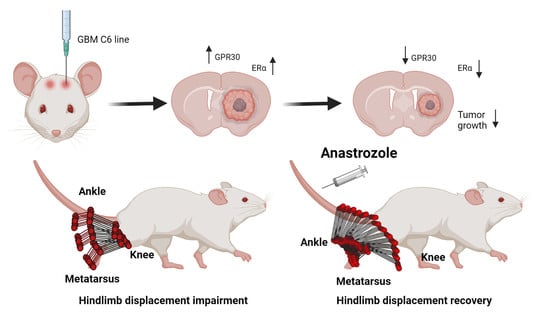
Locomotion Outcome Improvement in Mice with Glioblastoma Multiforme after Treatment with Anastrozole
Abstract
:1. Introduction
2. Methods
2.1. Cells Culture
2.2. Animals
2.3. Glioblastoma Xenograft and Mice Treatment
2.4. Body Weight in Mice
2.5. Hematoxylin & Eosin Staining
2.6. Immunofluorescence
2.7. Tunnel Walk Recordings
2.8. Dissimilarity Factor Analysis
2.9. Vertical/Horizontal Displacement Analysis
3. Statistics
4. Results
4.1. Body Weight in GBM and GBM+Anastrozole Groups
4.2. Histopathological Changes in the Striatal Area of GBM and GBM+Anastrozole Mice
4.3. Expression of ERα and GPR30 Receptors in the Study Groups
4.4. Changes in Mice Locomotion with Glioblastoma and Those Treated with Anastrozole
4.5. The Horizontal Displacement among Different Study Groups
4.6. Changes in Vertical Displacement
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghosh, D.; Nandi, S.; Bhattacharjee, S. Combination Therapy to Checkmate Glioblastoma: Clinical Challenges and Advances. Clin. Transl. Med. 2018, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.B.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus Concomitant and Adjuvant Temozolomide for Glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Lonardi, S.; Tosoni, A.; Brandes, A.A. Adjuvant Chemotherapy in the Treatment of High Grade Gliomas. Cancer Treat. Rev. 2005, 31, 79–89. [Google Scholar] [CrossRef]
- Birch, J.L.; Strathdee, K.; Gilmour, L.; Vallatos, A.; McDonald, L.; Kouzeli, A.; Vasan, R.; Qaisi, A.H.; Croft, D.R.; Crighton, D.; et al. A Novel Small-Molecule Inhibitor of MRCK Prevents Radiation-Driven Invasion in Glioblastoma. Cancer Res. 2018, 78, 6509–6522. [Google Scholar] [CrossRef] [PubMed]
- Miyai, M.; Tomita, H.; Soeda, A.; Yano, H.; Iwama, T.; Hara, A. Current Trends in Mouse Models of Glioblastoma. J. Neurooncol. 2017, 135, 423–432. [Google Scholar] [CrossRef]
- Shi, L.H.; Luo, F.; Woodward, D.J.; Chang, J.Y. Neural Responses in Multiple Basal Ganglia Regions during Spontaneous and Treadmill Locomotion Task in Rats. Exp. Brain Res. 2004, 157, 303–314. [Google Scholar] [CrossRef] [PubMed]
- Gonzales, K.K.; Smith, Y. Cholinergic Interneurons in the Dorsal and Ventral Striatum: Anatomical and Functional Considerations in Normal and Diseased Conditions. Ann. N. Y. Acad. Sci. 2015, 1349, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Yuldasheva, N.Y.; Batten, T.F.C.; Pickles, A.R.; Kellett, K.A.B.; Saha, S. Tau Pathology and Neurochemical Changes Associated with Memory Dysfunction in an Optimised Murine Model of Global Cerebral Ischaemia-A Potential Model for Vascular Dementia? Neurochem. Int. 2018, 118, 134–144. [Google Scholar] [CrossRef]
- Yague, J.G.; Lavaque, E.; Carretero, J.; Azcoitia, I.; Garcia-Segura, L.M. Aromatase, the Enzyme Responsible for Estrogen Biosynthesis, Is Expressed by Human and Rat Glioblastomas. Neurosci. Lett. 2004, 368, 279–284. [Google Scholar] [CrossRef]
- Dueñas Jiménez, J.M.; Candanedo Arellano, A.; Santerre, A.; Orozco Suárez, S.; Sandoval Sánchez, H.; Feria Romero, I.; López-Elizalde, R.; Alonso Venegas, M.; Netel, B.; de la Torre Valdovinos, B.; et al. Aromatase and Estrogen Receptor Alpha MRNA Expression as Prognostic Biomarkers in Patients with Astrocytomas. J. Neurooncol. 2014, 119, 275–284. [Google Scholar] [CrossRef]
- Amir, E.; Seruga, B.; Niraula, S.; Carlsson, L.; Ocaña, A. Toxicity of Adjuvant Endocrine Therapy in Postmenopausal Breast Cancer Patients: A Systematic Review and Meta-Analysis. J. Natl. Cancer Inst. 2011, 103, 1299–1309. [Google Scholar] [CrossRef] [PubMed]
- Ascencio-Piña, C.; Pérez-Cisneros, M.; Dueñaz-Jimenez, S.; Mendizabal-Ruiz, G. A System for High-Speed Synchronized Acquisition of Video Recording of Rodents during Locomotion. In VIII Latin American Conference on Biomedical Engineering and XLII National Conference on Biomedical Engineering, Proceedings of CLAIB-CNIB 2019, Cancún, México, 2–5 October 2019; Springer: Cham, Switzerland, 2020; pp. 309–314. [Google Scholar]
- Kubatka, P.; Sadlonova, V.; Kajo, K.; Machalekova, K.; Ostatnikova, D.; Nosalova, G.; Fetisovova, Z. Neoplastic Effects of Exemestane in Premenopausal Breast Cancer Model. Neoplasma 2008, 55, 538–543. [Google Scholar]
- Sadlonova, V.; Kubatka, P.; Kajo, K.; Ostatnikova, D.; Nosalova, G.; Adamicova, K.; Sadlonova, J. Side Effects of Anastrozole in the Experimental Pre-Menopausal Mammary Carcinogenesis. Neoplasma 2009, 56, 124–129. [Google Scholar] [CrossRef] [PubMed]
- Overk, C.R.; Borgia, J.A.; Mufson, E.J. A Novel Approach for Long-Term Oral Drug Administration in Animal Research. J. Neurosci. Methods 2011, 195, 194–199. [Google Scholar] [CrossRef]
- Sestak, I.; Cuzick, J. Preventive Therapy for Breast Cancer. Curr. Oncol. Rep. 2012, 14, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Broniscer, A.; Tatevossian, R.G.; Sabin, N.D.; Klimo, P.; Dalton, J.; Lee, R.; Gajjar, A.; Ellison, D.W. Clinical, Radiological, Histological and Molecular Characteristics of Paediatric Epithelioid Glioblastoma. Neuropathol. Appl. Neurobiol. 2014, 40, 327–336. [Google Scholar] [CrossRef]
- Weinberg, O.K.; Marquez-Garban, D.C.; Fishbein, M.C.; Goodglick, L.; Garban, H.J.; Dubinett, S.M.; Pietras, R.J. Aromatase Inhibitors in Human Lung Cancer Therapy. Cancer Res. 2005, 65, 11287–11291. [Google Scholar] [CrossRef]
- Forbes, J.F.; Sestak, I.; Howell, A.; Bonanni, B.; Bundred, N.; Levy, C.; Von Minckwitz, G.; Eiermann, W.; Neven, P.; Stierer, M.; et al. Anastrozole versus Tamoxifen for the Prevention of Locoregional and Contralateral Breast Cancer in Postmenopausal Women with Locally Excised Ductal Carcinoma in Situ (IBIS-II DCIS): A Double-Blind, Randomised Controlled Trial. Lancet 2016, 387, 866–873. [Google Scholar] [CrossRef]
- Rothenberger, N.; Somasundaram, A.; Stabile, L. The Role of the Estrogen Pathway in the Tumor Microenvironment. Int. J. Mol. Sci. 2018, 19, 611. [Google Scholar] [CrossRef]
- Hönikl, L.S.; Lämmer, F.; Gempt, J.; Meyer, B.; Schlegel, J.; Delbridge, C. High Expression of Estrogen Receptor Alpha and Aromatase in Glial Tumor Cells Is Associated with Gender-Independent Survival Benefits in Glioblastoma Patients. J. Neurooncol. 2020, 147, 567–575. [Google Scholar] [CrossRef]
- Demark-Wahnefried, W.; Peterson, B.L.; Winer, E.P.; Marks, L.; Aziz, N.; Marcom, P.K.; Blackwell, K.; Rimer, B.K. Changes in Weight, Body Composition, and Factors Influencing Energy Balance Among Premenopausal Breast Cancer Patients Receiving Adjuvant Chemotherapy. J. Clin. Oncol. 2001, 19, 2381–2389. [Google Scholar] [CrossRef]
- Winer, E.P.; Hudis, C.; Burstein, H.J.; Wolff, A.C.; Pritchard, K.I.; Ingle, J.N.; Chlebowski, R.T.; Gelber, R.; Edge, S.B.; Gralow, J.; et al. American Society of Clinical Oncology Technology Assessment on the Use of Aromatase Inhibitors As Adjuvant Therapy for Postmenopausal Women With Hormone Receptor–Positive Breast Cancer: Status Report 2004. J. Clin. Oncol. 2005, 23, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Smollich, M.; Götte, M.; Fischgräbe, J.; Radke, I.; Kiesel, L.; Wülfing, P. Differential Effects of Aromatase Inhibitors and Antiestrogens on Estrogen Receptor Expression in Breast Cancer Cells. Anticancer Res. 2009, 29, 2167–2171. [Google Scholar] [PubMed]
- Vivacqua, A.; Lappano, R.; De Marco, P.; Sisci, D.; Aquila, S.; De Amicis, F.; Fuqua, S.A.W.; AndoÒ, S.; Maggiolini, M. G Protein-Coupled Receptor 30 Expression Is Up-Regulated by EGF and TGFα in Estrogen Receptor α-Positive Cancer Cells. Mol. Endocrinol. 2009, 23, 1815–1826. [Google Scholar] [CrossRef] [PubMed]
- Tian, S.; Zhan, N.; Li, R.; Dong, W. Downregulation of G Protein-Coupled Estrogen Receptor (GPER) Is Associated with Reduced Prognosis in Patients with Gastric Cancer. Med. Sci. Monit. 2019, 25, 3115–3126. [Google Scholar] [CrossRef]
- Tutzauer, J.; Sjöström, M.; Bendahl, P.O.; Rydén, L.; Fernö, M.; Fredrik Leeb-Lundberg, L.M.; Alkner, S. Plasma Membrane Expression of G Protein-Coupled Estrogen Receptor (GPER)/G Protein-Coupled Receptor 30 (GPR30) Is Associated with Worse Outcome in Metachronous Contralateral Breast Cancer. PLoS ONE 2020, 15, e0231786. [Google Scholar] [CrossRef]
- Li, Y.; Jia, Y.; Bian, Y.; Tong, H.; Qu, J.; Wang, K.; Wan, X.-P. Autocrine Motility Factor Promotes Endometrial Cancer Progression by Targeting GPER-1. Cell Commun. Signal. 2019, 17, 22. [Google Scholar] [CrossRef]
- Gutiérrez-Almeida, C.; Santerre, A.; León-Moreno, L.; Aguilar-García, I.; Castañeda-Arellano, R.; Dueñas-Jiménez, S.; Dueñas-jiménez, J. Proliferation and Apoptosis Regulation by G Protein-coupled Estrogen Receptor in Glioblastoma C6 Cells. Oncol. Lett. 2022, 24, 217. [Google Scholar] [CrossRef]
- Lang, S.; Cadeaux, M.; Opoku-Darko, M.; Gaxiola-Valdez, I.; Partlo, L.A.; Goodyear, B.G.; Federico, P.; Kelly, J. Assessment of Cognitive, Emotional, and Motor Domains in Patients with Diffuse Gliomas Using the National Institutes of Health Toolbox Battery. World Neurosurg. 2017, 99, 448. [Google Scholar] [CrossRef]
- IJzerman-Korevaar, M.; Snijders, T.J.; de Graeff, A.; Teunissen, S.C.C.M.; de Vos, F.Y.F. Prevalence of Symptoms in Glioma Patients throughout the Disease Trajectory: A Systematic Review. J. Neurooncol. 2018, 140, 485–496. [Google Scholar] [CrossRef]
- Cochereau, J.; Herbet, G.; Duffau, H. Patients with Incidental WHO Grade II Glioma Frequently Suffer from Neuropsychological Disturbances. Acta Neurochir. 2016, 158, 305–312. [Google Scholar] [CrossRef]
- Liouta, E.; Koutsarnakis, C.; Liakos, F.; Stranjalis, G. Effects of Intracranial Meningioma Location, Size, and Surgery on Neurocognitive Functions: A 3-Year Prospective Study. J. Neurosurg. 2016, 124, 1578–1584. [Google Scholar] [CrossRef]
- Liouta, E.; Katsaros, V.K.; Stranjalis, G.; Leks, E.; Klose, U.; Bisdas, S. Motor and Language Deficits Correlate with Resting State Functional Magnetic Resonance Imaging Networks in Patients with Brain Tumors. J. Neuroradiol. 2019, 46, 199–206. [Google Scholar] [CrossRef]
- Felix Souza, T.K.; Nucci, M.P.; Mamani, J.B.; Rodrigues da Silva, H.; Carvalho Fantacini, D.M.; Botelho de Souza, L.E.; Picanço-Castro, V.; Covas, D.T.; Vidoto, E.L.; Tannús, A.; et al. Image and Motor Behavior for Monitoring Tumor Growth in C6 Glioma Model. PLoS ONE 2018, 13, e0201453. [Google Scholar] [CrossRef]
- Sciacero, P.; Girelli, G.F.; Cante, D.; Franco, P.; Borca, V.C.; Grosso, P.; Marra, A.; Bombaci, S.; Tofani, S.; La Porta, M.R.; et al. Cerebellar Glioblastoma Multiforme in an Adult Woman. Tumori 2014, 100, 74–78. [Google Scholar] [CrossRef]
- Kushner, D.S.; Amidei, C. Rehabilitation of Motor Dysfunction in Primary Brain Tumor Patients. Neuro-Oncol. Pract. 2015, 2, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, V.L.; Valdes, P.A.; Hickey, W.F.; de Leo, J.A. Current Review of in Vivo GBM Rodent Models: Emphasis on the CNS-1 Tumour Model. ASN Neuro 2011, 3, 171–181. [Google Scholar] [CrossRef]
- Garcia-Rill, E. The Basal Ganglia and the Locomotor Regions. Brain Res. Rev. 1986, 11, 47–63. [Google Scholar] [CrossRef]
- Wagner, J.; Stephan, T.; Kalla, R.; Brückmann, H.; Strupp, M.; Brandt, T.; Jahn, K. Mind the Bend: Cerebral Activations Associated with Mental Imagery of Walking along a Curved Path. Exp. Brain Res. 2008, 191, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Gosgnach, S. Synaptic Connectivity amongst Components of the Locomotor Central Pattern Generator. Front. Neural Circuits 2022, 16, 1–6. [Google Scholar] [CrossRef]
- Grillner, S.; El Manira, A. Current Principles of Motor Control, with Special Reference to Vertebrate Locomotion. Physiol. Rev. 2020, 100, 271–320. [Google Scholar] [CrossRef]
- Taccola, G.; Ichiyama, R.M.; Edgerton, V.R.; Gad, P. Stochastic Spinal Neuromodulation Tunes the Intrinsic Logic of Spinal Neural Networks. Exp. Neurol. 2022, 355, 114138. [Google Scholar] [CrossRef] [PubMed]
- Rybak, I.A.; Dougherty, K.J.; Shevtsova, N.A. Organization of the Mammalian Locomotor CPG: Review of Computational Model and Circuit Architectures Based on Genetically Identified Spinal Interneurons. Eneuro 2015, 2, ENEURO.0069-15.2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.; Pallucchi, I.; Ausborn, J.; Ampatzis, K.; Bertuzzi, M.; Fontanel, P.; Picton, L.D.; El Manira, A. Multiple Rhythm-Generating Circuits Act in Tandem with Pacemaker Properties to Control the Start and Speed of Locomotion. Neuron 2020, 105, 1048–1061.e4. [Google Scholar] [CrossRef] [PubMed]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-García, I.G.; Jiménez-Estrada, I.; Castañeda-Arellano, R.; Alpirez, J.; Mendizabal-Ruiz, G.; Dueñas-Jiménez, J.M.; Gutiérrez-Almeida, C.E.; Osuna-Carrasco, L.P.; Ramírez-Abundis, V.; Dueñas-Jiménez, S.H. Locomotion Outcome Improvement in Mice with Glioblastoma Multiforme after Treatment with Anastrozole. Brain Sci. 2023, 13, 496. https://doi.org/10.3390/brainsci13030496
Aguilar-García IG, Jiménez-Estrada I, Castañeda-Arellano R, Alpirez J, Mendizabal-Ruiz G, Dueñas-Jiménez JM, Gutiérrez-Almeida CE, Osuna-Carrasco LP, Ramírez-Abundis V, Dueñas-Jiménez SH. Locomotion Outcome Improvement in Mice with Glioblastoma Multiforme after Treatment with Anastrozole. Brain Sciences. 2023; 13(3):496. https://doi.org/10.3390/brainsci13030496
Chicago/Turabian StyleAguilar-García, Irene Guadalupe, Ismael Jiménez-Estrada, Rolando Castañeda-Arellano, Jonatan Alpirez, Gerardo Mendizabal-Ruiz, Judith Marcela Dueñas-Jiménez, Coral Estefania Gutiérrez-Almeida, Laura Paulina Osuna-Carrasco, Viviana Ramírez-Abundis, and Sergio Horacio Dueñas-Jiménez. 2023. "Locomotion Outcome Improvement in Mice with Glioblastoma Multiforme after Treatment with Anastrozole" Brain Sciences 13, no. 3: 496. https://doi.org/10.3390/brainsci13030496