Embryoid Body Cells from Human Embryonic Stem Cells Overexpressing Dopaminergic Transcription Factors Survive and Initiate Neurogenesis via Neural Rosettes in the Substantia Nigra
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Pluripotent Stem Cells Lines
2.2. Embryoid Body Culture
2.3. 6-Hydroxydopamine Lesion and Cell Transplantation
2.4. Rotational Behavior
2.5. Histology Procedures
2.6. Image Acquisition and Cell Counting
2.7. Statistical Analysis
3. Results
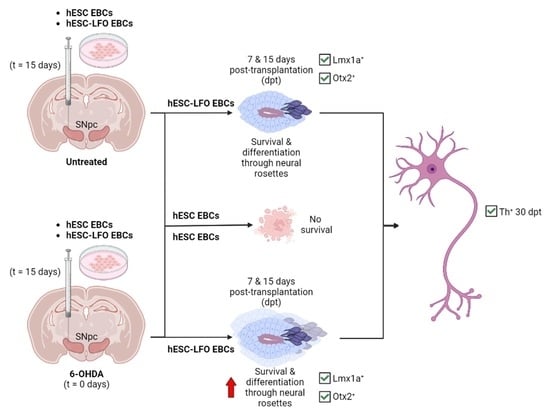
3.1. EBCs from hESC-LFO Cells Survive and Differentiate toward Neural Rosette Formation in the SNpc
3.2. Neural Rosettes Express Dopaminergic Lineage Markers in the Substantia Nigra
3.3. Neural Rosettes Are Mainly at the Differentiation Stage of Lumen Formation
3.4. hESC-LFO EBCs Grafts Express Tyrosine Hydroxylase and Neuromelanin at 30 Days Post-Transplantation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Surguchov, A. Biomarkers in Parkinson’s Disease. In Neurodegenerative Diseases Biomarkers; Peplow, P.V., Martinez, B., Gennarelli, T.A., Eds.; Springer Protocols: New York, NY, USA, 2022; pp. 155–180. [Google Scholar]
- Langston, J.W.; Schüle, B.; Rees, L.; Nichols, R.J.; Barlow, C. Multisystem Lewy Body Disease and the Other Parkinsonian Disorders. Nat. Genet. 2015, 47, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Pfeiffer, R.F. Non-Motor Symptoms in Parkinson’s Disease. Park. Relat. Disord. 2016, 22, S119–S122. [Google Scholar] [CrossRef] [PubMed]
- Morato-Torres, A.C.; Wassouf, Z.; Zafar, F.; Sastre, D.; Outeiro, T.F.; Schüle, B. The Role of Alpha-Synuclein and Other Parkinson’s Genes in Neurodevelopmental and Neurodegenerative Disorders. Int. J. Mol. Sci. 2020, 21, 5724. [Google Scholar] [CrossRef] [PubMed]
- Stoker, T.B.; Barker, R.A. Cell Therapies for Parkinson’s Disease: How Far Have We Come? Regen. Med. 2016, 11, 777–786. [Google Scholar] [CrossRef]
- Qingxi, Z.; Wanling, C.; Sheng, T.; Tongxiang, L. Stem Cells for Modeling and Therapy of Parkinson’s Disease. Hum. Gene 2017, 28, 85–98. [Google Scholar]
- Boronat-García, A.; Guerra-Crespo, M.; Drucker-Colín, R. Historical Perspective of Cell Transplantation in Parkinson’s Disease. World J. Transpl. 2017, 7, 179–192. [Google Scholar] [CrossRef]
- Grealish, S.; Diguet, E.; Kirkeby, A.; Mattsson, B.; Heuer, A.; Bramoulle, Y.; Van Camp, N.; Perrier, A.L.; Hantraye, P.; Björklund, A.; et al. Human ESC-Derived Dopamine Neurons Show Similar Preclinical Efficacy and Potency to Fetal Neurons When Grafted in a Rat Model of Parkinson’s Disease. Cell Stem. Cell 2014, 15, 653–665. [Google Scholar] [CrossRef]
- Gaillard, A.; Decressac, M.; Frappé, I.; Fernagut, P.O.; Prestoz, L.; Besnard, S.; Jaber, M. Anatomical and Functional Reconstruction of the Nigrostriatal Pathway by Intranigral Transplants. Neurobiol. Dis. 2009, 35, 477–488. [Google Scholar] [CrossRef]
- Thompson, L.H.; Grealish, S.; Kirik, D.; Björklund, A. Reconstruction of the Nigrostriatal Dopamine Pathway in the Adult Mouse Brain. Eur. J. Neurosci. 2009, 30, 625–638. [Google Scholar] [CrossRef]
- Wictorin, K.; Brundin, P.; Sauer, H.; Lindvall, O.; Björklund, A. Long Distance Directed Axonal Growth from Human Dopaminergic Mesencephalic Neuroblasts Implanted along the Nigrostriatal Pathway in 6-Hydroxydopamine Lesioned Adult Rats. J. Comp. Neurol. 1992, 323, 475–494. [Google Scholar] [CrossRef] [PubMed]
- Droguerre, M.; Brot, S.; Vitrac, C.; Benoit-Marand, M.; Belnoue, L.; Patrigeon, M.; Lainé, A.; Béré, E.; Jaber, M.; Gaillard, A. Better Outcomes with Intranigral versus Intrastriatal Cell Transplantation: Relevance for Parkinson’s Disease. Cells 2022, 11, 1191. [Google Scholar] [CrossRef] [PubMed]
- Brot, S.; Thamrin, N.P.; Bonnet, M.-L.; Francheteau, M.; Patrigeon, M.; Belnoue, L.; Gaillard, A. Long-Term Evaluation of Intranigral Transplantation of Human IPSC-Derived Dopamine Neurons in a Parkinson’s Disease Mouse Model. Cells 2022, 11, 1596. [Google Scholar] [CrossRef]
- Kim, T.W.; Koo, S.Y.; Studer, L. Pluripotent Stem Cell Therapies for Parkinson Disease: Present Challenges and Future Opportunities. Front. Cell Dev. Biol. 2020, 8, 729. [Google Scholar] [CrossRef] [PubMed]
- Björklund, A.; Parmar, M. Dopamine Cell Therapy: From Cell Replacement to Circuitry Repair. J. Park. Dis. 2021, 11, S159–S165. [Google Scholar] [CrossRef] [PubMed]
- Maya-Espinosa, G.; Collazo-Navarrete, O.; Millán-Aldaco, D.; Palomero-Rivero, M.; Guerrero-Flores, G.; Drucker-Colín, R.; Covarrubias, L.; Guerra-Crespo, M. Mouse Embryonic Stem Cell-Derived Cells Reveal Niches That Support Neuronal Differentiation in the Adult Rat Brain. Stem. Cells 2015, 33, 491–502. [Google Scholar] [CrossRef]
- Collazo-Navarrete, O.; Hernández-García, D.; Guerrero-Flores, G.; Drucker-Colín, R.; Guerra-Crespo, M.; Covarrubias, L. The Substantia Nigra Is Permissive and Gains Inductive Signals When Lesioned for Dopaminergic Differentiation of Embryonic Stem Cells. Stem. Cells Dev. 2019, 28, 1104–1115. [Google Scholar] [CrossRef]
- Itskovitz-Eldor, J.; Schuldiner, M.; Karsenti, D.; Eden, A.; Yanuka, O.; Amit, M.; Soreq, H.; Benvenisty, N. Differentiation of Human Embryonic Stem Cells into Embryoid Bodies Compromising the Three Embryonic Germ Layers. Mol. Med. 2000, 6, 88–95. [Google Scholar] [CrossRef]
- Tomov, M.L.; Olmsted, Z.T.; Paluh, J.L. The Human Embryoid Body Cystic Core Exhibits Architectural Complexity Revealed by Use of High Throughput Polymer Microarrays. Macromol. Biosci. 2015, 15, 892–900. [Google Scholar] [CrossRef]
- Baizabal, J.M.; Covarrubias, L. The Embryonic Midbrain Directs Neuronal Specification of Embryonic Stem Cells at Early Stages of Differentiation. Dev. Biol. 2009, 325, 49–59. [Google Scholar] [CrossRef]
- Arenas, E.; Denham, M.; Villaescusa, J.C. How to Make a Midbrain Dopaminergic Neuron. Development 2015, 142, 1918–1936. [Google Scholar] [CrossRef]
- Zhu, F.; Gamboa, M.; Farruggio, A.P.; Hippenmeyer, S.; Tasic, B.; Schüle, B.; Chen-Tsai, Y.; Calos, M.P. DICE, an Efficient System for Iterative Genomic Editing in Human Pluripotent Stem Cells. Nucleic. Acids Res. 2014, 42, e34. [Google Scholar] [CrossRef]
- Thomson, J.A.; Itskovitz-Eldor, J.; Shapiro, S.S.; Waknitz, M.A.; Swiergiel, J.J.; Marshall, V.S.; Jones, J.M. Embryonic Stem Cell Lines Derived from Human Blastocysts. Science 1998, 282, 1145–1147. [Google Scholar] [CrossRef]
- Guerra-Crespo, M.; Collazo-Navarrete, O.; Ramos-Acevedo, R.; Morato-Torres, C.A.; Schüle, B. Embryoid Body Formation from Mouse and Human Pluripotent Stem Cells for Transplantation to Study Brain Microenvironment and Cellular Differentiation. In Embryonic Stem Cells: Methods and Protocols; Turksen, K., Ed.; Springer Nature: New York, NY, USA, 2021; pp. 1–18. [Google Scholar]
- National Academies Press. The National Academies Collection: Reports Funded by National Institutes of Health; National Academies Press: Washington, DC, USA, 1975. [Google Scholar]
- Deumens, R.; Blokland, A.; Prickaerts, J. Modeling Parkinson’s Disease in Rats: An Evaluation of 6-OHDA Lesions of the Nigrostriatal Pathway. Exp. Neurol. 2002, 175, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Boronat-García, A.; Palomero-Rivero, M.; Guerra-Crespo, M.; Millán-Aldaco, D.; Drucker-Colín, R. Intrastriatal Grafting of Chromospheres: Survival and Functional Effects in the 6-OHDA Rat Model of Parkinson’s Disease. PLoS ONE 2016, 11, e0160854. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 5th ed.; Elsevier Academic Press: Boston, MA, USA, 2005. [Google Scholar]
- Crawford, M.; Leclerc, V.; Dagnino, L. A Reporter Mouse Model for in Vivo Tracing and in Vitro Molecular Studies of Melanocytic Lineage Cells and Their Diseases. Biol. Open 2017, 6, 1219–1228. [Google Scholar] [CrossRef]
- Grabiec, M.; Hříbková, H.; Vařecha, M.; Střítecká, D.; Hampl, A.; Dvořák, P.; Sun, Y.M. Stage-Specific Roles of FGF2 Signaling in Human Neural Development. Stem. Cell Res. 2016, 17, 330–341. [Google Scholar] [CrossRef]
- Elkabetz, Y.; Panagiotakos, G.; al Shamy, G.; Socci, N.D.; Tabar, V.; Studer, L. Human ES Cell-Derived Neural Rosettes Reveal a Functionally Distinct Early Neural Stem Cell Stage. Genes Dev. 2008, 22, 152–165. [Google Scholar] [CrossRef] [PubMed]
- Satomi, K.; Morishita, Y.; Sakashita, S.; Kondou, Y.; Furuya, S.; Minami, Y.; Noguchi, M. Specific Expression of ZO-1 and N-Cadherin in Rosette Structures of Various Tumors: Possible Recapitulation of Neural Tube Formation in Embryogenesis and Utility as a Potentially Novel Immunohistochemical Marker of Rosette Formation in Pulmonary Neuroendo. Virchows Arch. 2011, 459, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Hříbková, H.; Grabiec, M.; Klemová, D.; Slaninová, I.; Sun, Y.-M. Calcium Signaling Mediates Five Types of Cell Morphological Changes to Form Neural Rosettes. J. Cell. Sci. 2018, 131, jcs206896. [Google Scholar] [CrossRef]
- Chung, S.; Leung, A.; Han, B.-S.; Chang, M.-Y.; Moon, J.-I.; Kim, C.-H.; Hong, S.; Pruszak, J.; Isacson, O.; Kim, K.-S. Wnt1-Lmx1a Forms a Novel Autoregulatory Loop and Controls Midbrain Dopaminergic Differentiation Synergistically with the SHH-FoxA2 Pathway. Cell Stem. Cell 2009, 5, 646–658. [Google Scholar] [CrossRef]
- Brown, J.P.; Couillard-Després, S.; Cooper-Kuhn, C.M.; Winkler, J.; Aigner, L.; Kuhn, H.G. Transient Expression of Doublecortin during Adult Neurogenesis. J. Comp. Neurol. 2003, 467, 1–10. [Google Scholar] [CrossRef]
- Segura-Aguilar, J.; Paris, I.; Muñoz, P.; Ferrari, E.; Zecca, L.; Zucca, F.A. Protective and Toxic Roles of Dopamine in Parkinson’s Disease. J. Neurochem. 2014, 129, 898–915. [Google Scholar] [CrossRef]
- Bishop, J.A.; Nelson, A.M.; Merz, W.G.; Askin, F.B.; Riedel, S. Evaluation of the Detection of Melanin by the Fontana-Masson Silver Stain in Tissue with a Wide Range of Organisms Including Cryptococcus. Hum. Pathol. 2012, 43, 898–903. [Google Scholar] [CrossRef]
- Kiyohara, T.; Nakamaru, S.; Miyamoto, M.; Shijimaya, T.; Nagano, N.; Makimura, K.; Tanimura, H. Site-specific Acral Nevus Histologically Reminiscent of Melanoma: Recognition of the Utility of the Fontana–Masson Stain. J. Derm. 2018, 46, e183–e185. [Google Scholar] [CrossRef] [PubMed]
- Doucet-Beaupré, H.; Gilbert, C.; Profes, M.S.; Chabrat, A.; Pacelli, C.; Giguère, N.; Rioux, V.; Charest, J.; Deng, Q.; Laguna, A.; et al. Lmx1a and Lmx1b Regulate Mitochondrial Functions and Survival of Adult Midbrain Dopaminergic Neurons. Proc. Natl. Acad. Sci. 2016, 113, E4387–E4396. [Google Scholar] [CrossRef]
- Domanskyi, A.; Alter, H.; Vogt, M.A.; Gass, P.; Vinnikov, I.A. Transcription Factors Foxa1 and Foxa2 Are Required for Adult Dopamine Neurons Maintenance. Front. Cell Neurosci. 2014, 8, 275. [Google Scholar] [CrossRef]
- Puelles, E.; Annino, A.; Tuorto, F.; Usiello, A.; Acampora, D.; Czerny, T.; Brodski, C.; Ang, S.-L.; Wurst, W.; Simeone, A. Otx2 Regulates the Extent, Identity and Fate of Neuronal Progenitor Domains in the Ventral Midbrain. Development 2004, 131, 2037–2048. [Google Scholar] [CrossRef] [PubMed]
- Knight, G.T.; Lundin, B.F.; Iyer, N.; Ashton, L.M.T.; Sethares, W.A.; Willett, R.M.; Ashton, R.S. Engineering Induction of Singular Neural Rosette Emergence within HPSC-Derived Tissues. Elife 2018, 7, e37549. [Google Scholar] [CrossRef] [PubMed]
- Jo, J.; Xiao, Y.; Sun, A.X.; Cukuroglu, E.; Tran, H.D.; Göke, J.; Tan, Z.Y.; Saw, T.Y.; Tan, C.P.; Lokman, H.; et al. Midbrain-like Organoids from Human Pluripotent Stem Cells Contain Functional Dopaminergic and Neuromelanin-Producing Neurons. Cell Stem. Cell 2016, 19, 248–257. [Google Scholar] [CrossRef]
- Olivatti, T.O.F.; Alcantara, G.P.; Lemos, A.C.C.E.; da Silva, M.G.; Miot, H.A. Standardization of Organoid Culture for Evaluation of Melanogenesis Induced by UVB, UVA and Visible Light. Bras. Derm. 2020, 95, 46–51. [Google Scholar] [CrossRef]
- Grealish, S.; Heuer, A.; Cardoso, T.; Kirkeby, A.; Jönsson, M.; Johansson, J.; Björklund, A.; Jakobsson, J.; Parmar, M. Monosynaptic Tracing Using Modified Rabies Virus Reveals Early and Extensive Circuit Integration of Human Embryonic Stem Cell-Derived Neurons. Stem. Cell Rep. 2015, 4, 975–983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, T.; Adler, A.F.; Mattsson, B.; Hoban, D.B.; Nolbrant, S.; Wahlestedt, J.N.; Kirkeby, A.; Grealish, S.; Björklund, A.; Parmar, M. Target-Specific Forebrain Projections and Appropriate Synaptic Inputs of HESC-Derived Dopamine Neurons Grafted to the Midbrain of Parkinsonian Rats. J. Comp. Neurol. 2018, 526, 2133–2146. [Google Scholar] [CrossRef] [PubMed]
- Björklund, L.M.; Sánchez-Pernaute, R.; Chung, S.; Andersson, T.; Chen, I.Y.C.; McNaught, K.S.P.; Brownell, A.-L.; Jenkins, B.G.; Wahlestedt, C.; Kim, K.-S.; et al. Embryonic Stem Cells Develop into Functional Dopaminergic Neurons after Transplantation in a Parkinson Rat Model. Proc. Natl. Acad. Sci. 2002, 99, 2344–2349. [Google Scholar] [CrossRef] [PubMed]
- Villadiego, J.; Romo-Madero, S.; García-Swinburn, R.; Suárez-Luna, N.; Bermejo-Navas, A.; Echevarría, M.; Toledo-Aral, J.J. Long-Term Immunosuppression for CNS Mouse Xenotransplantation: Effects on Nigrostriatal Neurodegeneration and Neuroprotective Carotid Body Cell Therapy. Xenotransplantation 2018, 25, e12410. [Google Scholar] [CrossRef] [PubMed]
- Hyman, C.; Hofer, M.; Barde, Y.-A.; Juhasz, M.; Yancopoulos, G.D.; Squinto, S.P.; Lindsay, R.M. BDNF Is a Neurotrophic Factor for Dopaminergic Neurons of the Substantia Nigra. Nature 1991, 350, 230–232. [Google Scholar] [CrossRef]
- Porritt, M.J.; Batchelor, P.E.; Howells, D.W. Inhibiting BDNF Expression by Antisense Oligonucleotide Infusion Causes Loss of Nigral Dopaminergic Neurons. Exp. Neurol. 2005, 192, 226–234. [Google Scholar] [CrossRef]
- Kim, H.I.; Lee, S.; Lim, J.; Chung, S.; Koo, T.-S.; Ji, Y.-G.; Suh, Y.-G.; Son, W.S.; Kim, S.-H.; Choi, H.J. ERRγ Ligand HPB2 Upregulates BDNF-TrkB and Enhances Dopaminergic Neuronal Phenotype. Pharm. Res. 2021, 165, 105423. [Google Scholar] [CrossRef]
- Palasz, E.; Wysocka, A.; Gasiorowska, A.; Chalimoniuk, M.; Niewiadomski, W.; Niewiadomska, G. BDNF as a Promising Therapeutic Agent in Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 1170. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, K.-C.; Pruszak, J.; Yoshizaki, T.; van Arensbergen, J.; Sanchez-Pernaute, R.; Isacson, O. Enhanced Yield of Neuroepithelial Precursors and Midbrain-Like Dopaminergic Neurons from Human Embryonic Stem Cells Using the Bone Morphogenic Protein Antagonist Noggin. Stem. Cells 2007, 25, 411–418. [Google Scholar] [CrossRef]
- Erceg, S.; Ronaghi, M.; Stojković, M. Human Embryonic Stem Cell Differentiation Toward Regional Specific Neural Precursors. Stem. Cells 2009, 27, 78–87. [Google Scholar] [CrossRef]
- Lara-Rodarte, R.; Cortés, D.; Soriano, K.; Carmona, F.; Rocha, L.; Estudillo, E.; López-Ornelas, A.; Velasco, I. Mouse Embryonic Stem Cells Expressing GDNF Show Enhanced Dopaminergic Differentiation and Promote Behavioral Recovery After Grafting in Parkinsonian Rats. Front Cell Dev. Biol. 2021, 9, 661656. [Google Scholar] [CrossRef] [PubMed]
- Delgado-Minjares, K.M.; Martinez-Fong, D.; Martínez-Dávila, I.A.; Bañuelos, C.; Gutierrez-Castillo, M.E.; Blanco-Alvarez, V.M.; Cardenas-Aguayo, M.-C.; Luna-Muñoz, J.; Pacheco-Herrero, M.; Soto-Rojas, L.O. Mechanistic Insight from Preclinical Models of Parkinson’s Disease Could Help Redirect Clinical Trial Efforts in GDNF Therapy. Int. J. Mol. Sci. 2021, 22, 11702. [Google Scholar] [CrossRef]
- Rolletschek, A.; Chang, H.; Guan, K.; Czyz, J.; Meyer, M.; Wobus, A.M. Differentiation of Embryonic Stem Cell-Derived Dopaminergic Neurons Is Enhanced by Survival-Promoting Factors. Mech. Dev. 2001, 105, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Roussa, E.; Krieglstein, K. GDNF Promotes Neuronal Differentiation and Dopaminergic Development of Mouse Mesencephalic Neurospheres. Neurosci. Lett. 2004, 361, 52–55. [Google Scholar] [CrossRef]
- Pascual, A.; Hidalgo-Figueroa, M.; Gómez-Díaz, R.; López-Barneo, J. GDNF and Protection of Adult Central Catecholaminergic Neurons. J. Mol. Endocrinol. 2011, 46, R83–R92. [Google Scholar] [CrossRef]
- Piltonen, M.; Planken, A.; Leskelä, O.; Myöhänen, T.T.; Hänninen, A.-L.; Auvinen, P.; Alitalo, K.; Andressoo, J.-O.; Saarma, M.; Männistö, P.T. Vascular Endothelial Growth Factor C Acts as a Neurotrophic Factor for Dopamine Neurons in Vitro and in Vivo. Neuroscience 2011, 192, 550–563. [Google Scholar] [CrossRef]
- Caballero, B.; Sherman, S.J.; Falk, T. Insights into the Mechanisms Involved in Protective Effects of VEGF-B in Dopaminergic Neurons. Park Dis. 2017, 2017, 4263795. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, T.; Shingo, T.; Kobayashi, K.; Takeuchi, A.; Yano, A.; Muraoka, K.; Matsui, T.; Miyoshi, Y.; Hamada, H.; Date, I. Neuroprotective Effects of Vascular Endothelial Growth Factor (VEGF) upon Dopaminergic Neurons in a Rat Model of Parkinson’s Disease. Eur. J. Neurosci. 2004, 19, 1494–1504. [Google Scholar] [CrossRef] [PubMed]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE Guidelines 2.0: Updated Guidelines for Reporting Animal Research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ramos-Acevedo, R.; Morato-Torres, C.A.; Padilla-Godínez, F.J.; Bernal-Conde, L.D.; Palomero-Rivero, M.; Zafar, F.; Collazo-Navarrete, O.; Soto-Rojas, L.O.; Schüle, B.; Guerra-Crespo, M. Embryoid Body Cells from Human Embryonic Stem Cells Overexpressing Dopaminergic Transcription Factors Survive and Initiate Neurogenesis via Neural Rosettes in the Substantia Nigra. Brain Sci. 2023, 13, 329. https://doi.org/10.3390/brainsci13020329
Ramos-Acevedo R, Morato-Torres CA, Padilla-Godínez FJ, Bernal-Conde LD, Palomero-Rivero M, Zafar F, Collazo-Navarrete O, Soto-Rojas LO, Schüle B, Guerra-Crespo M. Embryoid Body Cells from Human Embryonic Stem Cells Overexpressing Dopaminergic Transcription Factors Survive and Initiate Neurogenesis via Neural Rosettes in the Substantia Nigra. Brain Sciences. 2023; 13(2):329. https://doi.org/10.3390/brainsci13020329
Chicago/Turabian StyleRamos-Acevedo, Rodrigo, Carmen Alejandra Morato-Torres, Francisco J. Padilla-Godínez, Luis Daniel Bernal-Conde, Marcela Palomero-Rivero, Faria Zafar, Omar Collazo-Navarrete, Luis O. Soto-Rojas, Birgitt Schüle, and Magdalena Guerra-Crespo. 2023. "Embryoid Body Cells from Human Embryonic Stem Cells Overexpressing Dopaminergic Transcription Factors Survive and Initiate Neurogenesis via Neural Rosettes in the Substantia Nigra" Brain Sciences 13, no. 2: 329. https://doi.org/10.3390/brainsci13020329