Pharmacological Modulation of the MIP-1 Family and Their Receptors Reduces Neuropathic Pain Symptoms and Influences Morphine Analgesia: Evidence from a Mouse Model
Abstract
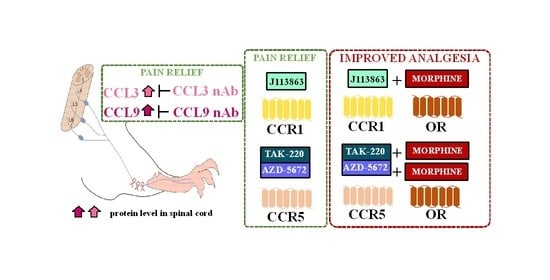
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Chronic Constriction Injury
2.3. Biochemical Tests
2.3.1. Analysis of Gene Expression via RT-qPCR
2.3.2. Enzyme-Linked Immunosorbent Assay (ELISA) Analysis
2.3.3. Western Blot Analysis
2.4. Single Intrathecal Drug Administration in the Mouse Model of Neuropathy
2.4.1. Administration of CCL3 and CCL9 Neutralizing Antibodies
2.4.2. Administration of CCL3 Neutralizing Antibody with Morphine
2.4.3. Administration of CCL9 Neutralizing Antibody with Morphine
2.4.4. Administration of CCR1 and CCR5 Antagonists
2.4.5. Administration of CCR1 Antagonist with Morphine
2.4.6. Administration of CCR5 Antagonists with Morphine
2.4.7. Coadministration of CCR1 and CCR5 Antagonists
2.5. Behavioral Tests
2.5.1. Von Frey Test
2.5.2. Cold Plate Test
2.6. Statistical Analysis
3. Results
3.1. Temporal Changes in the mRNA and/or Protein Levels of Olig2, TMEM119, IBA1, and GFAP Measured in Parallel with Pain-Related Behavior after Chronic Constriction Injury of the Sciatic Nerve in Mice
3.2. Temporal Changes in the mRNA and Protein Levels of CCL3, CCL4, and CCL9 after Chronic Constriction Injury of the Sciatic Nerve in Mice
3.3. Effects of a Single Intrathecal Administration of CCL3 nAb on Pain-Related Behavior and Morphine Analgesia 7 Days after Chronic Constriction Injury of the Sciatic Nerve in Mice
3.4. Effects of a Single Intrathecal Administration of CCL9 nAb on Pain-Related Behavior and Morphine Analgesia 7 Days after Chronic Constriction Injury of the Sciatic Nerve in Mice
3.5. Temporal Changes in the mRNA and Protein Levels of CCR1 and CCR5 after Chronic Constriction Injury of the Sciatic Nerve in Mice
3.6. Effects of a Single Intrathecal J113863 Administration on Pain-Related Behavior and Morphine Analgesia 7 Days after Chronic Constriction Injury of the Sciatic Nerve in Mice
3.7. Effects of a Single Intrathecal TAK-220 Administration on Pain-Related Behavior and Morphine Analgesia 7 Days after Chronic Constriction Injury of the Sciatic Nerve in Mice
3.8. Effects of a Single Intrathecal AZD-5672 Administration on Pain-Related Behavior and Morphine Analgesia 7 Days after Chronic Constriction Injury of the Sciatic Nerve in Mice
3.9. Comparison of the Effects of Intrathecal Administration of Substances Targeting CCR1 (J113863), CCR5 (TAK-220/AZD-5672), and Their Combination (J11 + TAK-220 or J11 + AZD-5672) on Pain-Related Behavior 7 Days after Chronic Constriction Injury of the Sciatic Nerve in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef]
- Martin, T.J.; Eisenach, J.C. Pharmacology of opioid and nonopioid analgesics in chronic pain states. J. Pharmacol. Exp. Ther. 2001, 299, 811–817. [Google Scholar]
- Finnerup, N.B.; Kuner, R.; Jensen, T.S. Neuropathic pain: From mechanisms to treatment. Physiol. Rev. 2021, 101, 259–301. [Google Scholar] [CrossRef]
- von Bartheld, C.S.; Bahney, J.; Herculano-Houzel, S. The search for true numbers of neurons and glial cells in the human brain: A review of 150 years of cell counting. J. Comp. Neurol. 2016, 524, 3865–3895. [Google Scholar] [CrossRef] [Green Version]
- Mika, J.; Zychowska, M.; Popiolek-Barczyk, K.; Rojewska, E.; Przewlocka, B. Importance of glial activation in neuropathic pain. Eur. J. Pharmacol. 2013, 716, 106–119. [Google Scholar] [CrossRef]
- Ji, R.R.; Donnelly, C.R.; Nedergaard, M. Astrocytes in chronic pain and itch. Nat. Rev. Neurosci. 2019, 20, 667–685. [Google Scholar] [CrossRef]
- Zarpelon, A.C.; Rodrigues, F.C.; Lopes, A.H.; Souza, G.R.; Carvalho, T.T.; Pinto, L.G.; Xu, D.; Ferreira, S.H.; Alves-Filho, J.C.; McInnes, I.B.; et al. Spinal cord oligodendrocyte-derived alarmin IL-33 mediates neuropathic pain. FASEB J. 2016, 30, 54–65. [Google Scholar] [CrossRef] [Green Version]
- Charo, I.F.; Ransohoff, R.M. The many roles of chemokines and chemokine receptors in inflammation. N. Engl. J. Med. 2006, 354, 610–621. [Google Scholar] [CrossRef]
- Zychowska, M.; Rojewska, E.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Spinal CCL1/CCR8 signaling interplay as a potential therapeutic target—Evidence from a mouse diabetic neuropathy model. Int. Immunopharmacol. 2017, 52, 261–271. [Google Scholar] [CrossRef]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Nalepa, I.; Mika, J. Pharmacological blockade of spinal CXCL3/CXCR2 signaling by NVP CXCR2 20, a selective CXCR2 antagonist, reduces neuropathic pain following peripheral nerve injury. Front. Immunol. 2019, 10, 2198. [Google Scholar] [CrossRef] [Green Version]
- Zychowska, M.; Rojewska, E.; Piotrowska, A.; Kreiner, G.; Mika, J. Microglial inhibition influences XCL1/XCR1 expression and cause analgesic effects in a mouse model of diabetic neuropathy. Anethesiology 2016, 125, 573–589. [Google Scholar] [CrossRef] [Green Version]
- Ciechanowska, A.; Rojewska, E.; Piotrowska, A.; Barut, J.; Pawlik, K.; Ciapała, K.; Kreiner, G.; Mika, J. New insights into the analgesic properties of the XCL1/XCR1 and XCL1/ITGA9 axes modulation under neuropathic pain conditions—Evidence from animal studies. Front. Immunol. 2022, 13, 1058204. [Google Scholar] [CrossRef]
- Rojewska, E.; Zychowska, M.; Piotrowska, A.; Kreiner, G.; Nalepa, I.; Mika, J. Involvement of Macrophage Inflammatory Protein-1 Family Members in the Development of Diabetic Neuropathy and Their Contribution to Effectiveness of Morphine. Front. Immunol. 2018, 9, 494. [Google Scholar] [CrossRef] [Green Version]
- Kwiatkowski, K.; Ciapała, K.; Rojewska, E.; Makuch, W.; Mika, J. Comparison of the beneficial effects of RS504393, maraviroc and cenicriviroc on neuropathic pain-related symptoms in rodents: Behavioral and biochemical analyses. Int. Immunopharmacol. 2020, 84, 106540. [Google Scholar] [CrossRef]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Kwiatkowski, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. CCR4 Antagonist (C021) Administration Diminishes Hypersensitivity and Enhances the Analgesic Potency of Morphine and Buprenorphine in a Mouse Model of Neuropathic Pain. Front. Immunol. 2020, 11, 1241. [Google Scholar] [CrossRef]
- Pawlik, K.; Ciapała, K.; Ciechanowska, A.; Kwiatkowski, K.; Mika, J. Pharmacological Evidence of the Important Roles of CCR1 and CCR3 and Their Endogenous Ligands CCL2/7/8 in Hypersensitivity Based on a Murine Model of Neuropathic Pain. Cells 2022, 12, 98. [Google Scholar] [CrossRef]
- Sun, S.; Chen, D.; Lin, F.; Chen, M.; Yu, H.; Hou, L.; Li, C. Role of interleukin-4, the chemokine CCL3 and its receptor CCR5 in neuropathic pain. Mol. Immunol. 2016, 77, 184–192. [Google Scholar] [CrossRef]
- Pawlik, K.; Piotrowska, A.; Kwiatkowski, K.; Ciapała, K.; Popiolek-Barczyk, K.; Makuch, W.; Mika, J. The blockade of CC chemokine receptor type 1 influences the level of nociceptive factors and enhances opioid analgesic potency in a rat model of neuropathic pain. Immunology 2020, 159, 413–428. [Google Scholar] [CrossRef]
- Bennett, G.J.; Xie, Y.K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain 1988, 33, 87–107. [Google Scholar] [CrossRef]
- Piotrowska, A.; Ciapała, K.; Pawlik, K.; Kwiatkowski, K.; Rojewska, E.; Mika, J. Comparison of the effects of chemokine receptors CXCR2 and CXCR3 pharmacological modulation in neuropathic pain model—In vivo and in vitro study. Int. J. Mol. Sci. 2021, 22, 11074. [Google Scholar] [CrossRef]
- Chomczynski, P.; Sacchi, N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 1987, 162, 156–159. [Google Scholar] [CrossRef]
- CCL3 Antibody. Available online: https://www.rndsystems.com/products/mouse-ccl3-mip-1alpha-antibody_af-450-na (accessed on 7 March 2023).
- CCL9 Antibody. Available online: https://www.rndsystems.com/products/mouse-ccl9-10-mip-1gamma-antibody_af463 (accessed on 7 March 2023).
- Pawlik, K.; Ciechanowska, A.; Ciapała, K.; Rojewska, E.; Makuch, W.; Mika, J. Blockade of CC Chemokine Receptor Type 3 Diminishes Pain and Enhances Opioid Analgesic Potency in a Model of Neuropathic Pain. Front. Immunol. 2021, 12, 781310. [Google Scholar] [CrossRef]
- J 113863. Available online: https://www.tocris.com/products/j-113863_2595 (accessed on 7 March 2023).
- TAK-220. Available online: https://www.medchemexpress.com/TAK-220.html (accessed on 7 March 2023).
- AZD-5672. Available online: https://www.medchemexpress.com/azd-5672.html (accessed on 7 March 2023).
- Colloca, L.; Ludman, T.; Bouhassira, D.; Baron, R.; Dickenson, A.H.; Yarnitsky, D.; Freeman, R.; Truini, A.; Attal, N.; Finnerup, N.B.; et al. Neuropathic Pain. Nat. Rev. Dis. Primers 2017, 3, 17002. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.J.; Ji, R.R. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol. Ther. 2010, 126, 56–68. [Google Scholar] [CrossRef] [Green Version]
- Biber, K.; Boddeke, E. Neuronal CC chemokines: The distinct roles of CCL21 and CCL2 in neuropathic pain. Front. Cell. Neurosci. 2014, 8, 210. [Google Scholar] [CrossRef] [Green Version]
- Brion, C.; Lutz, S.M.; Albert, F.W. Simultaneous quantification of mrna and protein in single cells reveals post-transcriptional effects of genetic variation. eLife 2020, 9, 60645. [Google Scholar] [CrossRef]
- Ellis, A.; Bennett, D.L.H. Neuroinflammation and the generation of neuropathic pain. Br. J. Anaesth. 2013, 111, 128. [Google Scholar] [CrossRef] [Green Version]
- Kohno, H.; Maeda, T.; Perusek, L.; Pearlman, E.; Maeda, A. CCL3 Production by Microglial Cells Modulates Disease Severity in Murine Models of Retinal Degeneration. J. Immunol. 2014, 192, 3816–3827. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, A.; Tozaki-Saitoh, H.; Koga, Y.; Tsuda, M.; Inoue, K. Activation of P2X7 receptors induces CCL3 production in microglial cells through transcription factor NFAT. J. Neurochem. 2009, 108, 115–125. [Google Scholar] [CrossRef]
- Simpson, J.E.; Newcombe, J.; Cuzner, M.L.; Woodroofe, M.N. Expression of monocyte chemoattractant protein-1 and other beta-chemokines by resident glia and inflammatory cells in multiple sclerosis lesions. J. Neuroimmunol. 1998, 84, 238–249. [Google Scholar] [CrossRef]
- Ciechanowska, A.; Popiolek-Barczyk, K.; Pawlik, K.; Ciapała, K.; Oggioni, M.; Mercurio, D.; De Simoni, M.G.; Mika, J. Changes in macrophage inflammatory protein-1 (MIP-1) family members expression induced by traumatic brain injury in mice. Immunobiology 2020, 225, 151911. [Google Scholar] [CrossRef] [PubMed]
- Guzik-Kornacka, A.; Sliwa, A.; Plucinska, G.; Lukasiuk, K. Status epilepticus evokes prolonged increase in the expression of CCL3 and CCL4 mRNA and protein in the rat brain. Acta Neurobiol. Exp. 2011, 71, 193–207. [Google Scholar]
- Liu, C.; Cui, G.; Zhu, M.; Kang, X.; Guo, H. Neuroinflammation in Alzheimer’s disease: Chemokines produced by astrocytes and chemokine receptors. Int. J. Clin. Exp. Pathol. 2014, 7, 8342–8355. [Google Scholar] [PubMed]
- Xia, M.Q.; Qin, S.X.; Wu, L.J.; Mackay, C.R.; Hyman, B.T. Immunohistochemical study of the beta-chemokine receptors CCR3 and CCR5 and their ligands in normal and Alzheimer’s disease brains. Am. J. Pathol. 1998, 153, 31–37. [Google Scholar] [CrossRef]
- Shehadeh, N.; Pollack, S.; Wildbaum, G.; Zohar, Y.; Shafat, I.; Makhoul, R.; Daod, E.; Hakim, F.; Perlman, R.; Karin, N. Selective Autoantibody Production against CCL3 Is Associated with Human Type 1 Diabetes Mellitus and Serves As a Novel Biomarker for Its Diagnosis. J. Immunol. 2009, 182, 8104–8109. [Google Scholar] [CrossRef] [Green Version]
- Bäckryd, E.; Lind, A.-L.; Thulin, M.; Larsson, A.; Gerdle, B.; Gordh, T. High levels of cerebrospinal fluid chemokines point to the presence of neuroinflammation in peripheral neuropathic pain: A cross-sectional study of 2 cohorts of patients compared with healthy controls. Pain 2017, 158, 2487–2495. [Google Scholar] [CrossRef]
- Rottman, J.B.; Ganley, K.P.; Williams, K.; Wu, L.; Mackay, C.R.; Ringler, D.J. Cellular localization of the chemokine receptor CCR5. Correlation to cellular targets of HIV-1 infection. Am. J. Pathol. 1997, 151, 1341–1351. [Google Scholar]
- Zhang, N.; Inan, S.; Cowan, A.; Sun, R.; Wang, J.M.; Rogers, T.J.; Caterina, M.; Oppenheim, J.J. A proinflammatory chemokine, CCL3, sensitizes the heat- and capsaicin-gated ion channel TRPV1. Proc. Natl. Acad. Sci. USA 2005, 102, 4536–4541. [Google Scholar] [CrossRef] [Green Version]
- Eltayeb, S.; Berg, A.L.; Lassmann, H.; Wallström, E.; Nilsson, M.; Olsson, T.; Ericsson-Dahlstrand, A.; Sunnemark, D. Temporal expression and cellular origin of CC chemokine receptors CCR1, CCR2 and CCR5 in the central nervous system: Insight into mechanisms of MOG-induced EAE. J. Neuroinflam. 2007, 4, 14. [Google Scholar] [CrossRef] [Green Version]
- Knerlich-Lukoschus, F. Chemokines and their receptors: Important mediators to be aware of in neuroregenerative approaches for spinal cord injury. Neural Regen. Res. 2015, 10, 562–564. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Jurga, A.; Slusarczyk, J.; Trojan, E.; Basta-Kaim, A.; Mika, J. Beneficial properties of maraviroc on neuropathic pain development and opioid effectiveness in rats. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2016, 64, 68–78. [Google Scholar] [CrossRef] [PubMed]
- Piotrowska, A.; Kwiatkowski, K.; Rojewska, E.; Makuch, W.; Mika, J. Maraviroc reduces neuropathic pain through polarization of microglia and astroglia—Evidence from in vivo and in vitro studies. Neuropharmacology 2016, 108, 207–219. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, J.; Ciapała, K.; Pawlik, K.; Dobrogowski, J.; Przeklasa-Muszynska, A.; Mika, J. Blockade of CCR4 Diminishes Hypersensitivity and Enhances Opioid Analgesia—Evidence from a Mouse Model of Diabetic Neuropathy. Neuroscience 2020, 441, 77–92. [Google Scholar] [CrossRef] [PubMed]
- Cowell, R.M.; Xu, H.; Galasso, J.M.; Silverstein, F.S. Hypoxic-ischemic injury induces macrophage inflammatory protein-1alpha expression in immature rat brain. Stroke 2002, 33, 795–801. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Wang, L.; Zhou, Y.; Gan, Y.; Zhu, W.; Xia, Y.; Jiang, X.; Watkins, S.; Vazquez, A.; Thomson, A.W.; et al. C-C chemokine receptor type 5 (CCR5)-mediated docking of transferred Tregs protects against early blood-brain barrier disruption after stroke. J. Am. Heart Assoc. 2017, 6, e006387. [Google Scholar] [CrossRef]
- Joy, M.T.; Ben Assayag, E.; Shabashov-Stone, D.; Liraz-Zaltsman, S.; Mazzitelli, J.; Arenas, M.; Abduljawad, N.; Kliper, E.; Korczyn, A.D.; Thareja, N.S.; et al. CCR5 Is a Therapeutic Target for Recovery after Stroke and Traumatic Brain Injury. Cell 2019, 176, 1143–1157.e13. [Google Scholar] [CrossRef] [Green Version]
- Llorián-Salvador, M.; González-Rodríguez, S.; Lastra, A.; Fernández-García, M.T.; Hidalgo, A.; Menéndez, L.; Baamonde, A. Involvement of CC Chemokine Receptor 1 and CCL3 in Acute and Chronic Inflammatory Pain in Mice. Basic Clin. Pharmacol. Toxicol. 2016, 119, 12543. [Google Scholar] [CrossRef] [Green Version]
- Saika, F.; Kiguchi, N.; Kobayashi, Y.; Fukazawa, Y.; Kishioka, S. CC-chemokine ligand 4/macrophage inflammatory protein-1β participates in the induction of neuropathic pain after peripheral nerve injury. Eur. J. Pain 2012, 16, 1271–1280. [Google Scholar] [CrossRef]
- Kwiatkowski, K.; Popiolek-Barczyk, K.; Piotrowska, A.; Rojewska, E.; Ciapała, K.; Makuch, W.; Mika, J. Chemokines CCL2 and CCL7, but not CCL12, play a significant role in the development of pain-related behavior and opioid-induced analgesia. Cytokine 2019, 119, 202–213. [Google Scholar] [CrossRef]
- Pharmacodynamics: Molecular Mechanisms of Drug Action|Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13e|AccessMedicine |McGraw Hill Medical. Available online: https://accessmedicine.mhmedical.com/content.aspx?bookid=2189§ionid=170349571 (accessed on 7 February 2023).
- Kwiatkowski, K.; Mika, J. The importance of chemokines in neuropathic pain development and opioid analgesic potency. Pharmacol. Rep. 2018, 70, 821–830. [Google Scholar] [CrossRef]
- Rogers, T.J. Bidirectional Regulation of Opioid and Chemokine Function. Front. Immunol. 2020, 11, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, D.; Bu, H.; Guo, G.; Shu, B.; Wang, W.; Guan, X.; Yang, H.; Tian, X.; Xiang, H.; Gao, F. Activation of CXCL10/CXCR3 Signaling Attenuates Morphine Analgesia: Involvement of Gi Protein. J. Mol. Neurosci. 2014, 53, 571–579. [Google Scholar] [CrossRef] [PubMed]
- Johnston, I.N.; Milligan, E.D.; Wieseler-Frank, J.; Frank, M.G.; Zapata, V.; Campisi, J.; Langer, S.; Martin, D.; Green, P.; Fleshner, M.; et al. A role for proinflammatory cytokines and fractalkine in analgesia, tolerance, and subsequent pain facilitation induced by chronic intrathecal morphine. J. Neurosci. 2004, 24, 7353–7365. [Google Scholar] [CrossRef] [Green Version]
- Zhao, C.; Guo, R.X.; Hu, F.; Meng, J.L.; Mo, L.Q.; Chen, P.X.; Liao, X.X.; Cui, Y.; Feng, J.Q. Spinal MCP-1 contributes to the development of morphine antinociceptive tolerance in rats. Am. J. Med. Sci. 2012, 344, 473–479. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, K.; Piotrowska, A.; Rojewska, E.; Makuch, W.; Mika, J. The RS504393 Influences the Level of Nociceptive Factors and Enhances Opioid Analgesic Potency in Neuropathic Rats. J. Neuroimmune Pharmacol. 2017, 12, 402–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piotrowska, A.; Rojewska, E.; Pawlik, K.; Kreiner, G.; Ciechanowska, A.; Makuch, W.; Zychowska, M.; Mika, J. Pharmacological blockade of CXCR3 by (±)-NBI-74330 reduces neuropathic pain and enhances opioid effectiveness—Evidence from in vivo and in vitro studies. BBA Mol. Basis Dis. 2018, 1864, 3418–3437. [Google Scholar] [CrossRef]
- Inan, S.; Eisenstein, T.K.; Watson, M.N.; Doura, M.; Meissler, J.J.; Tallarida, C.S.; Chen, X.; Geller, E.B.; Rawls, S.M.; Cowan, A.; et al. Coadministration of chemokine receptor antagonists with morphine potentiates morphine’s analgesic effect on incisional pain in rats. J. Pharmacol. Exp. Ther. 2018, 367, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Eisenstein, T.K.; Chen, X.; Inan, S.; Meissler, J.J.; Tallarida, C.S.; Geller, E.B.; Rawls, S.M.; Cowan, A.; Adler, M.W. Chemokine Receptor Antagonists in Combination with Morphine as a Novel Strategy for Opioid Dose Reduction in Pain Management. Mil. Med. 2020, 185, 130–135. [Google Scholar] [CrossRef] [Green Version]
- Afonso, A.S.; Carnaval, T.; Cés, S.V. Combination therapy for neuropathic pain: A review of recent evidence. J. Clin. Med. 2021, 10, 3533. [Google Scholar] [CrossRef]
- Inan, S.; Chen, X.; Eisenstein, E.M.; Meissler, J.J.; Geller, E.B.; Tallarida, C.; Watson, M.; Doura, M.; Barrett, J.E.; Cowan, A.; et al. Chemokine receptor antagonists enhance morphine’s antinociceptive effect but not respiratory depression. Life Sci. 2021, 285, 120014. [Google Scholar] [CrossRef]
- Pathan, H.; Williams, J. Basic opioid pharmacology: An update. Br. J. Pain 2012, 6, 11–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Afsharimani, B.; Cabot, P.J.; Parat, M.O. Morphine use in cancer surgery. Front. Pharmacol. 2011, 2, 46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dworkin, R.H.; Backonja, M.; Rowbotham, M.C.; Allen, R.R.; Argoff, C.R.; Bennett, G.J.; Bushnell, M.C.; Farrar, J.T.; Galer, B.S.; Haythornthwaite, J.A.; et al. Advances in Neuropathic Pain: Diagnosis, Mechanisms, and Treatment Recommendations. Arch. Neurol. 2003, 60, 1524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fornasari, D. Pharmacotherapy for Neuropathic Pain: A Review. Pain Ther. 2017, 6, 25–33. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Geller, E.B.; Rogers, T.J.; Adler, M.W. Rapid heterologous desensitization of antinociceptive activity between mu or delta opioid receptors and chemokine receptors in rats. Drug Alcohol Depend. 2007, 88, 36–41. [Google Scholar] [CrossRef] [Green Version]
- Szabo, I.; Chen, X.H.; Xin, L.; Adler, M.W.; Howard, O.M.Z.; Oppenheim, J.J.; Rogers, T.J. Heterologous desensitization of opioid receptors by chemokines inhibits chemotaxis and enhances the perception of pain. Proc. Natl. Acad. Sci. USA 2002, 99, 10276–10281. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Li, J.; Bot, G.; Szabo, I.; Rogers, T.J.; Liu-Chen, L.Y. Heterodimerization and cross-desensitization between the μ-opioid receptor and the chemokine CCR5 receptor. Eur. J. Pharmacol. 2004, 483, 175–186. [Google Scholar] [CrossRef]
- Ferré, S.; Casadó, V.; Devi, L.A.; Filizola, M.; Jockers, R.; Lohse, M.J.; Milligan, G.; Pin, J.P.; Guitart, X. G protein-coupled receptor oligomerization revisited: Functional and pharmacological perspectives. Pharmacol. Rev. 2014, 66, 413–434. [Google Scholar] [CrossRef] [Green Version]
- Gaborit, M.; Massotte, D. Therapeutic potential of opioid receptor heteromers in chronic pain and associated comorbidities. Br. J. Pharmacol. 2021, 180, 994–1013. [Google Scholar] [CrossRef]
- Suzuki, S.; Chuang, L.F.; Yau, P.; Doi, R.H.; Chuang, R.Y. Interactions of opioid and chemokine receptors: Oligomerization of mu, kappa, and delta with CCR5 on immune cells. Exp. Cell Res. 2002, 280, 192–200. [Google Scholar] [CrossRef]
- Szabo, I.; Wetzel, M.A.; Zhang, N.; Steele, A.D.; Kaminsky, D.E.; Chen, C.; Liu-Chen, L.-Y.; Bednar, F.; Henderson, E.E.; Howard, O.M.Z.; et al. Selective inactivation of CCR5 and decreased infectivity of R5 HIV-1 strains mediated by opioid-induced heterologous desensitization. J. Leukoc. Biol. 2003, 74, 1074–1082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, C.; Rahim, R.T.; Davey, P.C.; Bednar, F.; Bardi, G.; Zhang, L.; Zhang, N.; Oppenheim, J.J.; Rogers, T.J. Protein kinase Cζ mediates μ-opioid receptor-induced cross-desensitization of chemokine receptor CCR5. J. Biol. Chem. 2011, 286, 20354–20365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnatt, C.K.; Falls, B.A.; Yuan, Y.; Raborg, T.J.; Masvekar, R.R.; El-Hage, N.; Selley, D.E.; Nicola, A.V.; Knapp, P.E.; Hauser, K.F.; et al. Exploration of bivalent ligands targeting putative mu opioid receptor and chemokine receptor CCR5 dimerization. Bioorganic Med. Chem. 2016, 24, 5969–5987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Rogers, T.J.; Caterina, M.; Oppenheim, J.J. Proinflammatory chemokines, such as C-C chemokine ligand 3, desensitize mu-opioid receptors on dorsal root ganglia neurons. J. Immunol. 2004, 173, 594–599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Prisco, S.; Summa, M.; Chellakudam, V.; Rossi, P.I.A.; Pittaluga, A. RANTES-mediated control of excitatory amino acid release in mouse spinal cord. J. Neurochem. 2012, 121, 428–437. [Google Scholar] [CrossRef]
- Kramp, B.K.; Megens, R.T.A.; Sarabi, A.; Winkler, S.; Projahn, D.; Weber, C.; Koenen, R.R.; von Hundelshausen, P. Exchange of extracellular domains of CCR1 and CCR5 reveals confined functions in CCL5-mediated cell recruitment. Thromb. Haemost. 2013, 110, 795–806. [Google Scholar] [CrossRef]
- Eliav, E.; Gracely, R.H. Measuring and assessing pain. In Orofacial Pain and Headache; Elsevier Health Sciences: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Gonçalves dos Santos, G.; Delay, L.; Yaksh, T.L.; Corr, M. Neuraxial Cytokines in Pain States. Front. Immunol. 2020, 10, 3061. [Google Scholar] [CrossRef] [Green Version]
- Steinberg, B.E.; Silverman, H.A.; Robbiati, S.; Gunasekaran, M.K.; Tsaava, T.; Battinelli, E.; Stiegler, A.; Bouton, C.E.; Chavan, S.S.; Tracey, K.J.; et al. Cytokine-specific Neurograms in the Sensory Vagus Nerve. Bioelectron. Med. 2016, 3, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Ji, G.; Zhou, S.; Kochukov, M.Y.; Westlund, K.N.; Carlton, S.M. Plasticity in intact Aδ- and C-fibers contributes to cold hypersensitivity in neuropathic rats. Neuroscience 2007, 150, 182–193. [Google Scholar] [CrossRef] [Green Version]
- Kania, B.F.; Bracha, U.; Lonc, G.; Wojnar, T. Rola antagonistów glutaminianergicznych receptorów metabotropowych w eksperymentalnym bólu neuropatycznym u zwierząt. Med. Weter 2020, 76, 564–571. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciechanowska, A.; Pawlik, K.; Ciapała, K.; Mika, J. Pharmacological Modulation of the MIP-1 Family and Their Receptors Reduces Neuropathic Pain Symptoms and Influences Morphine Analgesia: Evidence from a Mouse Model. Brain Sci. 2023, 13, 579. https://doi.org/10.3390/brainsci13040579
Ciechanowska A, Pawlik K, Ciapała K, Mika J. Pharmacological Modulation of the MIP-1 Family and Their Receptors Reduces Neuropathic Pain Symptoms and Influences Morphine Analgesia: Evidence from a Mouse Model. Brain Sciences. 2023; 13(4):579. https://doi.org/10.3390/brainsci13040579
Chicago/Turabian StyleCiechanowska, Agata, Katarzyna Pawlik, Katarzyna Ciapała, and Joanna Mika. 2023. "Pharmacological Modulation of the MIP-1 Family and Their Receptors Reduces Neuropathic Pain Symptoms and Influences Morphine Analgesia: Evidence from a Mouse Model" Brain Sciences 13, no. 4: 579. https://doi.org/10.3390/brainsci13040579