Antioxidant Activities and Mechanisms of Tomentosin in Human Keratinocytes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Cell Culture
2.2. Assay for Cell Viability
2.3. ARE and XRE Luciferase Reporter Assay and β-Galactosidase Assay
2.4. Dichlorofluorescin Diacetate (DCFDA) Cellular ROS Detection Assay
2.5. Diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging Assay
[(Absorbance of control − Absorbance of sample)/Absorbance of control] × 100
2.6. Real-Time RT-PCR Analysis of mRNA Levels
2.7. Measurement of Protein Levels Using Western Blot Analysis
2.8. Preparation of the Nuclear and Cytoplasmic Cell Fractions
2.9. Detection of Target Proteins Using Immunocytochemistry
2.10. Enzyme-Linked Immunosorbent Assay (ELISA) for Target Proteins
2.11. Statistical Analysis for Data Significance
3. Results
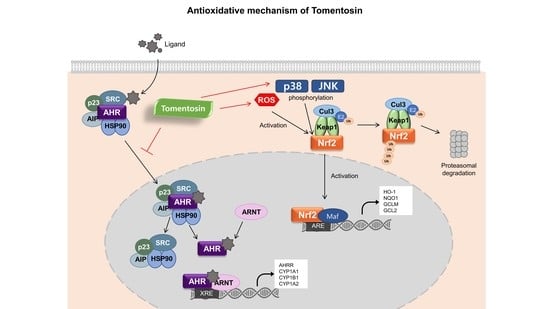
3.1. Tomentosin Exerts Antioxidant Activity in HaCaT Cells
3.2. Tomentosin Activates the Nrf2 Signaling Pathway
3.3. Tomentosin Induces Nrf2 Activation by Phosphorylating p38 MAPK and JNK in HaCaT Cells
3.4. Tomentosin-Induced ROS Production Mediates Nrf2 Activation
3.5. Tomentosin Suppresses B[a]P-Induced AhR Signaling
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mylona, P.V.; Polidoros, A.N.; Scandalios, J.G. Antioxidant gene responses to ROS-generating xenobiotics in developing and germinated scutella of maize. J. Exp. Bot. 2007, 58, 1301–1312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupont, E.; Gomez, J.; Bilodeau, D. Beyond UV radiation: A skin under challenge. Int. J. Cosmet. Sci. 2013, 35, 224–232. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.E.; Cho, D.; Park, H.J. Air pollution and skin diseases: Adverse effects of airborne particulate matter on various skin diseases. Life Sci. 2016, 152, 126–134. [Google Scholar] [PubMed]
- Bottai, G.; Mancina, R.; Muratori, M.; Di Gennaro, P.; Lotti, T. 17β-estradiol protects human skin fibroblasts and keratinocytes against oxidative damage. J. Eur. Acad. Dermatol. Venereol. 2013, 27, 1236–1243. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Athar, M. Oxidative stress in the pathogenesis of skin disease. J. Investig. Dermatol. 2006, 126, 2565–2575. [Google Scholar] [PubMed] [Green Version]
- D’Autréaux, B.; Toledano, M.B. ROS as signalling molecules: Mechanisms that generate specificity in ROS homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [PubMed]
- Tsuji, G.; Takahara, M.; Uchi, H.; Matsuda, T.; Chiba, T.; Takeuchi, S.; Yasukawa, F.; Moroi, Y.; Furue, M. Identification of ketoconazole as an AhR-Nrf2 activator in cultured human keratinocytes: The basis of its anti-inflammatory effect. J. Investig. Dermatol. 2012, 132, 59–68. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, P.; Unni, S.; Krishnappa, G.; Padmanabhan, B. The Keap1–Nrf2 pathway: Promising therapeutic target to counteract ROS-mediated damage in cancers and neurodegenerative diseases. Biophys. Rev. 2017, 9, 41–56. [Google Scholar]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and-independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar]
- Huang, H.-C.; Nguyen, T.; Pickett, C.B. Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J. Biol. Chem. 2002, 277, 42769–42774. [Google Scholar] [CrossRef] [Green Version]
- Costa, C.; Catania, S.; De Pasquale, R.; Stancanelli, R.; Scribano, G.; Melchini, A. Exposure of human skin to benzo [a] pyrene: Role of CYP1A1 and aryl hydrocarbon receptor in oxidative stress generation. Toxicology 2010, 271, 83–86. [Google Scholar] [CrossRef]
- Verma, N.; Pink, M.; Rettenmeier, A.W.; Schmitz-Spanke, S. Review on proteomic analyses of benzo [a] pyrene toxicity. Proteomics 2012, 12, 1731–1755. [Google Scholar]
- El Yaagoubi, O.; Lahmadi, A.; Bouyahya, A.; Filali, H.; Samaki, H.; El Antri, S.; Aboudkhil, S. Antitumor Effect of Inula viscosa Extracts on DMBA-Induced Skin Carcinoma Are Mediated by Proteasome Inhibition. Biomed. Res. Int. 2021, 2021, 6687589. [Google Scholar] [CrossRef]
- Lee, C.M.; Lee, J.; Nam, M.J.; Choi, Y.S.; Park, S.-H. Tomentosin displays anti-carcinogenic effect in human osteosarcoma MG-63 cells via the induction of intracellular reactive oxygen species. Int. J. Mol. Sci. 2019, 20, 1508. [Google Scholar] [CrossRef] [Green Version]
- Maoz, M.; Neeman, I. Antimicrobial effects of aqueous plant extracts on the fungi Microsporum canis and Trichophyton rubrum and on three bacterial species. Lett. Appl. Microbiol. 1998, 26, 61–63. [Google Scholar] [CrossRef]
- Hernández, V.; Recio, M.C.; Máñez, S.; Giner, R.M.; Ríos, J.-L. Effects of naturally occurring dihydroflavonols from Inula viscosa on inflammation and enzymes involved in the arachidonic acid metabolism. Life Sci. 2007, 81, 480–488. [Google Scholar]
- Merghoub, N.; El Btaouri, H.; Benbacer, L.; Gmouh, S.; Trentesaux, C.; Brassart, B.; Attaleb, M.; Madoulet, C.; Wenner, T.; Amzazi, S. Tomentosin induces telomere shortening and caspase-dependant apoptosis in cervical cancer cells. J. Cell. Biochem. 2017, 118, 1689–1698. [Google Scholar] [CrossRef]
- Zucker, S.N.; Fink, E.E.; Bagati, A.; Mannava, S.; Bianchi-Smiraglia, A.; Bogner, P.N.; Wawrzyniak, J.A.; Foley, C.; Leonova, K.I.; Grimm, M.J. Nrf2 amplifies oxidative stress via induction of Klf9. Mol. Cell 2014, 53, 916–928. [Google Scholar] [CrossRef] [Green Version]
- De Vries, H.E.; Witte, M.; Hondius, D.; Rozemuller, A.J.; Drukarch, B.; Hoozemans, J.; Van Horssen, J. Nrf2-induced antioxidant protection: A promising target to counteract ROS-mediated damage in neurodegenerative disease? Free Radic. Biol. Med. 2008, 45, 1375–1383. [Google Scholar]
- Kobayashi, M.; Yamamoto, M. Nrf2-Keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzym. Regul. 2006, 46, 113–140. [Google Scholar]
- Machala, M.; Vondráček, J.; Bláha, L.; Ciganek, M.; Neča, J. Aryl hydrocarbon receptor-mediated activity of mutagenic polycyclic aromatic hydrocarbons determined using in vitro reporter gene assay. Mutat. Res. /Genet. Toxicol. Environ. Mutagenesis 2001, 497, 49–62. [Google Scholar] [CrossRef]
- Tsuchiya, Y.; Nakajima, M.; Itoh, S.; Iwanari, M.; Yokoi, T. Expression of aryl hydrocarbon receptor repressor in normal human tissues and inducibility by polycyclic aromatic hydrocarbons in human tumor-derived cell lines. Toxicol. Sci. 2003, 72, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Tsuji, G.; Takahara, M.; Uchi, H.; Takeuchi, S.; Mitoma, C.; Moroi, Y.; Furue, M. An environmental contaminant, benzo (a) pyrene, induces oxidative stress-mediated interleukin-8 production in human keratinocytes via the aryl hydrocarbon receptor signaling pathway. J. Dermatol. Sci. 2011, 62, 42–49. [Google Scholar] [CrossRef]
- Rawlings, A.; Harding, C. Moisturization and skin barrier function. Dermatol. Ther. 2004, 17, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Krutmann, J.; Liu, W.; Li, L.; Pan, X.; Crawford, M.; Sore, G.; Seite, S. Pollution and skin: From epidemiological and mechanistic studies to clinical implications. J. Dermatol. Sci. 2014, 76, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Nickoloff, B.; Turka, L. Keratinocytes: Key immunocytes of the integument. Am. J. Pathol. 1993, 143, 325. [Google Scholar] [PubMed]
- Sekhar, K.R.; Yan, X.X.; Freeman, M.L. Nrf2 degradation by the ubiquitin proteasome pathway is inhibited by KIAA0132, the human homolog to INrf2. Oncogene 2002, 21, 6829–6834. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.D.; Hannink, M. Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol. Cell. Biol. 2003, 23, 8137–8151. [Google Scholar] [CrossRef] [Green Version]
- Ishii, T.; Itoh, K.; Takahashi, S.; Sato, H.; Yanagawa, T.; Katoh, Y.; Bannai, S.; Yamamoto, M. Transcription factor Nrf2 coordinately regulates a group of oxidative stress-inducible genes in macrophages. J. Biol. Chem. 2000, 275, 16023–16029. [Google Scholar] [CrossRef] [Green Version]
- Omiecinski, C.J.; Vanden Heuvel, J.P.; Perdew, G.H.; Peters, J.M. Xenobiotic metabolism, disposition, and regulation by receptors: From biochemical phenomenon to predictors of major toxicities. Toxicol. Sci. 2011, 120, S49–S75. [Google Scholar]
- Van Den Bogaard, E.H.; Podolsky, M.A.; Smits, J.P.; Cui, X.; John, C.; Gowda, K.; Desai, D.; Amin, S.G.; Schalkwijk, J.; Perdew, G.H. Genetic and pharmacological analysis identifies a physiological role for the AHR in epidermal differentiation. J. Investig. Dermatol. 2015, 135, 1320–1328. [Google Scholar] [CrossRef] [Green Version]
- Lawal, A.O. Air particulate matter induced oxidative stress and inflammation in cardiovascular disease and atherosclerosis: The role of Nrf2 and AhR-mediated pathways. Toxicol. Lett. 2017, 270, 88–95. [Google Scholar]
- Connor, K.T.; Aylward, L.L. Human response to dioxin: Aryl hydrocarbon receptor (AhR) molecular structure, function, and dose-response data for enzyme induction indicate an impaired human AhR. J. Toxicol. Environ. Health Part B Crit. Rev. 2006, 9, 147–171. [Google Scholar] [CrossRef]
- Gualtieri, M.; Øvrevik, J.; Mollerup, S.; Asare, N.; Longhin, E.; Dahlman, H.-J.; Camatini, M.; Holme, J.A. Airborne urban particles (Milan winter-PM2. 5) cause mitotic arrest and cell death: Effects on DNA, mitochondria, AhR binding and spindle organization. Mutat. Res./Fundam. Mol. Mech. Mutagenesis 2011, 713, 18–31. [Google Scholar] [CrossRef]
- Mathew, L.K.; Simonich, M.T.; Tanguay, R.L. AHR-dependent misregulation of Wnt signaling disrupts tissue regeneration. Biochem. Pharmacol. 2009, 77, 498–507. [Google Scholar]
- Jenner, P. Oxidative stress in Parkinson’s disease. Ann. Neurol. Off. J. Am. Neurol. Assoc. Child Neurol. Soc. 2003, 53, S26–S38. [Google Scholar]
- Lefer, D.J.; Granger, D.N. Oxidative stress and cardiac disease. Am. J. Med. 2000, 109, 315–323. [Google Scholar]
- Nichols, J.A.; Katiyar, S.K. Skin photoprotection by natural polyphenols: Anti-inflammatory, antioxidant and DNA repair mechanisms. Arch. Dermatol. Res. 2010, 302, 71–83. [Google Scholar]
- García-Pérez, M.-E.; Royer, M.; Duque-Fernandez, A.; Diouf, P.N.; Stevanovic, T.; Pouliot, R. Antioxidant, toxicological and antiproliferative properties of Canadian polyphenolic extracts on normal and psoriatic keratinocytes. J. Ethnopharmacol. 2010, 132, 251–258. [Google Scholar] [CrossRef]
- Takei, K.; Hashimoto-Hachiya, A.; Takahara, M.; Tsuji, G.; Nakahara, T.; Furue, M. Cynaropicrin attenuates UVB-induced oxidative stress via the AhR–Nrf2–Nqo1 pathway. Toxicol. Lett. 2015, 234, 74–80. [Google Scholar] [CrossRef]
- Lee, S.E.; Kwon, K.; Oh, S.W.; Park, S.J.; Yu, E.; Kim, H.; Yang, S.; Park, J.Y.; Chung, W.-J.; Cho, J.Y. Mechanisms of Resorcinol Antagonism of Benzo [a] pyrene-Induced Damage to Human Keratinocytes. Biomol. Ther. 2021, 29, 227. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Park, S.-H.; Oh, S.W.; Kwon, K.; Yu, E.; Lee, C.W.; Son, Y.K.; Kim, C.; Lee, B.-H.; Cho, J.Y.; et al. Antioxidant Activities and Mechanisms of Tomentosin in Human Keratinocytes. Antioxidants 2022, 11, 990. https://doi.org/10.3390/antiox11050990
Yang S, Park S-H, Oh SW, Kwon K, Yu E, Lee CW, Son YK, Kim C, Lee B-H, Cho JY, et al. Antioxidant Activities and Mechanisms of Tomentosin in Human Keratinocytes. Antioxidants. 2022; 11(5):990. https://doi.org/10.3390/antiox11050990
Chicago/Turabian StyleYang, Seyoung, See-Hyoung Park, Sae Woong Oh, Kitae Kwon, Eunbi Yu, Chae Won Lee, Youn Kyoung Son, Changmu Kim, Byoung-Hee Lee, Jae Youl Cho, and et al. 2022. "Antioxidant Activities and Mechanisms of Tomentosin in Human Keratinocytes" Antioxidants 11, no. 5: 990. https://doi.org/10.3390/antiox11050990