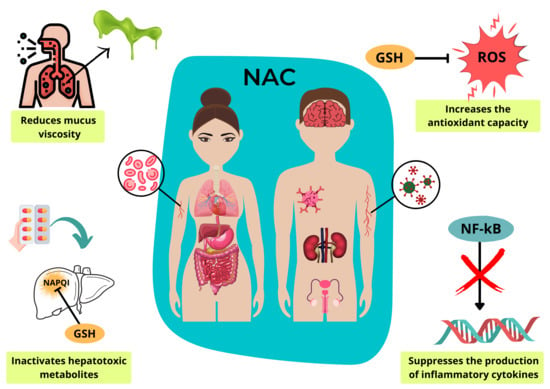
N-Acetylcysteine (NAC): Impacts on Human Health
Abstract
:1. Introduction
2. Pharmacokinetics and Bioavailability
| Summary of the Compound N-Acetylcysteine | |||||
| Drug indication NAC is used mainly as a mucolytic and in the management of acetaminophen (paracetamol) overdose. | |||||
Chemical structure | Molecular formula C5H9NO3S | Synonyms | |||
| |||||
| pKa 3.24: –COOH and 9.5: –SH | |||||
| Molecular weight 163.2 g/mol | Protein binding 66–97% (usually to albumin) | WHO essential medicines Antidotes and other substances used in poisonings. | |||
| Drug classes | Therapeutic uses | Taste Characteristic sour taste | |||
|
| ||||
| Color/Form White crystalline powder | |||||
| Odor Slight acetic odor | |||||
| Drug warnings | |||||
| |||||
| Absorption | |||||
| |||||
| Metabolism | |||||
| |||||
| Half-life | Clearance | Volume of distrib | Excretion | ||
| 0.11 L/h/kg | ution 0.47 L/kg |
| ||
| Dosage forms | Overdosage | ||||
| Single intravenous doses of NAC that were lethal:
| ||||
| Drug–drug interactions | |||||
| |||||
| Adapted from PubChem (2021) [31] and DrugBank (2021) [32]. | |||||
3. Mechanism of Molecular Action
4. Clinical Indications
4.1. Lung Diseases
4.2. Cardiovascular Diseases
4.3. Psychiatric Illnesses
4.4. Neurodegenerative Diseases
4.5. Liver Diseases
| Management of paracetamol poisoning |
| New recommendation for standard regimen of two acetylcysteine bags ab |
| Initial infusion |
|
| Second NAC infusion |
|
| If ongoing NAC is required, continue at the rate of the second infusion (i.e., 100 mg/kg over 16 h). Higher ongoing infusion rates (i.e., 200 mg/kg over 16 h) may be required for massive paracetamol ingestions and a clinical toxicologist should be consulted c. |
| a NAC is also compatible with 0.45% saline + 5% dextrose. b For adults (aged ≥14 years), dosing should be based on actual body weight rounded up to the nearest 10 kg, with a ceiling weight of 110 kg. For children (aged < 14 years), actual body weight should be used. c If the initial paracetamol concentration was more than double the nomogram line following an acute ingestion, acetylcysteine dose should be increased to 200 mg/kg (maximum 22 g) in glucose 5% 1000 mL (child, 14 mL/kg up to 1000 mL) or sodium chloride 0.9% 1000 mL (child, 14 mL/kg up to 1000 mL) intravenously, over 16 h. Adapted from Chiew et al. (2020) [82]. |
4.6. Kidney Diseases
4.7. Gastrointestinal Diseases
4.8. Infectious Diseases
4.9. Cancer Prevention and Treatment
4.10. Other Conditions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Tardiolo, G.; Bramanti, P.; Mazzon, E. Overview on the effects of n-acetylcysteine in neurodegenerative diseases. Molecules 2018, 23, 3305. [Google Scholar] [CrossRef] [Green Version]
- Ooi, S.L.; Green, R.; Pak, S.C. N-Acetylcysteine for the treatment of psychiatric disorders: A review of current evidence. BioMed Res. Int. 2018, 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Aldini, G.; Altomare, A.; Baron, G.; Vistoli, G.; Carini, M.; Borsani, L.; Sergio, F. N-Acetylcysteine as an antioxidant and disulphide breaking agent: The reasons why. Free Radic. Res. 2018, 52, 751–762. [Google Scholar] [CrossRef]
- Dodd, S.; Dean, O.; Copolov, D.L.; Malhi, G.S.; Berk, M. N-acetylcysteine for antioxidant therapy: Pharmacology and clinical utility. Expert Opin. Biol. Ther. 2008, 8, 1955–1962. [Google Scholar] [CrossRef]
- Ezerina, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl cysteine functions as a fast-acting antioxidant by triggering intracellular h2s and sulfane sulfur production. Cell Chem. Biol. 2018, 25, 447–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuzzocrea, S.; Mazzon, E.; Costantino, G.; Serraino, I.; Dugo, L.; Calabrò, G.; Cucinotta, G.; De Sarro, A.; Caputi, A.P. Beneficial effects of n-acetylcysteine on ischaemic brain injury. Br. J. Pharmacol. 2000, 130, 1219–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crupi, R.; Gugliandolo, E.; Siracusa, R.; Impellizzeri, D.; Cordaro, M.; Di Paola, R.; Britti, D.; Cuzzocrea, S. N-acetyl-L-cysteine reduces Leishmania amazonensis-induced inflammation in BALB/c mice. BMC Vet. Res. 2020, 16, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poncin, S.; Colin, I.M.; Decallonne, B.; Clinckspooor, I.; Many, M.C.; Denef, J.F.; Gérard, A.C. N-acetylcysteine and 15 deoxy-{delta}12,14-prostaglandin J2 exert a protective effect against autoimmune thyroid destruction in vivo but not against interleukin-1{alpha}/interferon {gamma}-induced inhibitory effects in thyrocytes in vitro. Am. J. Pathol. 2010, 177, 219–228. [Google Scholar] [CrossRef] [PubMed]
- De Andrade, K.Q.; Moura, F.A.; Dos Santos, J.M.; De Araújo, O.R.; De Farias Santos, J.C.; Goulart, M.O.F. Oxidative stress and inflammation in hepatic diseases: Therapeutic possibilities of N-Acetylcysteine. Int. J. Mol. Sci. 2015, 16, 30269–30308. [Google Scholar] [CrossRef]
- Shahripour, R.B.; Harrigan, M.R.; Alexandrov, A.V. N-acetylcysteine (NAC) in neurological disorders: Mechanisms of action and therapeutic opportunities. Brain Behav. 2014, 4, 108–122. [Google Scholar] [CrossRef]
- Rushworth, G.F.; Megson, I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014, 141, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Holdiness, M.R. Clinical pharmacokinetics of N-Acetylcysteine. Clin. Pharmacokinet. 1991, 20, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Borgstrom, L.; Kagedal, B.; Paulsen, O. Pharmacokinetics of N-acetylcysteine in man. Eur. J. Clin. Pharmacol. 1986, 31, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Olsson, B.; Johansson, M.; Gabrielsson, J.; Bolme, P. Pharmacokinetics and bioavailability of reduced and oxidized N-acetylcysteine. Eur. J. Clin. Pharmacol. 1988, 34, 77–82. [Google Scholar] [CrossRef]
- Jones, A.L.; Jarvie, D.R.; Simpson, D.; Hayes, P.C.; Prescott, L.F. Pharmacokinetics of N-acetylcysteine are altered in patients with chronic liver disease. Aliment. Pharmacol. Ther. 1997, 11, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Villeneuve, E.; Gosselin, S. N-Acetylcysteine. In Critical Care Toxicology; Brent, J., Burkhart, K., Dargan, P., Hatten, B., Megarbane, B., Palmer, R., White, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar]
- Prescott, L.F.; Donovan, J.W.; Jarvie, D.R.; Proudfoot, A.T. The disposition and kinetics of intravenous N-acetylcysteine in patients with paracetamol overdosage. Eur. J. Clin. Pharmacol. 1989, 37, 501–506. [Google Scholar] [CrossRef]
- Nolin, T.D.; Ouseph, R.; Himmelfarb, J.; McMenamin, M.E.; Ward, R.A. Multiple-dose pharmacokinetics and pharmacodynamics of n-acetylcysteine in patients with end-stage renal disease. Clin. J. Am. Soc. Nephrol. 2010, 5, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, L.; Gazzaniga, A. Toxicological, pharmacokinetic and metabolic studies on acetylcysteine. Eur. J. Respir. Dis. Suppl. 1980, 111, 45–51. [Google Scholar]
- Rodenstein, D.; De Coster, A.; Gazzaniga, A. Pharmacokinetics of oral acetylcysteine. Clin. Pharmacokinet. 1978, 3, 247–254. [Google Scholar] [CrossRef]
- Papi, A.; Di Stefano, A.F.D.; Radicioni, M. Pharmacokinetics and safety of single and multiple doses of oral n-acetylcysteine in healthy chinese and caucasian volunteers: An open-label, phase i clinical study. Adv. Ther. 2021, 38, 468–478. [Google Scholar] [CrossRef]
- Toxicology Investigator Network. A multicenter comparison of the safety of oral versus intravenous acetylcysteine for treatment of acetaminophen overdose. Clin. Toxicol. 2010, 48, 424–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greene, S.C.; Noonan, P.K.; Sanabria, C.; Peacock, W.F. Effervescent N-Acetylcysteine tablets versus oral solution n-acetylcysteine in fasting healthy adults: An open-label, randomized, single-dose, crossover, relative bioavailability study. Curr. Ther. Res. 2016, 83, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yarema, M.; Chopra, P.; Sivilotti, M.L.A.; Johnson, D.; Nettel-Aguirre, A.; Bailey, B.; Victorino, C.; Gosselin, S.; Purssell, R.; Thompson, M.; et al. Anaphylactoid reactions to intravenous n-acetylcysteine during treatment for acetaminophen poisoning. J. Med. Toxicol. 2018, 14, 120–127. [Google Scholar] [CrossRef]
- Homma, S.; Azuma, A.; Taniguiche, H.; Ogura, T.; Mochiuki, Y.; Sugiyama, Y.; Nakata, K.; Yoshimura, K.; Takeuchi, M.; Kudoh, S. Efficacy of inhaled N-acetylcysteine monotherapy in patients with early stage idiopathic pulmonary fibrosis. Respirology 2012, 17, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Kuzmenko, N.; Oleg, I.; Dmytro, D.; Roman, I.; Galyna, G. The efficacy and safety of inhaled acetylcysteine in comparison with oral acetylcysteine in chronic obstructive pulmonary disease: A randomized single-center study. Pol. Ann. Medicine. 2020, 27, 108–114. [Google Scholar] [CrossRef]
- Calverley, P.; Rogliani, P.; Papi, A. Safety of N-Acetylcysteine at high doses in chronic respiratory diseases: A review. Drug Saf. 2021, 44, 273–290. [Google Scholar] [CrossRef] [PubMed]
- Feng, F.; Zhang, J.; Wang, Z.; Wu, Q.; Zhou, X. Efficacy and safety of N-acetylcysteine therapy for idiopathic pulmonary fibrosis: An updated systematic review and meta-analysis. Exp. Ther. Med. 2019, 18, 802–816. [Google Scholar] [CrossRef] [PubMed]
- Liou, G.G.; Hsieh, C.C.; Lee, Y.J.; Li, P.H.; Tsai, M.S.; Li, C.T.; Wang, S.H. N-Acetyl cysteine overdose inducing hepatic steatosis and systemic inflammation in both propacetamol-induced hepatotoxic and normal mice. Antioxidants 2021, 10, 442. [Google Scholar] [CrossRef]
- Mahmoudi, G.A.; Astaraki, P.; Mohtashami, A.Z.; Ahadi, M. N-acetylcysteine overdose after acetaminophen poisoning. Int. Med. Case Rep. J. 2015, 8, 65–69. [Google Scholar] [CrossRef] [Green Version]
- PubChem [Internet]. Bethesda (MD): National Library of Medicine (US), National Center for Biotechnology Information; 2004-PubChem Compound Summary for CID 12035, Acetylcysteine. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Acetylcysteine (accessed on 29 April 2021).
- Drugbank [Internet]. Available online: https://go.drugbank.com/drugs/DB06151 (accessed on 5 April 2021).
- Pfaff, A.R.; Beltz, J.; King, E.; Ercal, N. Medicinal thiols: Current status and new perspectives. Mini Rev. Med. Chem. 2020, 20, 513–529. [Google Scholar] [CrossRef]
- Samuni, Y.; Goldstein, S.; Dean, O.M.; Berk, M. The chemistry and biological activities of N-acetylcysteine. Biochim. Biophys. Acta. 2013, 1830, 4117–4129. [Google Scholar] [CrossRef] [PubMed]
- Radomska-Lesnniewska, D.M.; Skopinski, P. N-acetylcysteine as an anti-oxidant and anti-inflammatory drug and its some clinical applications. Centr. Eur. J. Immunol. 2012, 37, 57–66. [Google Scholar]
- Pei, Y.; Liu, H.; Yang, Y.; Yang, Y.; Jiao, Y.; Tay, F.R.; Chen, J. Biological activities and potential oral applications of n-acetylcysteine: Progress and prospects. Oxidative Med. Cell. Longev. 2018, 2018, 1–14. [Google Scholar] [CrossRef]
- Aruoma, O.I.; Halliwell, B.; Hoey, B.M.; Butler, J. The antioxidant action of N-acetylcysteine: Its reaction with hydrogen peroxide, hydroxyl radical, superoxide, and hypochlorous acid. Free Radic. Biol. Med. 1989, 6, 593–597. [Google Scholar] [CrossRef]
- Prutz, W.A.; Monig, H.; Butler, J.; Land, E.J. Reactions of nitrogen-dioxide in aqueous model systems—Oxidation of tyrosine units in peptides and proteins. Arch. Biochem. Biophys. 1985, 243, 125–134. [Google Scholar] [CrossRef]
- Ford, E.; Hughes, M.N.; Wardman, P. Kinetics of the reactions of nitrogen dioxide with glutathione, cysteine, and uric acid at physiological pH. Free Radic. Biol. Med. 2002, 32, 1314–1323. [Google Scholar] [CrossRef]
- Chen, S.N.; Hoffman, M.Z. Effect of pH on the reactivity of carbonate radicals in aqueous solution. Radiat. Res. 1975, 62, 18–27. [Google Scholar] [CrossRef]
- Miranda, K.M.; Paolocci, N.; Katori, T.; Thomas, D.D.; Ford, E.; Bartberger, M.D.; Espey, M.G.; Kass, D.A.; Feelisch, M.; Fukuto, J.M.; et al. A biochemical rationale for the discrete behavior of nitroxyl and nitric oxide in the cardiovascular system. Proc. Natl. Acad. Sci. USA 2003, 100, 9196–9201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benrahmoune, M.; Therond, P.; Abedinzadeh, Z. The reaction of superoxide radical with N-acetylcysteine. Free Radic. Biol. Med. 2000, 29, 775–782. [Google Scholar] [CrossRef]
- Winterbourn, C.C.; Metodiewa, D. Reactivity of biologically important thiol compounds with superoxide and hydrogen peroxide. Free Radic. Biol. Med. 1999, 27, 322–328. [Google Scholar] [CrossRef]
- Trujillo, M.; Radi, R. Peroxynitrite reaction with the reduced and the oxidized forms of lipoic acid: New insights into the reaction of peroxynitrite with thiols. Arch. Biochem. Biophys. 2002, 397, 91–98. [Google Scholar] [CrossRef]
- Kasperczyk, S.; Dobrakowski, M.; Kasperczyk, A.; Romuk, E.; Rykaczewska-Czerwińska, M.; Pawlas, N.; Birkner, E. Effect of N-acetylcysteine administration on homocysteine level, oxidative damage to proteins, and levels of iron (Fe) and Fe-related proteins in lead-exposed workers. Toxicol. Ind. Health 2016, 32, 1607–1618. [Google Scholar] [CrossRef]
- Dhouib, I.E.; Jallouli, M.; Annabi, A.; Gharbi, N.; Elfazaa, S.; Lasram, M.M. A minireview on N-acetylcysteine: An old drug with new approaches. Life Sci. 2016, 151, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Ullian, M.E.; Gelasco, A.K.; Fitzgibbon, W.R.; Beck, C.N.; Morinelli, T.A. N-acetylcysteine decreases angiotensin II receptor binding in vascular smooth muscle cells. J. Am. Soc. Nephrol. 2005, 16, 2346–2353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hayakawa, M.; Miyashita, H.; Sakamoto, I.; Kitagawa, M.; Tanaka, H.; Yasuda, H.; Karin, M.; Kikugawa, K. Evidence that reactive oxygen species do not mediate NF-kB activation. EMBO J. 2003, 22, 3356–3366. [Google Scholar] [CrossRef] [Green Version]
- Meurer, S.K.; Lahme, B.; Tihaa, L.; Weiskirchen, R.; Gressner, A.M. N-acetyl-L-cysteine suppresses TGF-beta signaling at distinct molecular steps: The biochemical and biological efficacy of a multifunctional, antifibrotic drug. Biochem. Pharmacol. 2005, 70, 1026–1034. [Google Scholar] [CrossRef] [PubMed]
- Palacio, J.R.; Markert, U.R.; Martínez, P. Anti-inflammatory properties of N-acetylcysteine on lipopolysaccharide-activated macrophages. Inflamm. Res. 2011, 60, 695–704. [Google Scholar] [CrossRef]
- Decramer, M.; Rutten-van, M.M.; Dekhuijzen, P.N.R.; Troosters, T.; van Herwaarden, C.; Pellegrino, R.; van Schayck, C.P.O.; Olivieri, D.; Donno, M.D.; De Backer, W.; et al. Effects of N-acetylcysteine on outcomes in chronic obstructive pulmonary disease (Bronchitis Randomized on NAC Cost-Utility Study, BRONCUS): A randomised placebo-controlled trial. Lancet 2005, 365, 1552–1560. [Google Scholar] [CrossRef]
- Schermer, T.; Chavannes, N.; Dekhuijzen, R.; Wouters, E.; Muris, J.; Akkermans, R.; van Schayck, O.; van Weel, C. Fluticasone and N-acetylcysteine in primary care patients with COPD or chronic bronchitis. Respir. Med. 2009, 103, 542–551. [Google Scholar] [CrossRef] [Green Version]
- Tse, H.N.; Raiteri, L.; Wong, K.Y.; Yee, K.S.; Ng, L.Y.; Wai, K.Y.; Loo, C.K.; Chan, M.H. High-dose n-acetylcysteine in stable COPD. The 1-year, double-blind, randomized, placebo-controlled HIACE Study. Chest 2013, 144, 106–118. [Google Scholar] [CrossRef]
- Zheng, J.P.; Wen, F.Q.; Bai, C.X.; Wan, H.Y.; Kang, J.; Chen, P.; Yao, W.Z.; Ma, L.J.; Li, X.; Raiteri, L.; et al. Twice daily N-acetylcysteine 600 mg for exacerbations of chronic obstructive pulmonary disease (PANTHEON): A randomised, double-blind placebo-controlled trial. Lancet Respiriratory Med. 2014, 2, 187–194. [Google Scholar] [CrossRef]
- Johnson, K.; McEvoy, C.E.; Naqvi, S.; Wendt, C.; Reilkoff, R.A.; Wetherbee, E.E.; Nelson, D.; Tirouvanziam, R.; Niewoehner, D.E. High-dose oral N-acetylcysteine fails to improve respiratory health status in patients with chronic obstructive pulmonary disease and chronic bronchitis: A randomized, placebo-controlled trial. Int. J. COPD. 2016, 11, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Shen, Y.; Cai, W.; Lei, S.; Zhang, Z. Effect of high/low dose n-acetylcysteine on chronic obstructive pulmonary disease: A systematic review and meta-analysis. COPD 2013, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Cazzola, M.; Calzetta, L.; Page, C.; Jardim, J.; Chuchalin, A.G.; Rogliani, P.; Matera, M.G. Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: A meta-analysis. Europ. Respir. Rev. 2015, 24, 451–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fowdar, K.; Chen, H.; He, Z.; Zhang, J.; Zhong, X.; Zhang, J.; Li, M.; Bai, J. The effect of N-acetylcysteine on exacerbations of chronic obstructive pulmonary disease: A meta-analysis and systematic review. Heart Lung 2017, 46, 120–128. [Google Scholar] [CrossRef] [PubMed]
- Ciofu, O.; Smith, S.; Lykkesfeldt, J. Antioxidant supplementation for lung disease in cystic fibrosis. Cochrane Database Syst. Rev. 2019, 10, CD007020. [Google Scholar] [CrossRef]
- Tirouvanziam, R.; Conrad, C.K.; Bottiglieri, T.; Herzenberg, L.A.; Moss, R.B.; Herzenberg, L.A. High-dose oral N-acetylcysteine, a glutathione prodrug, modulates inflammation in cystic fibrosis. Proc. Natl. Acad. Sci. USA 2006, 10, 4628–4633. [Google Scholar] [CrossRef] [Green Version]
- Skov, M.; Pressler, T.; Likkesfeldt, J.; Poulsen, H.E.; Jensen, P.O.; Johansen, H.K.; Qvist, T.; Kraemer, D.; Hoiby, N.; Ciofu, O. The effect of short-term, high-dose oral N-acetylcysteine treatment on oxidative stress markers in cystic fibrosis patients with chronic P. aeruginosa infection—A pilot study. J. Cyst. Fibros. 2015, 14, 211–218. [Google Scholar] [CrossRef] [Green Version]
- Rogliani, P.; Calzetta, L.; Cavalli, F.; Matera, M.G.; Cazzola, M. Pirfenidone, nintedanib and N-acetylcysteine for the treatment of idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Pulm. Pharmacol Ther. 2016, 40, 95–103. [Google Scholar] [CrossRef]
- Arstall, M.A.; Yang, J.; Stafford, I.; Betts, W.H.; Horowitz, J.D. N-acetylcysteine in combination with nitroglycerin and streptokinase for the treatment of evolving acute myocardial infarction: Safety and biochemical effects. Circulation 1995, 92, 2855–2862. [Google Scholar] [CrossRef] [PubMed]
- Yesilbursa, D.; Serdar, A.; Senturk, T.; Serdar, Z.; Sağ, S.; Cordan, J. Effect of N-acetylcysteine on oxidative stress and ventricular function in patients with myocardial infarction. Heart Vessel. 2006, 21, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.E.G.; El Dib, R.; Braz, L.G.; Escudero, J.; Hayes, J.; Johnston, B.C. N-acetylcysteine use among patients undergoing cardiac surgery: A systematic review and meta-analysis of randomized trials. PLoS ONE 2019, 14, e0213862. [Google Scholar] [CrossRef] [PubMed]
- Gu, W.J.; Wu, Z.J.; Wang, P.F.; Aung, L.H.H.; Yin, R.X. N-Acetylcysteine supplementation for the prevention of atrial fibrillation after cardiac surgery: A meta-analysis of eight randomized controlled trials. BMC Cardiovasc. Disord. 2012, 12, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ozaydin, M.; Peker, O.; Erdogan, D.; Kapan, S.; Turker, Y.; Varol, E.; Ozguner, F.; Dogan, A.; Ibrisim, E. N-acetylcysteine for the prevention of postoperative atrial fibrillation: A prospective, randomized, placebo-controlled pilot study. Eur. Heart J. 2008, 29, 625–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berk, M.; Copolov, D.; Dean, O.; Lu, K.; Jeavons, S.; Schapkaitz, I.; Anderson-Hunt, M.; Judd, F.; Katz, F.; Katz, P.; et al. N-acetyl cysteine as a glutathione precursor for schizophrenia--a double-blind, randomized, placebo-controlled trial. Biol. Psychiatr. 2008, 64, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Sepehrmanesh, Z.; Heidary, M.; Akasheh, N.; Akbari, H.; Heidary, M. Therapeutic effect of adjunctive N-acetyl cysteine (NAC) on symptoms of chronic schizophrenia: A double-blind, randomized clinical trial. Prog. Neuropsychopharmacol. Biol. Psychiatr. 2018, 82, 289–296. [Google Scholar] [CrossRef]
- Yolland, C.O.B.; Phillipou, A.; Castle, D.J.; Neill, E.; Hughes, M.E.; Galletly, C.; Smith, Z.M.; Francis, P.S.; Dean, O.M.; Sarris, J.; et al. Improvement of cognitive function in schizophrenia with N-acetylcysteine: A theoretical review. Nutr. Neurosci. 2020, 23, 139–148. [Google Scholar] [CrossRef]
- Conus, P.; Seidman, L.J.; Fournier, M.; Xin, L.; Cleusix, M.; Baumann, P.S.; Ferrari, C.; Cousins, A.; Alameda, L.; Gholam-Rezaee, M.; et al. N-acetylcysteine in a double-blind randomized placebo-controlled trial: Toward biomarker-guided treatment in early psychosis. Schizophr. Bull. 2018, 44, 317–327. [Google Scholar] [CrossRef] [Green Version]
- Berk, M.; Turner, A.; Malhi, G.S.; Ng, C.H.; Cotton, S.M.; Dodd, S.; Samuni, Y.; Tanious, M.; McAulay, C.; Dowling, N.; et al. A randomised controlled trial of a mitochondrial therapeutic target for bipolar depression: Mitochondrial agents, N-acetylcysteine, and placebo. BMC Med. 2019, 17, 1–11. [Google Scholar] [CrossRef]
- Magalhães, P.V.; Dean, O.M.; Bush, A.I.; Copolov, D.L.; Malhi, G.S.; Kohlmann, K.K.; Jeavons, S.; Schapkaitz, I.; Anderson-Hunt, M.D.; Berk, M. N-acetyl cysteine add-on treatment for bipolar II disorder: A subgroup analysis of a randomized placebo-controlled trial. J. Affect. Disord. 2011, 129, 317–320. [Google Scholar] [CrossRef]
- Berk, M.; Dean, O.M.; Cotton, S.M.; Jeavons, S.; Tanious, M.; Kohlmann, K.; Hewitt, K.; Moss, K.; Allwang, C.; Schapkaitz, I.; et al. The efficacy of adjunctive N-acetylcysteine in major depressive disorder: A double-blind, randomized, placebo-controlled trial. J. Clin. Psychiatr. 2014, 75, 628–636. [Google Scholar] [CrossRef] [Green Version]
- Kishi, T.; Miyake, N.; Okuya, M.; Sakuma, K.; Iwata, N. N-acetylcysteine as an adjunctive treatment for bipolar depression and major depressive disorder: A systematic review and meta-analysis of double-blind, randomized placebo-controlled trials. Psychopharmacology 2020, 237, 3481–3487. [Google Scholar] [CrossRef]
- Holmay, M.J.; Terpstra, M.; Coles, L.D.; Mishra, U.; Ahlskog, M.; Oz, G.; Cloyd, J.C.; Tuite, P.J. N-acetylcysteine boosts brain and blood glutathione in gaucher and Parkinson’s diseases. Clin. Neuropharmacol. 2013, 36, 103. [Google Scholar] [CrossRef] [Green Version]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.W.; Wintering, N.A.; Bazzan, A.J.; Bowens, B.K.; Chervoneva, I.; Intenzo, C.; Newberg, A.B. N-Acetyl cysteine is associated with dopaminergic improvement in Parkinson’s disease. Clin. Pharmacol Ther. 2019, 106, 884–890. [Google Scholar] [CrossRef]
- Coles, L.D.; Tuite, P.J.; Öz, G.; Mishra, U.R.; Kartha, R.V.; Sullivan, K.M.; Terpstra, M.; Cloyd, J.C. Repeated-dose oral N-acetylcysteine in Parkinson’s disease: Pharmacokinetics and effect on brain glutathione and oxidative stress. J. Clin. Pharmacol. 2018, 58, 158–167. [Google Scholar] [CrossRef]
- Remington, R.; Lortie, J.J.; Hoffmann, H.; Page, R.; Morrell, C.; Shea, T.B. A nutritional formulation for cognitive performance in mild cognitive impairment: A placebo-controlled trial with an open-label extension. J. Alzheimers Dis. 2015, 48, 591–595. [Google Scholar] [CrossRef] [Green Version]
- Krysko, K.M.; Bischof, A.; Nourbakhsh, B.; Henry, R.G.; Revirajan, N.; Manguinao, M.; Nguyen, K.; Akula, A.; Li, Y.; Waubant, E. A pilot study of oxidative pathways in MS fatigue: Randomized trial of N-acetyl cysteine. Ann. Clin. Translat. Neurol. 2021, 8, 811–824. [Google Scholar] [CrossRef]
- Monti, D.A.; Zabrecky, G.; Leist, T.P.; Wintering, N.; Bazzan, A.J.; Zhan, T.; Newberg, A.B. N-acetyl cysteine administration is associated with increased cerebral glucose metabolism in patients with multiple sclerosis: An exploratory study. Front. Neurol. 2020, 11, 88. [Google Scholar] [CrossRef]
- Chiew, A.L.; Reith, D.; Pomerleau, A.; Wong, A.; Isoardi, K.Z.; Soderstrom, J.; Buckley, N.A. Updated guidelines for the management of paracetamol poisoning in Australia and New Zealand. Med. J. Aust. 2020, 212, 175–183. [Google Scholar] [CrossRef]
- Nabi, T.; Nabi, S.; Rafiq, N.; Shah, A. Role of N-acetylcysteine treatment in non-acetaminophen-induced acute liver failure: A prospective study. Saudi J. Gastroenterol. 2017, 23, 169–175. [Google Scholar] [CrossRef]
- Chughlay, M.F.; Kramer, N.; Spearman, C.W.; Werfalli, M.; Cohen, K. N-acetylcysteine for non-paracetamol drug-induced liver injury: A systematic review. Br. J. Clin. Pharmacol. 2016, 81, 1021–1029. [Google Scholar] [CrossRef] [Green Version]
- Khoshbaten, M.; Aliasgarzadeh, A.; Masnadi, K.; Tarzamani, M.K.; Farhang, S.; Babaei, H.; Kiani, J.; Zaare, M.; Najafipoor, F. N-acetylcysteine improves liver function in patients with non-alcoholic fatty liver disease. Hep. Mon. 2010, 10, 12–16. [Google Scholar]
- Nguyen-Khac, E.; Thevenot, T.; Piquet, M.A.; Benferhat, S.; Goria, O.; Chatelain, D.; Tramier, B.; Dewaele, F.; Ghrib, S.; Rudler, M.; et al. Glucocorticoids plus N-Acetylcysteine in Severe Alcoholic Hepatitis. N. Engl. J. Med. 2011, 365, 1781–1789. [Google Scholar] [CrossRef] [Green Version]
- Moreno, C.; Langlet, P.; Hittelet, A.; Lasser, L.; Degre, D.; Evrard, S.; Colle, I.; Lemmers, A.; Deviere, J.; Le Moine, O. Enteral nutrition with or without N-acetylcysteine in the treatment of severe acute alcoholic hepatitis: A randomized multicenter controlled trial. J. Hepatol. 2010, 53, 1117–1122. [Google Scholar] [CrossRef] [PubMed]
- He, G.; Li, Q.; Li, W.; Wang, L.; Yang, J.; Zeng, F. N-Acetylcysteine for preventing of acute kidney injury in chronic kidney disease patients undergoing cardiac surgery: A meta analysis. Heart Surg. Forum 2018, 21, E513–E521. [Google Scholar] [CrossRef] [PubMed]
- Wittstock, A.; Burkert, M.; Zidek, W.; Tepel, M.; Scholze, A. N-acetylcysteine improves arterial vascular reactivity in patients with chronic kidney disease. Nephron J. 2009, 12, c184–c189. [Google Scholar] [CrossRef]
- Mainra, R.; Gallo, K.; Moist, L. Effect of N-acetylcysteine on renal function in patients with chronic kidney disease. Nephrology 2007, 12, 510–513. [Google Scholar] [CrossRef] [PubMed]
- Hamidian, S.M.T.; Aletaha, N.S.; Taslimi, R.; Montazeri, M. An additive effect of oral N-acetylcysteine on eradication of Hlicobacter pylori. J. Pathog. 2015, 2015, 540271. [Google Scholar] [CrossRef] [Green Version]
- Yoon, H.; Lee, D.H.; Jang, E.S.; Kim, J.; Shin, C.M.; Park, Y.S.; Hwang, J.H.; Kim, J.W.; Jeong, S.H.; Kim, N. Effects of N-acetylcysteine on first-line sequential therapy for Helicobacter pylori infection: A randomized controlled pilot trial. Gut Liver 2016, 10, 520–525. [Google Scholar] [CrossRef] [Green Version]
- Irrazabal, T.; Thakur, B.K.; Croitoru, K.; Martin, A. Preventing colitis-associated colon cancer with antioxidants: A systematic review. Cell. Mol. Gastroenterol. Hepatol. 2021, 11, 1177–1197. [Google Scholar] [CrossRef]
- Kuyumcu, A.; Akyol, A.; Buyuktuncer, Z.; Ozmen, M.M.; Besler, H.T. Improved oxidative status in major abdominal surgery patients after N-acetyl cysteine supplementation. Nutr. J. 2015, 14, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Estensen, R.D.; Levy, M.; Klopp, S.J.; Galbraith, A.R.; Mandel, J.S.; Blomquist, J.A.; Wattenberg, L.W. N-acetylcysteine suppression of the proliferative index in the colon of patients with previous adenomatous colonic polyps. Cancer Lett. 1999, 147, 109–114. [Google Scholar] [CrossRef]
- Guijarro, L.G.; Mate, J.; Gisbert, J.P.; Perez-Calle, J.L.; Marin-Jimenez, I.; Arriaza, E.; Olleros, T.; Delgado, M.; Castillejo, M.S.; Prieto-Merino, D.; et al. N-acetyl-L-cysteine combined with mesalamine in the treatment of ulcerative colitis: Randomized, placebo-controlled pilot study. World J. Gastroenterol. 2008, 14, 2851–2857. [Google Scholar] [CrossRef]
- Wang, N.; Shi, X.F.; Guo, S.H.; Zhang, D.Z.; Ren, H. A clinical study of N-acetylcysteine treatment in chronic hepatitis B patients. Zhonghua Gan Zang Bing Za Zhi. Chin. J. Hepatol. 2008, 16, 487–489. [Google Scholar]
- Mahakalkar, S.M.; Nagrale, D.; Gaur, S.; Urade, C.; Murhar, B.; Turankar, A. N-acetylcysteine as an add-on to directly observed therapy short-I therapy in fresh pulmonary tuberculosis patients: A randomized, placebo-controlled, double-blinded study. Perspect. Clin. Res. 2017, 8, 132. [Google Scholar] [CrossRef] [PubMed]
- Memorial Sloan Kettering Cancer Center. A study of N-Acetylcysteine in Patients with COVID-19 Infection (2020). Available online: https://clinicaltrials.gov/ct2/show/NCT04374461 (accessed on 5 April 2021).
- Wiest, D.B.; Chang, E.; Fanning, D.; Garner, S.; Cox, T.; Jenkins, D.D. Antenatal pharmacokinetics and placental transfer of N-acetylcysteine in chorioamnionitis for fetal neuroprotection. J. Pediatr. 2014, 165, 672–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buhimschi, C.S.; Bahtiyar, M.O.; Zhao, G.; Abdelghany, O.; Schneider, L.; Razeq, S.A.; Dulay, A.T.; Lipkind, H.S.; Mieth, S.; Rogers, L.; et al. Antenatal N-acetylcysteine to improve outcomes of premature infants with intra-amniotic infection and inflammation (Triple I): Randomized clinical trial. Pediatr. Res. 2021, 89, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Allameh, Z.; Karimi, A.; Rafiei Tabatabaei, S.; Sharifian, M.; Salamzadeh, J. Effect of N-acetylcysteine on inflammation biomarkers in pediatric acute pyelonephritis: A randomized controlled trial. Iran. J. Kidney Dis. 2015, 9, 454–462. [Google Scholar]
- Won, H.R.; Lee, G.H.; Kim, J.H.; Lee, S.H.; Kwon, S.Y.; Baek, S.K.; Ryu, C.H.; Lee, S.J.; Park, I.S.; Shin, S.C.; et al. Effects of N-acetylcysteine inhalation therapy on the quality of life of patients with head and neck cancer who are receiving radiation therapy: A prospective non-randomized controlled multi-center study. J. Cancer Res. Clin. Oncol. 2020, 147, 539–547. [Google Scholar] [CrossRef]
- Sio, T.T.; Blanchard, M.J.; Novotny, P.J.; Patel, S.H.; Rwigema, J.C.M.; Pederson, L.D.; McGee, L.A.; Gamez, M.E.; Seeger, G.R.; Manterson, J.A.; et al. N-acetylcysteine rinse for thick secretion and mucositis of head and neck chemoradiotherapy (Alliance MC13C2): A double-blind randomized clinical trial. In Mayo Clinic Proceedings; Elsevier: Amsterdam, The Netherlands, 2019; Volume 94, pp. 1814–1824. [Google Scholar] [CrossRef]
- Machado, R.C.B.R.; Vargas, H.O.; Urbano, M.R.; Verri, W.A., Jr.; Porcu, M.; Nunes, S.O.V. N-acetylcysteine as an adjunctive treatment for smoking cessation: A randomized clinical trial. Braz. J. Psychiatry 2020, 42, 519–526. [Google Scholar] [CrossRef]
- Jannatifar, R.; Parivar, K.; Roodbari, N.H.; Nasr-Esfahani, M.H. Effects of N-acetyl-cysteine supplementation on sperm quality, chromatin integrity and level of oxidative stress in infertile men. Reprod. Biol. Endocrinol. 2019, 17, 24. [Google Scholar] [CrossRef] [Green Version]
- Nur, E.; Brandjes, D.P.; Teerlink, T.; Otten, H.M.; Oude Elferink, R.P.J.; Muskiet, F.; Evers, L.M.; ten Cate, H.; Biemond, B.J.; Duits, A.J.; et al. N-acetylcysteine reduces oxidative stress in sickle cell patients. Ann. Hematol. 2012, 91, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Pace, B.S.; Shartava, A.; Pack-Mabien, A.; Mulekar, M.; Ardia, A.; Goodman, S.R. Effects of N-acetylcysteine on dense cell formation in sickle cell disease. Am. J. Hematol. 2003, 73, 26–32. [Google Scholar] [CrossRef]
- Motawei, S.M.; Attalla, S.M.; Gouda, H.E.; Harouny, M.A.; Elmansoury, A.M. The effects of N-acetyl cysteine on oxidative stress among patients with pre-eclampsia. Int. J. Gynecol Obstet. 2016, 135, 226–227. [Google Scholar] [CrossRef]
- Roes, E.M.; Raijmakers, M.T.; De Boo, T.M.; Zusterzeel, P.L.; Merkus, H.M.; Peters, W.H.; Steegers, E.A. Oral N-acetylcysteine administration does not stabilise the process of established severe preeclampsia. Eur. J. Obs. Gynecol. Reprod. Biol. 2006, 127, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Rani, M.; Aggarwal, R.; Vohra, K. Effect of N-Acetylcysteine on metabolic profile in metabolic syndrome patients. Metab. Synd. Relat. Disord. 2020, 18341–18346. [Google Scholar] [CrossRef]
- Yalçin, E.; Feyza, A.; Feriha, C.; Okan, A.M. N-acetylcysteine in chronic blepharitis. Cornea 2002, 21, 164–168. [Google Scholar] [CrossRef] [PubMed]
- Akyol-Salman, I.; Azizi, S.; Mumcu, U.; Baykal, O. Efficacy of topical N-acetylcysteine in the treatment of meibomian gland dysfunction. J. Ocul. Pharmacol. Ther. 2010, 26, 329–333. [Google Scholar] [CrossRef]
- Akyol-Salman, I.; Azizi, S.; Mumcu, U.; Baykal, O. Comparison of the efficacy of topical N-acetyl-cysteine and a topical steroid-antibiotic combination therapy in the treatment of meibomian gland dysfunction. J. Ocul. Pharmacol. Ther. 2012, 28, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Rabe, K.F.; Watz, H. Chronic obstructive pulmonary disease. Lancet 2017, 389, 1931–1940. [Google Scholar] [CrossRef]
- Santus, P.; Corsico, A.; Solidoro, P.; Braido, F.; Di Marco, F.; Scichilone, N. Oxidative stress and respiratory system: Pharmacological and clinical reappraisal of N-Acetylcysteine. COPD 2014, 11, 705–717. [Google Scholar] [CrossRef]
- Sadowska, A.M.; Manuel-Y-Keenoy, B.; De Backer, W.A. Antioxidant and anti-inflammatory efficacy of NAC in the treatment of COPD: Discordant in vitro and in vivo dose-effects: A review. Pulm. Pharm. Ther. 2007, 20, 9–22. [Google Scholar] [CrossRef]
- Cazzola, M.; Rogliani, P.; Calzetta, L.; Hanania, N.A.; Matera, M.G. Impact of mucolytic agents on COPD exacerbations: A pair-wise and Network meta-analysis. COPD J. Chronic Obstr. Pulm. Dis. 2017, 14, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Global Initiative for Chronic Obstructive Lung Disease (GOLD). Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Pulmonary Disease. 2021. Available online: https://goldcopd.org/wp-content/uploads/2020/11/GOLD-REPORT-2021-v1.1-25Nov20_WMV.pdf (accessed on 2 April 2021).
- Cantu-Gonzalez, G. 50 years ago in The Journal of Pediatrics: The use of N-acetylcysteine in the treatment of cystic fibrosis. J. Pediatr. 2014, 165, 721. [Google Scholar] [CrossRef]
- Tam, J.; Nash, E.F.; Ratjen, F.; Tullis, E.; Stephenson, A. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst. Rev. 2013, 7, CD007168. [Google Scholar] [CrossRef] [PubMed]
- Nash, E.F.; Stephenson, A.; Ratjen, F.; Tullis, E. Nebulized and oral thiol derivatives for pulmonary disease in cystic fibrosis. Cochrane Database Syst Rev. 2009, 1, CD007168. [Google Scholar] [CrossRef] [Green Version]
- Sun, T.; Liu, J.; Zhao, D.W. Efficacy of N-Acetylcysteine in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Medicine 2016, 95, e3629. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.M.; Nepali, K.; Liou, J.P. Idiopathic pulmonary fibrosis: Current status, recent progress, and emerging targets. J. Med. Chem. 2016, 60, 527–553. [Google Scholar] [CrossRef]
- Paliogiannis, P.; Fois, A.G.; Collu, C.; Bandinu, A.; Zinellu, E.; Carru, C.; Pirina, P.; Mangoni, A.A.; Zinellu, A. Oxidative stress-linked biomarkers in idiopathic pulmonary fibrosis: A systematic review and meta-analysis. Biomark. Med. 2018, 12, 1175–1184. [Google Scholar] [CrossRef]
- Cantin, A.M.; Hubbard, R.C.; Crystal, R.G. Glutathione deficiency in the epithelial lining fluid of the lower respiratory tract in idiopathic pulmonary fibrosis. Am. Rev. Respir. Dis. 1989, 139, 370–372. [Google Scholar] [CrossRef]
- Sochman, J. N-acetylcysteine in acute cardiology: 10 years later: What do we know and what would we like to know? J. Am. Coll. Cardiol. 2002, 39, 1422–1428. [Google Scholar] [CrossRef] [Green Version]
- Pasupathy, S.; Tavella, R.; Grover, S.; Raman, B.; Procter, N.E.K.; Du, Y.T.; Mahadavan, G.; Stafford, I.; Heresztyn, T.; Holmes, A.; et al. Early use of n-acetylcysteine with nitrate therapy in patients undergoing primary percutaneous coronary intervention for st-segment–elevation myocardial infarction reduces myocardial infarct size (the NACIAM Trial [N-acetylcysteine in Acute Myocardial Infarction]). Circulation 2017, 136, 894–903. [Google Scholar] [CrossRef]
- Horowitz, J.D.; Henry, C.A.; Syrjanen, M.L.; Louis, W.J.; Fish, R.D.; Smith, T.W.; Antman, E.M. Combined use of nitroglycerin and N-acetylcysteine in the management of unstable angina pectoris. Circulation 1988, 77, 787–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McColl, A.J.; Keeble, T.; Hadjinikolaou, L.; Cohen, A.; Aitkenhead, H.; Glenville, B.; Richmond, W. Plasma antioxidants: Evidence for a protective role against reactive oxygen species following cardiac surgery. Ann. Clin Biochem. 1998, 35, 616–623. [Google Scholar] [CrossRef] [PubMed]
- Sucu, N.; Cinel, I.; Unlu, A.; Aytacoglu, B.; Tamer, L.; Kocak, Z.; Karaca, K.; Gul, A.; Dikmengil, M.; Atik, U.; et al. N-acetylcysteine for preventing pump-induced oxide inflammatory response during cardiopulmonary bypass. Surg. Today 2004, 34, 237–242. [Google Scholar] [CrossRef]
- Cacciapuoti, F. N-Acetyl-Cysteine supplementation lowers high homocysteine plasma levels and increases Glutathione synthesis in the trans-sulfuration pathway. Ital. J. Med. 2019, 13, 234–240. [Google Scholar] [CrossRef]
- Baker, W.L.; Anglade, M.W.; Baker, E.L.; White, C.M.; Kluger, J.; Coleman, C.I. Use of N-acetylcysteine to reduce post-cardiothoracic surgery complications: A meta-analysis. Eur. J. Cardiothorac. Surg. 2009, 35, 521–527. [Google Scholar] [CrossRef] [PubMed]
- Dean, O.; Giorlando, F.; Berk, M. N-acetylcysteine in psychiatry: Current therapeutic evidence and potential mechanisms of action. J. Psychiatry Neurosci. 2011, 36, 78–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, Y.; Vadodaria, K.C.; Lenkei, Z.; Kato, T.; Gage, F.H.; Marchetto, M.C.; Santos, R. Mitochondria, metabolism, and redox mechanisms in psychiatric disorders. Antioxid. Redox Signal. 2019, 31, 275–317. [Google Scholar] [CrossRef]
- Farokhnia, M.; Azarkolah, A.; Adinehfar, F.; Khodaie-Ardakani, M.R.; Hosseini, S.M.R.; Yekehtaz, H.; Tabrizi, M.; Rezaei, F.; Salehi, B.; Sadeghi, S.M.R.; et al. N-acetylcysteine as an adjunct to risperidone for treatment of negative symptoms in patients with chronic schizophrenia: A randomized, double-blind, placebo-controlled study. Clin. Neuropharmacol. 2013, 36, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Lavoie, S.; Murray, M.M.; Deppen, P.; Knyazeva, M.G.; Berk, M.; Boulat, O.; Bovet, P.; Bush, A.I.; Conus, P.; Copolov, D.; et al. Glutathione precursor, N-acetyl-cysteine, improves mismatch negativity in schizophrenia patients. Neuropsychopharmacology 2008, 33, 2187–2199. [Google Scholar] [CrossRef] [Green Version]
- Retsa, C.; Knebel, J.F.; Geiser, E.; Ferrari, C.; Jenni, R.; Fournier, M.; Alameda, L.; Baumann, P.S.; Clarke, S.; Conus, P.; et al. Treatment in early psychosis with N-acetyl-cysteine for 6 months improves low-level auditory processing: Pilot study. Schizophr. Res. 2018, 191, 80–86. [Google Scholar] [CrossRef] [Green Version]
- Carmeli, C.; Knyazeva, M.G.; Cuénod, M.; Do, K.Q. Glutathione precursor N-acetyl-cysteine modulates EEG synchronization in schizophrenia patients: A double-blind, randomized, placebo-controlled trial. PLoS ONE 2012, 7, e29341. [Google Scholar] [CrossRef] [Green Version]
- McQueen, G.; Lally, J.; Collier, T.; Zelaya, F.; Lythgoe, D.; Barker, G.J.; Stone, J.M.; McGuire, P.; MacCabe, J.H.; Egerton, A. Effects of N-acetylcysteine on brain glutamate levels and resting perfusion in schizophrenia. Psychopharmacology 2018, 235, 3045–3054. [Google Scholar] [CrossRef] [Green Version]
- Moghaddam, B.; Javitt, D. From revolution to evolution: The glutamate hypothesis of schizophrenia and its implication for treatment. Neuropsychopharmacology 2012, 37, 4–15. [Google Scholar] [CrossRef] [Green Version]
- Willborn, R.J.; Hall, C.P.; Fuller, M.A. Recycling N-acetylcysteine: A review of evidence for adjunctive therapy in schizophrenia. Ment. Health Clin. 2019, 9, 116–123. [Google Scholar] [CrossRef]
- Matsuzawa, D.; Hashimoto, K. Magnetic resonance spectroscopy study of the antioxidant defense system in schizophrenia. Antioxid. Redox Signal. 2011, 15, 2057–2065. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Bosker, F.J.; Li, J.; Schoevers, R.A. N-acetylcysteine as add-on to antidepressant medication in therapy refractory major depressive disorder patients with increased inflammatory activity: Study protocol of a double-blind randomized placebo-controlled trial. BMC Psychiatry 2018, 18, 279. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slattery, J.; Kumar, N.; Delhey, L.; Berk, M.; Dean, O.; Spielholz, C.; Frye, R. Clinical trials of N-acetylcysteine in psychiatry and neurology: A systematic review. Neurosci. Biobehav. Rev. 2015, 55, 294–321. [Google Scholar] [CrossRef] [Green Version]
- Paoletti, P. Molecular basis of NMDA receptor functional diversity. Eur. J. Neurosci. 2011, 33, 1351–1365. [Google Scholar] [CrossRef]
- De Farias, C.C.; Maes, M.; Bonifácio, K.L.; Bortolasci, C.C.; Nogueira, A.S.; Brinholi, F.F.; Matsumoto, A.K.; do Nascimento, M.A.; Melo, L.B.; Nixdorf, S.L.; et al. Highly specific changes in antioxidant levels and lipid peroxidation in Parkinson’s disease and its progression: Disease and staging biomarkers and new drug targets. Neurosci. Lett. 2016, 617, 66–71. [Google Scholar] [CrossRef]
- Percário, S.; Barbosa, A.S.; Varela, E.L.P.; Gomes, A.R.Q.; Ferreira, M.E.S.; Moreira, T.N.A.; Dolabela, M.F. Oxidative stress in Parkinson’s disease: Potential benefits of antioxidant supplementation. Oxidative Med. Cell. Longev. 2020, 2020, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Hara, Y.; McKeehan, N.; Dacks, P.A.; Fillit, H.M. Evaluation of the neuroprotective potential of n-acetylcysteine for prevention and treatment of cognitive aging and dementia. J. Prev. Alzheimer’s Dis. 2017, 4, 201–206. [Google Scholar] [CrossRef]
- Choi, I.Y.; Lee, S.P.; Denney, D.R.; Lynch, S.G. Lower levels of glutathione in the brains of secondary progressive multiple sclerosis patients measured by 1H magnetic resonance chemical shift imaging at 3 T. Mult. Scler. 2011, 17, 289–296. [Google Scholar] [CrossRef] [Green Version]
- Goldenberg, M.M. Multiple sclerosis review. Pharm. Ther. 2012, 37, 175–184. [Google Scholar]
- Ibitoye, R.; Kemp, K.; Rice, C.; Hares, K.; Scolding, N.; Wilkins, A. Oxidative stress-related biomarkers in multiple sclerosis: A review. Biomark. Med. 2016, 10, 889–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Plemel, J.R.; Juzwik, C.A.; Benson, C.A.; Monks, M.; Harris, C.; Ploughman, M. Over-the-counter anti-oxidant therapies for use in multiple sclerosis: A systematic review. MS J. 2015, 21, 1485–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asrani, S.K.; Devarbhavi, H.; Eaton, J.; Kamath, P.S. Burden of liver diseases in the world. J. Hepatol. 2019, 70, 151–171. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Michalak, A. Oxidative stress as a crucial factor in liver diseases. World J. Gastroenterol. 2014, 20, 8082–8091. [Google Scholar] [CrossRef]
- Harrison, P.M.; Wendon, J.A.; Gimson, A.E.; Alexander, G.J.; Williams, R. Improvement by acetylcysteine of hemodynamics and oxygen transport in fulminant hepatic failure. N. Engl. J. Med. 1991, 324, 1852–1857. [Google Scholar] [CrossRef]
- Darweesh, S.K.; Ibrahim, M.F.; El-Tahawy, M.A. Effect of N-Acetylcysteine on mortality and liver transplantation rate in non-acetaminophen-induced acute liver failure: A multicenter study. Clin. Drug Investig. 2017, 37, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Walayat, S.; Shoaib, H.; Asghar, M.; Kim, M.; Dhillon, S. Role of N-acetylcysteine in non-acetaminophen-related acute liver failure: An updated meta-analysis and systematic review. Ann. Gastroenterol. 2021, 34, 235–240. [Google Scholar] [CrossRef]
- Dludla, P.V.; Nkambule, B.B.; Mazibuko-Mbeje, S.E.; Nyambuya, T.M.; Marcheggiani, F.; Cirilli, I.; Ziqubu, K.; Shabalala, S.C.; Johnson, R.; Louw, J.; et al. N-Acetyl cysteine targets hepatic lipid accumulation to curb oxidative stress and inflammation in NAFLD: A comprehensive analysis of the literature. Antioxidants 2020, 9, 1283. [Google Scholar] [CrossRef] [PubMed]
- Ayhan, B.; Pamuk, G.; Kantar, B.; Kanbak, M.; Celebioglu, B.; Aypar, U. Renal functional effects of using N-acetylcysteine in cardiac surgery. Anesth. J. 2012, 20, 159–167. [Google Scholar]
- Santana-Santos, E.; Gowdak, L.H.; Gaiotto, F.A.; Puig, L.B.; Hajjar, L.A.; Zeferino, S.P.; Drager, L.F.; Shimizu, M.H.M.; Bortolotto, L.A.; De Lima, J.J.G. High dose of N-acetylcysteine prevents acute kidney injury in chronic kidney disease patients undergoing myocardial revascularization. Ann. Thorac. Surg. 2014, 97, 1617–1623. [Google Scholar] [CrossRef]
- Mei, M.; Zhao, H.W.; Pan, Q.G.; Pu, Y.M.; Tang, M.Z.; Shen, B.B. Efficacy of N-acetylcysteine in preventing acute kidney injury after cardiac surgery: A meta-analysis study. J. Investig. Surg. 2018, 31, 14–23. [Google Scholar] [CrossRef]
- Leja, M.; Grinberga-Derica, I.; Bilgilier, C.; Steininger, C. Review: Epidemiology of Helicobacter pylori infection. Helicobacter 2019, 24, e12635. [Google Scholar] [CrossRef] [Green Version]
- Kotilea, K.; Bontems, P.; Touati, E. Epidemiology, diagnosis and risk factors of Helicobacter pylori infection. Helicobacter Pylori Hum. Dis. 2019, 1149, 17–33. [Google Scholar] [CrossRef]
- De Brito, B.B.; Da Silva, F.A.F.; Soares, A.S.; Pereira, V.A.; Santos, M.L.C.; Sampaio, M.M.; Neves, P.H.M.; De Melo, F.F. Pathogenesis and clinical management of Helicobacter pylori gastric infection. World J. Gastroenterol. 2019, 25, 5578. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Leontiadis, G.; Howden, C.W.; Moss, S.F. ACG clinical guideline: Treatment of Helicobacter pylori infection. Am. J. Gastroenterol. 2017, 112, 212–239. [Google Scholar] [CrossRef] [PubMed]
- Malfertheiner, P.; Megraud, F.; O’Morain, C.A.; Gisbert, J.P.; Kuipers, E.J.; Axon, A.T.; Bazzoli, F.; Gasbarrini, A.; Atherton, J.; Graham, D.Y.; et al. Management of Helicobacter pylori infection—The Maastricht V/Florence consensus report. Gut 2017, 66, 6–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.J.; Kook, M.C.; Kim, Y.I.; Cho, S.J.; Lee, J.Y.; Kim, C.G.; Park, B.; Nam, B.H. Helicobacter pylori therapy for the prevention of metachronous gastric cancer. N. Engl. J. Med. 2018, 378, 1085–1095. [Google Scholar] [CrossRef]
- Makipour, K.; Friedenberg, F.K. The potential role of N-acetylcysteine for the treatment of Helicobacter pylori. J. Clin. Gastroenterol. 2011, 45, 841–843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, C.; Yi, J.; Nie, M.; Huang, M.; Rong, J.; Zhu, Z.; Chen, J.; Zhou, X.; Li, B.; Chen, H.; et al. N-acetylcysteine reduces ROS-mediated oxidative DNA damage and PI3K/Akt pathway activation induced by helicobacter pylori infection. Oxidative Med. Cell. Longev. 2018, 2018, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, S.; Bak, E.J.; Cha, J.H. N-acetylcysteine prevents the development of gastritis induced by Helicobacter pylori infection. J. Microbiol. 2017, 55, 396–402. [Google Scholar] [CrossRef]
- Fontes, L.E.S.; Martimbianco, A.L.C.; Zanin, C.; Riera, R. N-acetylcysteine as an adjuvant therapy for Helicobacter pylori eradication. Cochrane Database Syst. Rev. 2019, 2, 1–71. [Google Scholar] [CrossRef]
- Chen, C.C.; Luo, J.C.; Fang, Y.J.; Lee, J.Y.; Kuo, C.C.; Yang, T.H.; Chiu, M.C.; Yu, J.J.; Bair, M.J.; Chen, P.Y.; et al. Comparison of the effect of clarithromycin triple therapy with or without N-acetylcysteine in the eradication of Helicobacter pylori: A randomized controlled trial. Ther. Adv. Gastroenterol. 2020, 13, 1756284820927306. [Google Scholar] [CrossRef]
- Romagnoli, C.; Marcucci, T.; Picariello, L.; Tonelli, F.; Vincenzini, M.T.; Iantomasi, T. Role of N-acetylcysteine and GSH redox system on total and active MMP-2 in intestinal myofibroblasts of Crohn’s disease patients. Int. J. Colorectal Dis. 2013, 28, 915–924. [Google Scholar] [CrossRef] [Green Version]
- Fontani, F.; Marcucci, T.; Picariello, L.; Tonelli, F.; Vincenzini, M.T.; Iantomasi, T. Redox regulation of MMP-3/TIMP-1 ratio in intestinal myofibroblasts: Effect of N-acetylcysteine and curcumin. Exp. Cell Res. 2014, 323, 77–86. [Google Scholar] [CrossRef]
- Schauble, A.L.; Bisaccia, E.K.; Lee, G.; Nasr, S.Z. N-acetylcysteine for management of distal intestinal obstruction syndrome. J. Pediatric Pharmacol. Ther. 2019, 24, 390–397. [Google Scholar] [CrossRef]
- Chilvers, N.J.S.; Wheeler, J. Intraoperative intraluminal injection of N-acetylcysteine allowing treatment of distal intestinal obstruction syndrome without the need for enterotomy. Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef]
- Mccarty, M.F.; Lerner, A. Perspective: Prospects for nutraceutical support of intestinal barrier function. Adv. Nutr. 2021, 12, 316–324. [Google Scholar] [CrossRef]
- Koch, A.; Trautwein, C. N-acetylcysteine on its way to a broader application in patients with acute liver failure. Hepatology 2010, 51, 338–340. [Google Scholar] [CrossRef]
- Amaral, E.P.; Conceição, E.L.; Costa, D.L.; Rocha, M.S.; Marinho, J.M.; Cordeiro-Santos, M.; D’Império-Lima, M.R.; Barbosa, T.; Sher, A.; Andrade, B.B. N-acetyl-cysteine exhibits potent anti-mycobacterial activity in addition to its known anti-oxidative functions. BMC Microbiol. 2016, 16, 1–10. [Google Scholar] [CrossRef]
- Jorge-Aarón, R.M.; Rosa-Ester, M.P. N-acetylcysteine as a potential treatment for novel coronavirus disease 2019. Future Microbiol. 2020, 15, 959–962. [Google Scholar] [CrossRef]
- De Flora, S.; Balansky, R.; La Maestra, S. Rationale for the use of N-acetylcysteine in both prevention and adjuvant therapy of COVID-19. FASEB J. 2020, 34, 13185–13193. [Google Scholar] [CrossRef]
- Poe, F.L.; Corn, J. N-Acetylcysteine: A potential therapeutic agent for SARS-CoV-2. Med. Hypotheses 2020, 143, 109862. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, A.; Samimagham, H.R.; Azad, M.H.; Hooshyar, D.; Arabi, M.; KazemiJahromi, M. The efficacy of N-Acetylcysteine in severe COVID-19 patients: A structured summary of a study protocol for a randomised controlled trial. Trials 2021, 22, 271. [Google Scholar] [CrossRef]
- Jenkins, D.D.; Wiest, D.B.; Mulvihill, D.M.; Hlavacek, A.M.; Majstoravich, S.J.; Brown, T.R.; Taylor, J.J.; Buckley, J.R.; Turner, R.P.; Rollins, L.G.; et al. Fetal and neonatal effects of n-acetylcysteine when used for neuroprotection in maternal chorioamnionitis. J. Pediatr. 2016, 168, 67–76. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Outschoorn, U.E.; Peiris-Pages, M.; Pestell, R.G.; Sotgia, F.; Lisanti, M.P. Cancer metabolism: A therapeutic perspective. Nat. Rev. Clin. Oncol. 2016, 14, 11–31. [Google Scholar] [CrossRef]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Romero, I.L.; Mukherjee, A.; Kenny, H.A.; Litchfield, L.M.; Lengyel, E. Molecular pathways: Trafficking of metabolic resources in the tumor microenvironment. Clin. Cancer Res. 2015, 21, 680–686. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.; Hamilton, S.; Angel, D.; Fung, K.; Franklin, J.; Parnes, L.S.; Lewis, D.; Venkatesan, V.; Winquist, E. Cisplatin otoprotection using transtympanic L-N-acetylcysteine: A pilot randomized study in head and neck cancer patients. Laryngoscope 2014, 124, E87–E94. [Google Scholar] [CrossRef]
- Monti, D.; Sotgia, F.; Whitaker-Menezes, D.; Tuluc, M.; Birbe, R.; Berger, A.; Lazar, M.; Cotzia, P.; Draganova-Tacheva, R.; Lin, Z.; et al. Pilot study demonstrating metabolic and anti-proliferative effects of in vivo antioxidant supplementation with N-Acetylcysteine in breast cancer. Semin Oncol. 2017, 44, 226–232. [Google Scholar] [CrossRef] [PubMed]
- Manfredi, G.; Bertè, R.; Iiritano, S.; Londoni, C.; Brambilla, G.; Romeo, S.; Menozzi, F.; Griffanti, P.; Brandi, G.; Moreschi, O.; et al. Premedication with simethicone and N-acetylcysteine for improving mucosal visibility during upper gastrointestinal endoscopy in a Western population. Endosc. Int. Open 2021, 9, E190–E194. [Google Scholar] [CrossRef]
- Zhang, L.; Cheng, J.; Ji, M.; Wu, S.; Zhai, H.; Zhang, S. IDDF2019-ABS-0311 Efficacy and cost-effectiveness of premedication with N-acetylcysteine during upper gastrointestinal endoscopy examination: A single center, prospective, single blinded, randomized controlled trial. Gut 2019, 68, A117–A118. [Google Scholar]
- Lee, Y.J.; Lee, D.M.; Lee, C.H.; Heo, S.H.; Won, S.Y.; Im, J.H.; Cho, M.K.; Nam, H.S.; Lee, S.H. Suppression of human prostate cancer PC-3 cell growth by N-acetylcysteine involves over-expression of Cyr61. Toxicol. Vitro 2011, 25, 199–205. [Google Scholar] [CrossRef]
- Deng, J.; Liu, A.D.; Hou, G.Q.; Zhang, X.; Ren, K.; Chen, X.Z.; Li, S.S.C.; Wu, Y.S.; Cao, X. N-acetylcysteine decreases malignant characteristics of glioblastoma cells by inhibiting Notch2 signaling. J. Exp. Clin. Cancer Res. 2019, 38, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Adil, M.; Amin, S.S.; Mohtashim, M. N-acetylcysteine in dermatology. Indian J. Dermatol. Venereol. Leprol. 2018, 84, 652–659. [Google Scholar] [CrossRef]
- Lee, T.M.; Lee, K.M.; Lee, C.Y.; Lee, H.C.; Tam, K.W.; Loh, E.W. Effectiveness of N-acetylcysteine in autism spectrum disorders: A meta-analysis of randomized controlled trials. Aust. N. Z. J. Psychiatr. 2021, 55, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Ghafarizadeh, A.; Malmir, M.; Noreini, S.N.; Faraji, T. Antioxidant effects of N-acetylcysteine on the male reproductive system: A systematic review. Andrologia 2021, 53, e13898. [Google Scholar] [CrossRef] [PubMed]
- Sins, J.W.R.; Fijnvandraat, K.; Rijneveld, A.W.; Boom, M.B.; Kerkhoffs, J.L.; van Meurs, A.H.; De Groot, M.R.; Heijboer, H.; Dresse, M.F.; Ferster, A.; et al. N-Acetylcysteine in patients with sickle cell disease: A randomized controlled trial. Blood 2016, 128. [Google Scholar] [CrossRef]
- Moazzen, H.; Lu, X.; Ma, N.L.; Velenosi, T.J.; Urquhart, B.L.; Wisse, L.J.; Gittenberger-de Groot, A.C.; Feng, Q. N-Acetylcysteine prevents congenital heart defects induced by pregestational diabetes. Cardiovasc. Diabetol. 2014, 13, 46. [Google Scholar] [CrossRef] [Green Version]
- Braun, T.L.; Patel, V.; DeBord, L.C.; Rosen, T. A review of N-acetylcysteine in the treatment of grooming disorders. Int. J. Dermatol. 2019, 58, 502–510. [Google Scholar] [CrossRef]
- Pokupec, R.; Petricek, I.; Bradic, M.; Popovic-Suic, S.; Petricek, G. Comparison of local acetylcysteine and artificial tears in the management of dry eye syndrome. Acta Med. Croat. 2005, 59, 337–340. [Google Scholar]



| Reactive Species | Rate Constant (M−1·s−1) | Experimental Conditions | Ref. |
|---|---|---|---|
| •OH | 1.36 × 1010 | pH 7, rt | [37] |
| •NO2 | ≈2.4 × 108a ≈1.0 × 107 b | pH > pKa, rt pH 7.4, rt | [38] [39] |
| CO3•− | ≈1.0 × 107 1.8 × 108 | pH 7, rt pH 12, rt | [40] |
| HNO | 5 × 105 | pH 7.4, 37 °C | [41] |
| O2•− | 68 ± 6 × 103 | pH 7, rt pH 7.4, 25 °C | [37] [42] |
| H2O2 | 0.16 ± 0.01 0.85 ± 0.09 | pH 7.4, 37 °C pH 7.4, 25 °C | [37] [43] |
| ONOO− | 415 ± 10 | pH 7.4, 37 °C | [44] |
| Disease | Study Type | Dose | Treatment Type | Administration Routes | References |
|---|---|---|---|---|---|
| Lung diseases | |||||
| Chronic obstructive pulmonary disease | Clinical trial | 600 mg/day | 3 years | Oral | [51] |
| Clinical trial | 600 mg/day | 3 years | Oral | [52] | |
| Clinical trial NCT01136239 | 1200 mg/day | 1 year | Oral | [53] | |
| Clinical trial ChiCTR-TRC-09000460 | 1200 mg/day | 1 year | Oral | [54] | |
| Clinical trial NCT01599884 | 3600 mg/day | 8 weeks | Oral | [55] | |
| Systematic review with meta-analysis | >600 mg/day | Long term | Oral | [56] | |
| Systematic review with meta-analysis | ≥1200 mg/day | Long term | Oral | [57] | |
| Systematic review | Low doses: ≤600 mg/day High doses: >600 mg/day | Minimum of 6 months | Oral | [58] | |
| Cystic fibrosis | Systematic review with meta-analysis | 600 mg to 2800 mg/day | 3.9 and 12 months | Oral or inhalation | [59] |
| Clinical trial | 1800, 2400 and 3000 mg/day | 4 weeks | Oral | [60] | |
| Clinical trial 2007-001401-15 | 2400 mg/day | 4 weeks | Oral | [61] | |
| Idiopathic pulmonary fibrosis | Systematic review with meta-analysis | 704.8 to 1800 mg/day | - | Oral or inhalation | [62] |
| Systematic review with meta-analysis | Oral doses: 1800 mg/day Inhalation doses: 704.8 mg | 3 to 15 months | Oral or inhalation | [28] | |
| Cardiovascular diseases | Clinical trial | 15,000 mg/day | 24 h | Intravenous | [63] |
| Clinical trial | 15,000 mg/day | 24 h | Intravenous | [64] | |
| Cardiac surgery | Systematic review with meta-analysis | <100 mg/kg/ to ≥300 mg/kg/day | <24 and >48 h | Oral and/ or intravenous | [65] |
| Systematic review with meta-analysis | 50 mg/kg to 600 mg | Until 48 h | Oral and/ or intravenous | [66] | |
| Clinical trial | 50–150 mg/kg | 1 h preoperatively and 48 h postoperatively | intravenous | [67] | |
| Psychiatric illnesses | |||||
| Schizophrenia | Multicenter clinical trial | 2000 mg/day | 4 weeks | Oral | [68] |
| Clinical trial IRCT:2015080223463 | 1200 mg/day | 12 weeks | Oral | [69] | |
| Systematic review with meta-analysis | 600 mg to 3600 mg/day | ≥24 weeks | Oral | [70] | |
| Clinical trial NCT01354132 | 2700 mg/day | 6 months | Oral | [71] | |
| Bipolar disorder | Clinical trial ACTRN12612000830897 | 2000 mg/day | 16 weeks | Oral | [72] |
| Clinical trial 12605000362695 | 1000 mg/day | 24 weeks | Oral | [73] | |
| Depression | Clinical trial ACTRN12607000134426 | 1000 mg/day | 12 weeks | Oral | [74] |
| Systematic review with meta-analysis | 1000 mg to 3000 mg/day | - | Oral | [75] | |
| Neurodegenerative diseases | |||||
| Parkinson’s disease | Clinical trial NCT01427517 | 150 mg/kg | 1 h | Intravenous | [76] |
| Clinical trial NCT02445651 | Intravenous doses: 50 mg/kg Oral doses: 1000 mg/day | 3 months | Oral and intravenous | [77] | |
| Clinical trial NCT02212678 | 6000 mg/day | 4 weeks | Oral | [78] | |
| Alzheimer’s disease | Clinical trial NCT00903695 | 600 mg/day | 6 months | Oral | [79] |
| Multiple sclerosis | Clinical trial NCT02804594 | 1250 mg/day | 4 weeks | Oral | [80] |
| Clinical trial NCT03032601 | Intravenous doses: 50 mg/kg (once a week) Oral doses: 1000 mg/day (6 times a week) | 2 months | Oral and Intravenous | [81] | |
| Liver diseases | |||||
| Paracetamol poisoning | Guide clinical practice | 200 mg/kg in 4 h, then 100 mg/kg in 16 h | 20 h | Intravenous | [82] |
| Acute liver failure | Randomized case control study | 150 mg/kg over 1 h, followed by 12.5 mg/kg/h for 4 h and continuous infusion of 6.25 mg/kg/h for the remaining 67 h | 72 h | Intravenous | [83] |
| Systematic review | 150 mg/ kg over 1 h, followed by 12.5 mg/kg/h for 4 h, and continuous infusions of 6.25 mg/kg/h for the remaining 67 h | 72 h | Intravenous | [84] | |
| Nonalcoholic fatty liver disease | Clinical trial | 600 mg/day | 3 months | Oral | [85] |
| Acute alcoholic hepatitis | Multicenter clinical trial NCT00863785 | Day 1: at a dose of 150, 50, and 100 mg/kg a period of 30 min, 4 h, and 16 h, respectively; Days 2 through 5: 100 mg/kg | 28 days | Intravenous | [86] |
| Multicenter clinical trial | 300 mg/kg | 14 days | Intravenous | [87] | |
| Kidney diseases | |||||
| Chronic renal patients undergoing cardiac | Systematic review with meta-analysis | 150 mg/kg to 1200 mg | 6 h to 7 days | Intravenous | [88] |
| Chronic kidney disease | Clinical trial | 5000 mg | One hemodialysis session | Intravenous | [89] |
| Clinical trial | 600 mg/day | 2 doses with 1 week interval | Oral | [90] | |
| Gastrointestinal diseases | |||||
| Helicobacter pylori infection | Clinical trial | 600 mg/day | 14 days | Oral | [91] |
| Clinical trial | 800 mg/day | 5 days | Oral | [92] | |
| Colon cancer associated with colitis | Systematic review | 8000 mg to 1200 mg/day | 7 days to 12 weeks | Oral | [93] |
| Gastrointestinal cancer | Clinical trial | 1200 mg/day | 2 days before surgery until the fifth day postoperatively | Schematic parenteral | [94] |
| Clinical trial | 800 mg/day | 12 weeks | Oral | [95] | |
| Ulcerative colitis | Clinical trial | 800 mg/day | 4 weeks | Oral | [96] |
| Infectious diseases | |||||
| Chronic hepatitis B | Clinical trial | 8000 mg/day | 28 days | Intravenous | [97] |
| Pulmonary tuberculosis | Clinical trial | 600 mg/day | 2 months | Oral | [98] |
| SARS-CoV-2 | Clinical trial NCT04374461 | 6000 mg/day | 3 weeks | Intravenous | [99] |
| Chorioamnionitis | Clinical trial | 100 mg/kg/dose and 12.5–25 mg/kg/dose their infants | Diagnosis until delivery and every 12 h for 5 doses to their infants | Intravenous | [100] |
| Infection and/or intrauterine inflammation | Clinical trial NCT00397735 | 150 mg/kg administered in 1 h, followed by continuous infusion of NAC (50 mg/kg) for 4 h and infusion of 100 mg/kg of NAC in the next 16 h or until delivery | - | Intravenous | [101] |
| Acute pyelonephritis | Clinical trial NCT02080182 | Children with a body weight equal to or greater than 30 kg, 900 mg/day, children weighing between 8.5–30 kg, 600 mg/day, and those weighing less than 8.5 kg, 70 mg/kg/day | 5 days | Oral | [102] |
| Cancer prevention and treatment | |||||
| Radiation therapy, head and neck cancer | Prospective, controlled multicenter study | 2400 mg/day | 8 weeks | Inhalation therapy | [103] |
| Clinical trial NCT02123511 | 2500 mg/day | - | Rinsing | [104] | |
| Other conditions | |||||
| Smoking cessation | Clinical trial NCT02420418 | 1800 mg/day | 12 weeks | Oral | [105] |
| Male fertility | Clinical trial IRCT20170830035998N4 | 600 mg/day | 3 months | Oral | [106] |
| Sickle cell anemia | Clinical trial NTR1013 | 1200 or 2400 mg/day | 6 weeks | Oral | [107] |
| Clinical trial | 600 mg, 1200 mg or 2400 mg/day | 7 months | Oral | [108] | |
| Pre-eclampsia | Clinical trial | 400 mg/day | 6 weeks | Oral | [109] |
| Clinical trial | 600 mg/day | - | Oral | [110] | |
| Metabolic syndrome | Pilot study | 1200 mg/day | 6 weeks | Oral | [111] |
| Ocular condtions | |||||
| Chronic blepharitis | Clinical trial | 100 mg/day | 1 to 4 months | Oral | [112] |
| Meibomian gland dysfunction | Clinical trial | 5% | 1 month | Eye drops | [113] |
| Clinical trial | 5% | 1 month | Eye drops | [114] | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenório, M.C.d.S.; Graciliano, N.G.; Moura, F.A.; Oliveira, A.C.M.d.; Goulart, M.O.F. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants 2021, 10, 967. https://doi.org/10.3390/antiox10060967
Tenório MCdS, Graciliano NG, Moura FA, Oliveira ACMd, Goulart MOF. N-Acetylcysteine (NAC): Impacts on Human Health. Antioxidants. 2021; 10(6):967. https://doi.org/10.3390/antiox10060967
Chicago/Turabian StyleTenório, Micaely Cristina dos Santos, Nayara Gomes Graciliano, Fabiana Andréa Moura, Alane Cabral Menezes de Oliveira, and Marília Oliveira Fonseca Goulart. 2021. "N-Acetylcysteine (NAC): Impacts on Human Health" Antioxidants 10, no. 6: 967. https://doi.org/10.3390/antiox10060967