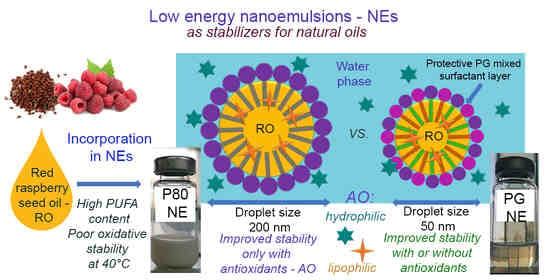
Red Raspberry Seed Oil Low Energy Nanoemulsions: Influence of Surfactants, Antioxidants, and Temperature on Oxidative Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Nanoemulsion Preparation
2.2.2. Nanoemulsion Characterization and Physical Stability
Particle Size Distribution
Electrical Conductivity and pH Value Measurements
2.3. Oxidative Stability Investigations
2.3.1. Red Raspberry Seed Oil (RO) Oxidative Stability
Peroxide Value (PV)
p-Anisidine Value (PA)
Thiobarbituric Reactive Substances (TBARS)
2.3.2. Nanoemulsion Oxidative Stability
Determination of Lipid Hydroperoxides (LH) in Nanoemulsions
Determination of TBARS in Nanoemulsions
2.4. Statistical Analysis
3. Results and Discussion
3.1. Influence of Red Raspberry Seed Oil, Antioxidants, and Temperature on Nanoemulsion Properties
3.1.1. The Nanoemulsion Droplet Size Distribution
3.1.2. The pH Value and Electrical Conductivity
3.2. Oxidative Stability of Red Raspberry Seed Oil
3.3. Oxidative Stability of Nanoemulsions with Red Raspeberry Seed Oil
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the skin: A new trend in food and cosmetics convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]
- Bialek, A.; Bialek, M.; Jelinska, M.; Tokarz, A. Fatty acid profile of new promising unconventional plant oils for cosmetic use. Int. J. Cosmet. Sci. 2016, 38, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Krasodomska, O.; Jungnickel, C. Viability of fruit seed oil O/W emulsions in personal care products. Colloids Surf. A Physicochem. Eng. Asp. 2015, 481, 468–475. [Google Scholar] [CrossRef]
- Bushman, B.S.; Phillips, B.; Isbell, T.; Ou, B.; Crane, J.M.; Knapp, S.J. Chemical composition of Caneberry (Rubus spp.) seeds and oils and their antioxidant potential. J. Agric. Food Chem. 2004, 52, 7982–7987. [Google Scholar] [CrossRef] [PubMed]
- Van Hoed, V.; de Clerq, N.; Echim, C.; Andjelkovic, M.; Leber, E.; Dewettnick, K.; Verhe, R. Berry seeds: A source of specialty oils with high content of bioactives and nutritional value. J. Food Lipids 2009, 16, 33–49. [Google Scholar] [CrossRef]
- Michalak, M.; Kiełtyka-Dadasiewicz, A. Oils from fruit seeds and their dietetic and cosmetic significance. Herba Pol. 2018, 64, 63–70. [Google Scholar] [CrossRef]
- Pilkington, S.M.; Watson, R.E.B.; Nicolaou, A.; Rhodes, L.E. Omega-3 polyunsaturated fatty acids: Photoprotective macronutrients. Exp. Dermatol. 2011, 20, 537–543. [Google Scholar] [CrossRef]
- Parry, J.; Su, L.; Luther, M.; Zhou, K.; Yurawecz, M.P.; Whittaker, P.; Yu, L. Fatty acid composition and antioxidant properties of cold-pressed marionberry, boysenberry, red raspberry, and blueberry seed oils. J. Agric. Food Chem. 2005, 53, 566–573. [Google Scholar] [CrossRef]
- Yang, B.; Ahotupa, M.; Petri Määttä, P.; Kallio, H. Composition and antioxidative activities of supercritical CO2-extracted oils from seeds and soft parts of northern berries. Food Res. Int. 2011, 44, 2009–2017. [Google Scholar] [CrossRef]
- Gledovic, A.; Janosevic Lezaic, A.; Krstonosic, V.; Djokovic, J.; Nikolic, I.; Bajuk-Bogdanovic, D.; Antic Stankovic, J.; Randjelovic, D.; Savic, S.M.; Filipovic, M.; et al. Low-energy nanoemulsions as carriers for red raspberry seed oil: Formulation approach based on Raman spectroscopy and textural analysis, physicochemical properties, stability and in vitro antioxidant/biological activity. PLoS ONE 2020, 15, e0230993. [Google Scholar] [CrossRef] [Green Version]
- Oomah, B.D.; Ladet, S.; Godfrey, D.V.; Liang, J.; Girard, B. Characteristics of raspberry (Rubus idaeus L.) seed oil. Food Chem. 2000, 69, 187–193. [Google Scholar] [CrossRef]
- Badea, G.; Lacatusu, I.; Badea, N.; Ott, C.; Meghea, A. Use of various vegetable oils in designing photoprotective nanostructured formulations for UV protection and antioxidant activity. Ind. Crops Prod. 2015, 67, 18–24. [Google Scholar] [CrossRef]
- Prescha, A.; Grajzer, M.; Dedyk, M.; Grajeta, H. The antioxidant activity and oxidative stability of cold-pressed oils. J. Am. Oil Chem. Soc. 2014, 91, 1291–1301. [Google Scholar] [CrossRef] [PubMed]
- Šućurović, A.; Vukelić, N.; Ignjatović, L.; Brčeski, I.; Jovanović, D. Physical-chemical characteristics and oxidative stability of oil obtained from lyophilized raspberry seed. Eur. J. Lipid Sci. Technol. 2009, 111, 1133–1141. [Google Scholar] [CrossRef]
- Ahmadi, O.; Jafarizadeh-Malmiri, H. Green approach in food nanotechnology based on subcritical water: Effects of thyme oil and saponin on characteristics of the prepared oil in water nanoemulsions. Food Sci. Biotechnol. 2020, 29, 783–792. [Google Scholar] [CrossRef] [PubMed]
- Demisli, S.; Theochari, I.; Christodoulo, P.; Zervou, M.; Xenakis, A.; Papadimitriou, V. Structure, activity and dynamics of extra virgin olive oil-in-water nanoemulsions loaded with vitamin D3 and calcium citrate. J. Mol. Liq. 2020, 306, 112908. [Google Scholar] [CrossRef]
- Pavoni, L.; Perinelli, D.R.; Bonacucina, G.; Cespi, M.; Palmieri, G.F. An Overview of micro- and nanoemulsions as vehicles for essential oils: Formulation, preparation and stability. Nanomaterials 2020, 10, 135. [Google Scholar] [CrossRef]
- Gupta, A.; Eral, H.B.; Hatton, T.A.; Doyle, P.S. Nanoemulsions: Formation, properties and applications. Soft Matter 2016, 12, 2826–2841. [Google Scholar] [CrossRef]
- Komaiko, J.S.; McClements, D.J. Formation of food-grade Nanoemulsions using low-energy preparation methods: A review of available methods. Compr. Rev. Food Sci. Food Saf. 2016, 15, 331–351. [Google Scholar] [CrossRef]
- Frankel, E.N.; Huang, S.-W.; Kanner, J.; German, J.B. Interfacial phenomena in the evaluation of antioxidants: Bulk oils vs emulsions. J. Agric. Food Chem. 1994, 42, 1054–1059. [Google Scholar] [CrossRef]
- Horn, A.F.; Nielsen, N.S.; Andersen, U.; Søgaard, L.H.; Horsewell, A.; Jacobsen, C. Oxidative stability of 70% fish oil-in-water emulsions: Impact of emulsifiers and pH. Eur. J. Lipid Sci. Technol. 2011, 113, 1243–1257. [Google Scholar] [CrossRef]
- Szumała, P.; Wysocka, I. Effect of gelation and storage conditions on the oxidative stability of microemulsion and nanoemulsion delivery systems. Eur. J. Pharm. Sci. 2018, 124, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Walker, R.; Decker, E.A.; McClements, D.J. Development of food-grade nanoemulsions and emulsions for delivery of omega-3 fatty acids: Opportunities and obstacles in the food industry. Food Funct. 2015, 6, 42–55. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Cheng, S.-F.; Bhattacharya, B.; Chakkaravarthi, S. Efficacy of free and encapsulated natural antioxidants in oxidative stability of edible oil: Special emphasis on nanoemulsion-based encapsulation. Trends Food Sci. Technol. 2019, 91, 305–318. [Google Scholar] [CrossRef]
- Rehman, A.; Jafari, S.M.; Tong, Q.; Karim, A.; Mahdi, A.A.; Iqbal, M.W.; Aadil, R.M.; Ali, A.; Manzoor, M.F. Role of peppermint oil in improving the oxidative stability and antioxidant capacity of borage seed oil-loaded nanoemulsions fabricated by modified starch. Int. J. Biol. Macromol. 2020, 153, 697–707. [Google Scholar] [CrossRef] [PubMed]
- Kampa, J.; Frazier, R.; Rodriguez-Garcia, J. Physical and chemical characterisation of conventional and nano/emulsions: Influence of vegetable oils from different origins. Foods 2022, 11, 681. [Google Scholar] [CrossRef]
- Costa, M.; Freiría-Gándara, J.; Losada-Barreiro, S.; Paiva-Martins, F.; Aliaga, C.; Bravo-Díaz, C. Interfacial kinetics in olive oil-in-water nanoemulsions: Relationships between rates of initiation of lipid peroxidation, induction times and effective interfacial antioxidant concentrations. J. Colloid Interface Sci. 2021, 604, 248–259. [Google Scholar] [CrossRef]
- Ferreira da Silveira, T.F.; Laguerre, M.; Bourlieu-Lacanal, C.; Lecomte, J.; Durand, E.; Figueroa-Espinoza, M.C.; Baréa, B.; Barouh, N.; Alves Castro, I.; Villeneuve, P. Impact of surfactant concentration and antioxidant mode of incorporation on the oxidative stability of oil-in-water nanoemulsions. LWT 2021, 141, 110892. [Google Scholar] [CrossRef]
- Gledovic, A.; Janosevic Lezaic, A.; Nikolic, I.; Tasic-Kostov, M.; Antic-Stankovic, J.; Krstonosic, V.; Randjelovic, D.; Bozic, D.; Ilic, D.; Tamburic, S.; et al. Polyglycerol ester-based low energy nanoemulsions with red raspberry reed oil and fruit extracts: Formulation development toward effective in vitro/in vivo bioperformance. Nanomaterials 2021, 11, 217. [Google Scholar] [CrossRef]
- Gledovic, A.; Bajuk-Bogdanović, D.; Uskoković-Marković, S.; Pavun, L.; Savic, S.; Janošević Ležaić, A. Low energy nanoemulsions as carriers for essential oils in topical formulations for antioxidant skin protection. Hem. Ind. 2022, 76, 29–42. [Google Scholar] [CrossRef]
- Drinić, Z.; Mudrić, J.; Zdunić, G.; Bigović, D.; Menković, N.; Šavikin, K. Effect of pomegranate peel extract on the oxidative stability of pomegranate seed oil. Food Chem. 2020, 333, 127501. [Google Scholar] [CrossRef] [PubMed]
- Malinowska, P.; Gliszczynska-Swigło, A.; Szymusiak, H. Protective effect of commercial acerola, willow, and rose extracts against oxidation of cosmetic emulsions containing wheat germ oil. Eur. J. Lipid Sci. Technol. 2014, 116, 1553–1562. [Google Scholar] [CrossRef]
- Shantha, N.; Decker, E.A. Rapid, sensitive, iron-based spectrophotometric methods for determination of peroxide values of food lipids. R J. AOAC Int. 1994, 77, 1994. [Google Scholar] [CrossRef]
- AOCS. Available online: https://www.aocs.org/attain-lab-services/methods/methods/search-results?method=111529 (accessed on 24 September 2022).
- Walker, R.M.; Gumus, C.E.; Decker, E.A.; McClements, D.J. Improvements in the formation and stability of fish oil-in-water nanoemulsions using carrier oils: MCT, thyme oil, & lemon oil. J. Food Eng. 2017, 211, 60–68. [Google Scholar]
- Azmi, N.A.N.; Elgharbawy, A.A.M.; Motlagh, S.R.; Samsudin, N.; Salleh, H.M. Nanoemulsions: Factory for food, pharmaceutical and cosmetics. Processes 2019, 7, 617. [Google Scholar] [CrossRef]
- Jurić, S.; Jurić, M.; Siddique, A.B.; Fathi, M. Vegetable oils rich in polyunsaturated fatty acids: Nanoencapsulation methods and stability enhancement. Food Rev. Int. 2022, 38, 32–69. [Google Scholar] [CrossRef]
- Solè, I.; Pey, C.M.; Maestro, A.; González, C.; Porras, M.; Solans, C.; Gutiérrez, J.M. Nano-emulsions prepared by the phase inversion composition method: Preparation variables and scale up. J. Colloid Interface Sci. 2010, 344, 417–423. [Google Scholar] [CrossRef]
- Chang, Y.Y.; McClements, D.J. Optimization of orange oil nanoemulsion formation by isothermal low-energy methods: Influence of the oil phase, surfactant, and temperature. J. Agric. Food Chem. 2014, 62, 2306–2312. [Google Scholar] [CrossRef]
- Hanno, I.; Centini, M.; Anselmi, C.; Bibiani, C. Green cosmetic surfactant from rice: Characterization and application. Cosmetics 2015, 2, 322–341. [Google Scholar] [CrossRef]
- Kato, T.; Nakamura, T.; Yamashita, M.; Kawaguchi, M.; Kato, T.; Itoh, T. Surfactants properties of purified polyglycerol monolaurates. J. Surfact. Deterg. 2003, 6, 331–337. [Google Scholar] [CrossRef]
- Klang, V.; Matsko, N.B.; Valenta, C.; Hofer, F. Electron microscopy of nanoemulsions: An essential tool for characterisation and stability assessment. Micron 2012, 43, 85–103. [Google Scholar] [CrossRef]
- Rebolleda, S.; Sanz, M.T.; Benito, J.M.; Beltran, S.; Escudero, I.; Gonzalez San-Jose, M.L. Formulation and characterisation of wheat bran oil-in-water nanoemulsions. Food Chem. 2015, 167, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, D.S.; Pereira, T.A.; Macie, N.R.; Bortoloto, J.; Viera, G.S.; Oliveira, G.C.; Rocha-Filho, P.A. Formation and stability of oil-in-water nanoemulsions containing rice bran oil: In vitro and in vivo assessments. J. Nanobiotechnol. 2011, 9, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Neves, M.A.; Wang, Z.; Kobayashi, I.; Nakajima, M. Assessment of oxidative stability in fish oil-in-water emulsions: Effect of emulsification process, droplet size and storage temperature. J. Food Process Eng. 2015, 40, e12316. [Google Scholar] [CrossRef]
- Kulisic, T.; Radonic, A.; Katalinic, V.; Milos, M. Use of different methods for testing antioxidative activity of oregano essential oil. Food Chem. 2004, 85, 633–640. [Google Scholar] [CrossRef]
- Dorman, H.J.D.; Surai, P.; Deans, S.G. In vitro antioxidant activity of a number of plant essential oils and phytoconstituents. J. Essent. Oil Rex. 2000, 12, 241–248. [Google Scholar] [CrossRef]
- Lee, J.; Choi, S.-J. Influence of blending of nonionic emulsifiers having various hydrophilic head sizes on lipid oxidation: Investigation of antioxidant polarity—Interfacial characteristics relationship. Antioxidants 2021, 10, 886. [Google Scholar] [CrossRef]
- Mc Clements, D.J.; Decker, E. Interfacial antioxidants: A review of natural and synthetic emulsifiers and coemulsifiers that can inhibit lipid oxidation. J. Agric. Food Chem. 2018, 66, 20–35. [Google Scholar] [CrossRef]
- Guo, X.; Sun, X.-T.; Liang, L.; Shi, L.-K.; Liu, R.-J.; Chang, M.; Wang, X.-G. Physical stability, oxidative stability, and bioactivity of nanoemulsion delivery systems incorporating lipophilic ingredients: Impact of oil saturation degree. J. Agric. Food Chem. 2021, 69, 5405–5415. [Google Scholar] [CrossRef]
- Marhamati, M.; Ranjbar, G.; Rezaie, M. Effects of emulsifiers on the physicochemical stability of oil-in-water nanoemulsions: A critical review. J. Mol. Liq. 2021, 340, 117218. [Google Scholar] [CrossRef]
- Weigel, F.; Weiss, J.; Decker, E.A.; Mc Clements, D.J. Lutein-enriched emulsion-based delivery systems: Influence of emulsifiers and antioxidants on physical and chemical stability. Food Chem. 2018, 242, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Zhong, J.; Liu, X.; Wang, Y.; Qin, X.; Li, Z. γ-Oryzanol nanoemulsions produced by a low-energy emulsification method: An evaluation of process parameters and physicochemical stability. Food Funct. 2017, 6, 2202–2211. [Google Scholar] [CrossRef] [PubMed]
- Nejadmansouri, M.; Hosseini, S.M.H.; Niakosari, M.; Yousefi, G.H.; Golmakani, M.T. Physicochemical properties and oxidative stability of fish oil nanoemulsions as affected by hydrophilic lipophilic balance, surfactant to oil ratio and storage temperature. Colloids Surf. A Physicochem. Eng. 2016, 506, 821–832. [Google Scholar] [CrossRef]









| Formulation Name | Surfactant (10 wt%) | Oil Phase (10 wt%) | Water Phase (80 wt%) |
|---|---|---|---|
| F0 P80 | 9.5 EP, 0.5 EUX | Water | |
| F1 P80 | 7.5 EP, 2 RO, 0.5 EUX | Water | |
| F1 P80 + BHT | P80 | 7.48 EP, 2 RO, 0.5 EUX, 0.02 BHT | Water |
| F1 P80 + ORE | 7.48 EP, 2 RO, 0.5 EUX, 0.02 ORE | Water | |
| F1 P80 + EDTA | 7.5 EP, 2 RO, 0.5 EUX | Water—79.8, EDTA—0.2 | |
| F1 P80 + OAK | 7.5 EP, 2 RO, 0.5 EUX | Water—79.0, OAK—1.0 | |
| F0 + PG | 9.5 EP, 0.5 EUX | Water—76, Glycerol—4 | |
| F1 + PG | 7.5 EP, 2 RO, 0.5 EUX | Water—56, Glycerol—24 | |
| F1 PG + BHT | PG | 7.48 EP, 2 RO, 0.5 EUX, 0.02 BHT | Water—56, Glycerol—24 |
| F1 PG + ORE | 7.48 EP, 2 RO, 0.5 EUX, 0.02 ORE | Water—56, Glycerol—24 | |
| F1 PG + EDTA | 7.5 EP, 2 RO, 0.5 EUX | Water—55.8, Glycerol—24, EDTA—0.2 | |
| F1 PG + OAK | 7.5 EP, 2 RO, 0.5 EUX | Water—55.8, Glycerol—24, OAK—1.0 |
| Formulation Name | Z-ave (nm) | PDI | pH | El. Cond. (µS/cm) |
|---|---|---|---|---|
| F0 P80 | 245.30 ± 3.12 | 0.121 ± 0.015 | 5.37 ± 0.006 | 159.33 ± 1.71 |
| F1 P80 | 207.63 ± 1.46 | 0.108 ± 0.017 | 5.45 ± 0.015 | 156.03 ± 0.76 |
| F1 P80 + BHT | 210.03 ± 1.62 | 0.125 ± 0.004 | 5.38 ± 0.021 | 167.37 ± 0.65 |
| F1 P80 + ORE | 203.50 ± 1.15 | 0.108 ± 0.014 | 5.40 ± 0.015 | 155.07 ± 0.49 |
| F1 P80 + EDTA | 206.03 ± 1.39 | 0.084 ± 0.027 | 4.98 ± 0.058 | 744.67 ± 0.58 |
| F1 P80 + OAK | 211.43 ± 1.66 | 0.116 ± 0.020 | 6.16 ± 0.025 | 242.67 ± 2.08 |
| F0 PG | 62.32 ± 0.32 | 0.060 ± 0.014 | 4.16 ± 0.006 | 167.73 ± 0.40 |
| F1 PG | 41.96 ± 0.72 | 0.059 ± 0.018 | 4.19 ± 0.010 | 81.07 ± 1.51 |
| F1 PG + BHT | 38.36 ± 0.73 | 0.110 ± 0.008 | 4.20 ± 0.017 | 75.77 ± 0.68 |
| F1 PG + ORE | 41.40 ± 0.95 | 0.079 ± 0.016 | 4.21 ± 0.026 | 71.27 ± 0.75 |
| F1 PG + EDTA | 46.56 ± 1.01 | 0.051 ± 0.013 | 4.59 ± 0.060 | 346.67 ± 1.53 |
| F1 PG + OAK | 41.57 ± 0.83 | 0.069 ± 0.024 | 4.35 ± 0.056 | 89.85 ± 0.23 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gledovic, A.; Janosevic-Lezaic, A.; Tamburic, S.; Savic, S. Red Raspberry Seed Oil Low Energy Nanoemulsions: Influence of Surfactants, Antioxidants, and Temperature on Oxidative Stability. Antioxidants 2022, 11, 1898. https://doi.org/10.3390/antiox11101898
Gledovic A, Janosevic-Lezaic A, Tamburic S, Savic S. Red Raspberry Seed Oil Low Energy Nanoemulsions: Influence of Surfactants, Antioxidants, and Temperature on Oxidative Stability. Antioxidants. 2022; 11(10):1898. https://doi.org/10.3390/antiox11101898
Chicago/Turabian StyleGledovic, Ana, Aleksandra Janosevic-Lezaic, Slobodanka Tamburic, and Snezana Savic. 2022. "Red Raspberry Seed Oil Low Energy Nanoemulsions: Influence of Surfactants, Antioxidants, and Temperature on Oxidative Stability" Antioxidants 11, no. 10: 1898. https://doi.org/10.3390/antiox11101898