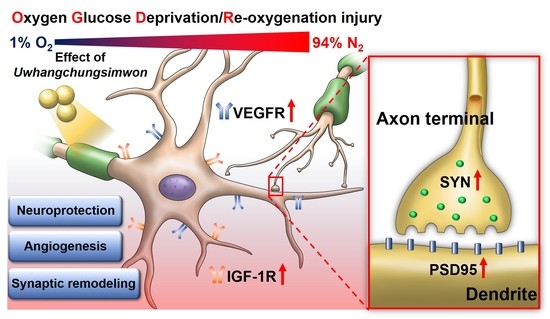
Uwhangchungsimwon Inhibits Oxygen Glucose Deprivation/Re-Oxygenation-Induced Cell Death through Neuronal VEGF and IGF-1 Receptor Signaling and Synaptic Remodeling in Cortical Neurons
Abstract
:1. Introduction
2. Materials and Methods
2.1. Primary Culture of Rat Cortical Neurons
2.2. UCW Preparation
2.3. UCW Pretreatment and Oxygen Glucose Deprivation/Re-Oxygenation (OGD/R)
2.4. Cell Counting Kit-8 (CCK-8) Assay
2.5. Live and Dead Assay
2.6. Live Imaging
2.7. Immunocytochemistry
2.8. Flow Cytometry
2.9. Real-Time PCR
2.10. Western Blotting
2.11. Statistics
3. Results
3.1. UCW Protects against the OGD/R-Induced Decrease in Cell Viability of Primary Cortical Neurons
3.2. UCW Attenuates OGD/R-Induced Oxidative Injury via Inhibition of iNOS-Mediated ROS Signaling in Primary Cortical Neurons
3.3. UCW Activates VEGF-VEGFR Signaling in OGD/R-Injured Primary Cortical Neurons
3.4. UCW Enhances MAP2 Signals Which Stained Neuronal Cell Bodies and Axons Exposed to OGD/R Injury in Primary Cortical Neurons
3.5. UCW Increases IGF-1R Expression on OGD/R-Induced Hypoxia Damage in Primary Cortical Neuron
3.6. UCW Prevents Synaptic Loss after OGD/R Injury in Primary Cortical Neurons
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Langhorne, P.; Coupar, F.; Pollock, A. Motor recovery after stroke: A systematic review. Lancet Neurol. 2009, 8, 741–754. [Google Scholar] [CrossRef]
- Kim, K.; Kim, Y.M.; Kim, E.K. Correlation between the Activities of Daily Living of Stroke Patients in a Community Setting and Their Quality of Life. J. Phys. Ther. Sci. 2014, 26, 417–419. [Google Scholar] [CrossRef] [Green Version]
- Langhorne, P.; Ramachandra, S.; Stroke Unit Trialists, C. Organised inpatient (stroke unit) care for stroke: Network meta-analysis. Cochrane Database Syst. Rev. 2020, 4, CD000197. [Google Scholar] [CrossRef]
- Font, M.A.; Arboix, A.; Krupinski, J. Angiogenesis, neurogenesis and neuroplasticity in ischemic stroke. Curr. Cardiol. Rev. 2010, 6, 238–244. [Google Scholar] [CrossRef] [Green Version]
- Rahman, A.A.; Amruta, N.; Pinteaux, E.; Bix, G.J. Neurogenesis After Stroke: A Therapeutic Perspective. Transl. Stroke Res. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Lee, H.S.; Han, J.; Bai, H.J.; Kim, K.W. Brain angiogenesis in developmental and pathological processes: Regulation, molecular and cellular communication at the neurovascular interface. FEBS J. 2009, 276, 4622–4635. [Google Scholar] [CrossRef]
- Zhang, H.; Vutskits, L.; Pepper, M.S.; Kiss, J.Z. VEGF is a chemoattractant for FGF-2-stimulated neural progenitors. J. Cell Biol. 2003, 163, 1375–1384. [Google Scholar] [CrossRef] [Green Version]
- Levy, M.J.F.; Boulle, F.; Steinbusch, H.W.; van den Hove, D.L.A.; Kenis, G.; Lanfumey, L. Neurotrophic factors and neuroplasticity pathways in the pathophysiology and treatment of depression. Psychopharmacology 2018, 235, 2195–2220. [Google Scholar] [CrossRef] [Green Version]
- Silva-Hucha, S.; Pastor, A.M.; Morcuende, S. Neuroprotective Effect of Vascular Endothelial Growth Factor on Motoneurons of the Oculomotor System. Int. J. Mol. Sci. 2021, 22, 814. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.Y.; Jang, S.W.; Lee, J.S.; Kim, Y.H.; Kim, H.G.; Han, J.M.; Kim, D.W.; Yi, M.H.; Choi, M.K.; Son, C.G. Uwhangchungsimwon, a traditional herbal medicine, protects brain against oxidative injury via modulation of hypothalamus-pituitary-adrenal (HPA) response in a chronic restraint mice model. J. Ethnopharmacol. 2014, 151, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Jang, M.; Kim, E.J.; Kim, H.; Ye, S.K.; Cho, I.H. Oriental Medicine Woohwangchungsimwon Attenuates Kainic Acid-Induced Seizures and Neuronal Cell Death in the Hippocampus. Rejuvenation Res. 2016, 19, 394–405. [Google Scholar] [CrossRef]
- Oh, H.M.; Lee, J.S.; Kim, S.W.; Oh, Y.T.; Kim, W.Y.; Lee, S.B.; Cho, Y.R.; Jeon, Y.J.; Cho, J.H.; Son, C.G. Uwhangchungsimwon, A Standardized Herbal Drug, Exerts an Anti-Depressive Effect in a Social Isolation Stress-Induced Mouse Model. Front. Pharmacol. 2019, 10, 1674. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.K.; Kim, Y.S.; Ko, C.N.; Bae, H.S.; Cho, K.H.; Lee, K.S. Effect of Uwhangchungsimwon on expression of nitric oxide synthase and vascular cell adhesion molecule-1 in human endothelial cells. Am. J. Chin. Med. 2003, 31, 389–402. [Google Scholar] [CrossRef]
- Seyedmajidi, M.; Shafaee, S.; Hashemipour, G.; Bijani, A.; Ehsani, H. Immunohistochemical Evaluation of Angiogenesis Related Markers in Pyogenic Granuloma of Gingiva. Asian Pac. J. Cancer Prev. 2015, 16, 7513–7516. [Google Scholar] [CrossRef] [Green Version]
- Kim, I.; Moon, S.O.; Park, S.K.; Chae, S.W.; Koh, G.Y. Angiopoietin-1 reduces VEGF-stimulated leukocyte adhesion to endothelial cells by reducing ICAM-1, VCAM-1, and E-selectin expression. Circ. Res. 2001, 89, 477–479. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Zhong, W.; Tang, X. Strategies to Improve the Efficiency of Transplantation with Mesenchymal Stem Cells for the Treatment of Ischemic Stroke: A Review of Recent Progress. Stem. Cells Int. 2021, 2021, 9929128. [Google Scholar] [CrossRef]
- Kim, H.; Jeon, W.; Hong, J.; Lee, J.; Yeo, C.; Lee, Y.; Baek, S.; Ha, I. Gongjin-Dan Enhances Neurite Outgrowth of Cortical Neuron by Ameliorating H2O2-Induced Oxidative Damage via Sirtuin1 Signaling Pathway. Nutrients 2021, 13, 4290. [Google Scholar] [CrossRef]
- Sahu, M.P.; Nikkila, O.; Lagas, S.; Kolehmainen, S.; Castren, E. Culturing primary neurons from rat hippocampus and cortex. Neuronal Signal. 2019, 3, NS20180207. [Google Scholar] [CrossRef] [Green Version]
- Rosenstein, J.M.; Krum, J.M.; Ruhrberg, C. VEGF in the nervous system. Organogenesis 2010, 6, 107–114. [Google Scholar] [CrossRef] [Green Version]
- Gazit, N.; Vertkin, I.; Shapira, I.; Helm, M.; Slomowitz, E.; Sheiba, M.; Mor, Y.; Rizzoli, S.; Slutsky, I. IGF-1 Receptor Differentially Regulates Spontaneous and Evoked Transmission via Mitochondria at Hippocampal Synapses. Neuron 2016, 89, 583–597. [Google Scholar] [CrossRef] [Green Version]
- Nieto Guil, A.F.; Oksdath, M.; Weiss, L.A.; Grassi, D.J.; Sosa, L.J.; Nieto, M.; Quiroga, S. IGF-1 receptor regulates dynamic changes in neuronal polarity during cerebral cortical migration. Sci. Rep. 2017, 7, 7703. [Google Scholar] [CrossRef] [Green Version]
- Bid, H.K.; Zhan, J.; Phelps, D.A.; Kurmasheva, R.T.; Houghton, P.J. Potent inhibition of angiogenesis by the IGF-1 receptor-targeting antibody SCH717454 is reversed by IGF-2. Mol. Cancer Ther. 2012, 11, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Lopez, C.; LeRoith, D.; Torres-Aleman, I. Insulin-like growth factor I is required for vessel remodeling in the adult brain. Proc. Natl. Acad. Sci. USA 2004, 101, 9833–9838. [Google Scholar] [CrossRef] [Green Version]
- Zheng, C.Y.; Seabold, G.K.; Horak, M.; Petralia, R.S. MAGUKs, synaptic development, and synaptic plasticity. Neuroscientist 2011, 17, 493–512. [Google Scholar] [CrossRef] [Green Version]
- Yang, P.; Sun, X.; Kou, Z.W.; Wu, K.W.; Huang, Y.L.; Sun, F.Y. VEGF Axonal Transport Dependent on Kinesin-1B and Microtubules Dynamics. Front. Mol. Neurosci. 2017, 10, 424. [Google Scholar] [CrossRef] [Green Version]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Wajngarten, M.; Silva, G.S. Hypertension and Stroke: Update on Treatment. Eur. Cardiol. 2019, 14, 111–115. [Google Scholar] [CrossRef] [Green Version]
- Kong, D.H.; Kim, Y.K.; Kim, M.R.; Jang, J.H.; Lee, S. Emerging Roles of Vascular Cell Adhesion Molecule-1 (VCAM-1) in Immunological Disorders and Cancer. Int. J. Mol. Sci. 2018, 19, 1057. [Google Scholar] [CrossRef] [Green Version]
- Yusuf-Makagiansar, H.; Anderson, M.E.; Yakovleva, T.V.; Murray, J.S.; Siahaan, T.J. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med. Res. Rev. 2002, 22, 146–167. [Google Scholar] [CrossRef]
- Saez, G.T.; Tormos, C.; Giner, V.; Chaves, J.; Lozano, J.V.; Iradi, A.; Redon, J. Factors related to the impact of antihypertensive treatment in antioxidant activities and oxidative stress by-products in human hypertension. Am. J. Hypertens 2004, 17, 809–816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wittko-Schneider, I.M.; Schneider, F.T.; Plate, K.H. Brain homeostasis: VEGF receptor 1 and 2-two unequal brothers in mind. Cell Mol. Life Sci. 2013, 70, 1705–1725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Liu, M.; Huang, M.; Chen, M.; Zhang, D.; Luo, L.; Ye, G.; Deng, L.; Peng, Y.; Wu, X.; et al. Ginsenoside F1 promotes angiogenesis by activating the IGF-1/IGF1R pathway. Pharmacol. Res. 2019, 144, 292–305. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Li, Y.; Chen, J.; Yang, M.; Katakowski, M.; Lu, M.; Chopp, M. Expression of insulin-like growth factor 1 and receptor in ischemic rats treated with human marrow stromal cells. Brain Res. 2004, 1030, 19–27. [Google Scholar] [CrossRef]
- De Geyter, D.; De Smedt, A.; Stoop, W.; De Keyser, J.; Kooijman, R. Central IGF-I Receptors in the Brain are Instrumental to Neuroprotection by Systemically Injected IGF-I in a Rat Model for Ischemic Stroke. CNS Neurosci. Ther. 2016, 22, 611–616. [Google Scholar] [CrossRef]
- Hayes, C.A.; Valcarcel-Ares, M.N.; Ashpole, N.M. Preclinical and clinical evidence of IGF-1 as a prognostic marker and acute intervention with ischemic stroke. J. Cereb. Blood Flow Metab. 2021, 41, 2475–2491. [Google Scholar] [CrossRef]
- Lee, M.S.; Lim, H.J.; Lee, M.S.; Jang, H.S. Perceptions, knowledge and misuse of an oriental herbal drug: A survey of 608 Korean female nursing college students. Complement. Ther. Clin. Pract. 2005, 11, 200–204. [Google Scholar] [CrossRef]







| Gene | 5′-3′ | Primer Sequence | NCBI Accession Numbers |
|---|---|---|---|
| iNOS | Forward | ATGGCTTGCCCCTGGAAGTT | NM_012611 |
| Reverse | TGTTGGGCTGGGAATAGCAC | ||
| VEGFR | Forward | AGAGACCCGCGTTTTCAGAG | NM_013062 |
| Reverse | TGAGGTAGGCAGGGAGAGTC | ||
| NF200 | Forward | AACACCACTTAGATGGCGGG | NM_012607 |
| Reverse | ACGTGGAGCGTTCAGCAATA | ||
| GAP43 | Forward | TGCCCTTTCTCAGATCCACT | NM_017195 |
| Reverse | TTGCCACACAGAGAGAGAGG | ||
| IGF-1R | Forward | GGGCCTGGTAATCATGCTGT | NM_052807 |
| Reverse | CATCCCGAAGGACCCTTGTC | ||
| VEGF | Forward | GTACCTCCACCATGCCAAGT | NM_031836 |
| Reverse | AATAGCTGCGCTGGTAGACG | ||
| GAPDH | Forward | CCCCCAATGTATCCGTTGTG | NM_017008 |
| Reverse | TAGCCCAGGATGCCCTTTAGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hong, J.Y.; Kim, H.; Yeo, C.; Jeon, W.-J.; Lee, J.; Baek, S.H.; Lee, Y.J.; Ha, I.-H. Uwhangchungsimwon Inhibits Oxygen Glucose Deprivation/Re-Oxygenation-Induced Cell Death through Neuronal VEGF and IGF-1 Receptor Signaling and Synaptic Remodeling in Cortical Neurons. Antioxidants 2022, 11, 1388. https://doi.org/10.3390/antiox11071388
Hong JY, Kim H, Yeo C, Jeon W-J, Lee J, Baek SH, Lee YJ, Ha I-H. Uwhangchungsimwon Inhibits Oxygen Glucose Deprivation/Re-Oxygenation-Induced Cell Death through Neuronal VEGF and IGF-1 Receptor Signaling and Synaptic Remodeling in Cortical Neurons. Antioxidants. 2022; 11(7):1388. https://doi.org/10.3390/antiox11071388
Chicago/Turabian StyleHong, Jin Young, Hyunseong Kim, Changhwan Yeo, Wan-Jin Jeon, Junseon Lee, Seung Ho Baek, Yoon Jae Lee, and In-Hyuk Ha. 2022. "Uwhangchungsimwon Inhibits Oxygen Glucose Deprivation/Re-Oxygenation-Induced Cell Death through Neuronal VEGF and IGF-1 Receptor Signaling and Synaptic Remodeling in Cortical Neurons" Antioxidants 11, no. 7: 1388. https://doi.org/10.3390/antiox11071388