Baseline (%) | 65 ± 5 | 67 ± 7 |
| 30–60 s (%) | 59 ± 3 | 61 ± 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rowland, S.N.; Da Boit, M.; Tan, R.; Robinson, G.P.; O’Donnell, E.; James, L.J.; Bailey, S.J. Dietary Nitrate Supplementation Enhances Performance and Speeds Muscle Deoxyhaemoglobin Kinetics during an End-Sprint after Prolonged Moderate-Intensity Exercise. Antioxidants 2023, 12, 25. https://doi.org/10.3390/antiox12010025
Rowland SN, Da Boit M, Tan R, Robinson GP, O’Donnell E, James LJ, Bailey SJ. Dietary Nitrate Supplementation Enhances Performance and Speeds Muscle Deoxyhaemoglobin Kinetics during an End-Sprint after Prolonged Moderate-Intensity Exercise. Antioxidants. 2023; 12(1):25. https://doi.org/10.3390/antiox12010025
Chicago/Turabian StyleRowland, Samantha N., Mariasole Da Boit, Rachel Tan, George P. Robinson, Emma O’Donnell, Lewis J. James, and Stephen J. Bailey. 2023. "Dietary Nitrate Supplementation Enhances Performance and Speeds Muscle Deoxyhaemoglobin Kinetics during an End-Sprint after Prolonged Moderate-Intensity Exercise" Antioxidants 12, no. 1: 25. https://doi.org/10.3390/antiox12010025