Mechanisms Underlying Cognitive Impairment Induced by Prenatal Alcohol Exposure
Abstract
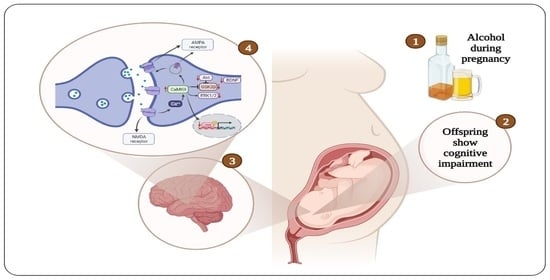
:1. Introduction
2. Behavioral Studies on the Effects of Prenatal Alcohol Exposure
3. Effect of Prenatal Alcohol Exposure on Body and Brain Weight
4. Effect of Prenatal Alcohol Exposure on Neurogenesis
5. Effect of Prenatal Alcohol Exposure on Synaptic Plasticity
6. Effect of Prenatal Alcohol Exposure on Mitochondrial Function
7. Activation of the GABA Receptor in the CNS of the Fetus in Response to Prenatal Alcohol Exposure
8. Effect of Prenatal Alcohol Exposure on Protein Expression and Activity
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rehm, J.; Parry, C.D. Alcohol consumption and infectious diseases in South Africa. Lancet 2009, 374, 2053. [Google Scholar] [CrossRef] [PubMed]
- Corrao, G. A meta-analysis of alcohol consumption and the risk of 15 diseases. Prev. Med. 2004, 38, 613–619. [Google Scholar] [CrossRef] [PubMed]
- Koch, M.; Fitzpatrick, A.L.; Rapp, S.R.; Nahin, R.L.; Williamson, J.D.; Lopez, O.L.; DeKosky, S.T.; Kuller, L.H.; Mackey, R.H.; Mukamal, K.J.; et al. Alcohol Consumption and Risk of Dementia and Cognitive Decline Among Older Adults With or Without Mild Cognitive Impairment. JAMA Netw. Open 2019, 2, e1910319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sachdeva, A.; Chandra, M.; Choudhary, M.; Dayal, P.; Anand, K.S. Alcohol-Related Dementia and Neurocognitive Impairment: A Review Study. Int. J. High Risk Behav. Addict. 2016, 5, e27976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Global prevalence of alcohol use and binge drinking during pregnancy, and fetal alcohol spectrum disorder. Biochem. Cell Biol. 2018, 96, 237–240. [Google Scholar] [CrossRef] [Green Version]
- Popova, S.; Lange, S.; Probst, C.; Gmel, G.; Rehm, J. Estimation of national, regional, and global prevalence of alcohol use during pregnancy and fetal alcohol syndrome: A systematic review and meta-analysis. Lancet Glob. Health 2017, 5, e290–e299. [Google Scholar] [CrossRef] [Green Version]
- Easey, K.E.; Dyer, M.L.; Timpson, N.J.; Munafò, M.R. Prenatal alcohol exposure and offspring mental health: A systematic review. Drug Alcohol Depend. 2019, 197, 344–353. [Google Scholar] [CrossRef]
- O’Leary, C.; Leonard, H.; Bourke, J.; D’Antoine, H.; Bartu, A.; Bower, C. Intellectual disability: Population-based estimates of the proportion attributable to maternal alcohol use disorder during pregnancy. Dev. Med. Child Neurol. 2013, 55, 271–277. [Google Scholar] [CrossRef]
- Charness, M.E.; Riley, E.P.; Sowell, E.R. Drinking During Pregnancy and the Developing Brain: Is Any Amount Safe? Trends Cogn. Sci. 2016, 20, 80–82. [Google Scholar] [CrossRef] [Green Version]
- Nulman, I.; Shulman, T.; Liu, F. Fetal Alcohol Spectrum Disorder. In Handbook of Developmental Neurotoxicology; Sciencedirect: Amsterdam, The Netherlands, 2018; pp. 427–437. [Google Scholar] [CrossRef]
- May, P.A.; Gossage, J.P.; Kalberg, W.O.; Robinson, L.K.; Buckley, D.; Manning, M.; Hoyme, H.E. Prevalence and epidemiologic characteristics of FASD from various research methods with an emphasis on recent in-school studies. Dev. Disabil. Res. Rev. 2009, 15, 176–192. [Google Scholar] [CrossRef]
- May, P.A.; Baete, A.; Russo, J.; Elliott, A.J.; Blankenship, J.; Kalberg, W.O.; Buckley, D.; Brooks, M.; Hasken, J.; Abdul-Rahman, O.; et al. Prevalence and Characteristics of Fetal Alcohol Spectrum Disorders. Pediatrics 2014, 134, 855–866. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, S.; Probst, C.; Gmel, G.; Rehm, J.; Burd, L.; Popova, S. Global Prevalence of Fetal Alcohol Spectrum Disorder Among Children and Youth. JAMA Pediatr. 2017, 171, 948–956. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.; Bhattacharya, D.; Dunaway, E.P.; Bhattacharya, S.; Bloemer, J.; Buabeid, M.; Escobar, M.; Suppiramaniam, V.; Dhanasekaran, M. Impaired ILK Function Is Associated with Deficits in Hippocampal Based Memory and Synaptic Plasticity in a FASD Rat Model. PLoS ONE 2015, 10, e0135700. [Google Scholar] [CrossRef] [Green Version]
- May, P.; Fiorentino, D.; Coriale, G.; Kalberg, W.; Hoyme, H.E.; Aragón, A.; Buckley, D.; Stellavato, C.; Gossage, J.P.; Robinson, L.; et al. Prevalence of Children with Severe Fetal Alcohol Spectrum Disorders in Communities Near Rome, Italy: New Estimated Rates Are Higher than Previous Estimates. Int. J. Environ. Res. Public Health 2011, 8, 2331–2351. [Google Scholar] [CrossRef]
- Petković, G.; Barišić, I. FAS prevalence in a sample of urban schoolchildren in Croatia. Reprod. Toxicol. 2010, 29, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Zuccolo, L.; Lewis, S.J.; Davey Smith, G.; Sayal, K.; Draper, E.S.; Fraser, R.; Barrow, M.; Alati, R.; Ring, S.; Macleod, J.; et al. Prenatal alcohol exposure and offspring cognition and school performance. A ‘Mendelian randomization’ natural experiment. Int. J. Epidemiol. 2013, 42, 1358–1370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sjölund, S.; Hemmingsson, T.; Allebeck, P. Survey of Swedish Conscripts. Alcohol. Clin. Exp. Res. 2015, 39, 548–555. [Google Scholar] [CrossRef] [Green Version]
- Kodituwakku, P.; Kodituwakku, E. Cognitive and Behavioral Profiles of Children with Fetal Alcohol Spectrum Disorders. Curr. Dev. Disord. Rep. 2014, 1, 149–160. [Google Scholar] [CrossRef] [Green Version]
- Hunt, P.S.; Jacobson, S.E.; Torok, E.J. Deficits in trace fear conditioning in a rat model of fetal alcohol exposure: Dose–response and timing effects. Alcohol 2009, 43, 465–474. [Google Scholar] [CrossRef]
- Sanchez, L.M.; Goss, J.; Wagner, J.; Davies, S.; Savage, D.D.; Hamilton, D.A.; Clark, B.J. Moderate prenatal alcohol exposure impairs performance by adult male rats in an object-place paired-associate task. Behav. Brain Res. 2019, 360, 228–234. [Google Scholar] [CrossRef]
- Gibbard, W.B.; Wass, P.; Clarke, M.E. The neuropsychological implications of prenatal alcohol exposure. Can. Child Adolesc. Psychiatr. Rev. 2003, 12, 72–76. [Google Scholar] [PubMed]
- Willford, J.A.; Richardson, G.A.; Leech, S.L.; Day, N.L. Verbal and Visuospatial Learning and Memory Function in Children With Moderate Prenatal Alcohol Exposure. Alcohol. Clin. Exp. Res. 2004, 28, 497–507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayal, K.; Heron, J.; Golding, J.; Alati, R.; Smith, G.D.; Gray, R.; Emond, A. Binge Pattern of Alcohol Consumption During Pregnancy and Childhood Mental Health Outcomes: Longitudinal Population-Based Study. Pediatrics 2009, 123, e289–e296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Easey, K.E.; Timpson, N.J.; Munafò, M.R. Association of Prenatal Alcohol Exposure and Offspring Depression: A Negative Control Analysis of Maternal and Partner Consumption. Alcohol. Clin. Exp. Res. 2020, 44, 1132–1140. [Google Scholar] [CrossRef] [Green Version]
- Niclasen, J.; Nybo Andersen, A.M.; Teasdale, T.W.; Strandberg-Larsen, K. Prenatal exposure to alcohol, and gender differences on child mental health at age seven years. J. Epidemiol. Community Health 2014, 68, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Sanou, A.S.; Diallo, A.H.; Holding, P.; Nankabirwa, V.; Engebretsen, I.M.S.; Ndeezi, G.; Tumwine, J.K.; Meda, N.; Tylleskar, T.; Kashala-Abotnes, E. Maternal alcohol consumption during pregnancy and child’s cognitive performance at 6–8 years of age in rural Burkina Faso: An observational study. PeerJ 2017, 5, e3507. [Google Scholar] [CrossRef] [Green Version]
- Carpita, B.; Migli, L.; Chiarantini, I.; Battaglini, S.; Montalbano, C.; Carmassi, C.; Cremone, I.M.; Dell’Osso, L. Autism Spectrum Disorder and Fetal Alcohol Spectrum Disorder: A Literature Review. Brain Sci. 2022, 12, 792. [Google Scholar] [CrossRef]
- Zhang, M.; Marjonen, H.; Sierra, A.; Nyman, A.; Rogojin, V.; Gröhn, O.; Linden, A.-M.; Hautaniemi, S.; Kaminen-Ahola, N. Early Maternal Alcohol Consumption Alters Hippocampal DNA Methylation, Gene Expression and Volume in a Mouse Model. PLoS ONE 2015, 10, e0124931. [Google Scholar] [CrossRef]
- Guelinckx, I.; Devlieger, R.; Vansant, G. Alcohol during pregnancy and lactation: Recommendations versus real intake. Arch. Public Health 2011, 68, 134–142. [Google Scholar] [CrossRef]
- Kaminen-Ahola, N. Fetal alcohol spectrum disorders: Genetic and epigenetic mechanisms. Prenat. Diagn. 2020, 40, 1185–1192. [Google Scholar] [CrossRef]
- Lee, X.A.; Mostafaie, Y. Acute alcohol exposure in early gestation programmes sex-specific insulin resistance in offspring: A shifting tide in prenatal alcohol exposure models. J. Physiol. 2020, 598, 1807–1808. [Google Scholar] [CrossRef]
- Olsen, R.W.; Liang, J. Role of GABAA receptors in alcohol use disorders suggested by chronic intermittent ethanol (CIE) rodent model. Mol. Brain 2017, 10, 45. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, N. Neurotransmitters in alcoholism: A review of neurobiological and genetic studies. Indian J. Hum. Genet. 2014, 20, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, I.A.; Harris, R.A. GABAA receptors and alcohol. Pharmacol. Biochem. Behav. 2008, 90, 90–94. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Sun, D. GABA receptors in brain development, function, and injury. Metab. Brain Dis. 2014, 30, 367–379. [Google Scholar] [CrossRef] [Green Version]
- Faber, D.S.; Pereda, A.E. Two Forms of Electrical Transmission Between Neurons. Front. Mol. Neurosci. 2018, 11, 427. [Google Scholar] [CrossRef] [PubMed]
- Cavanaugh, J.E.; Jaumotte, J.D.; Lakoski, J.M.; Zigmond, M.J. Neuroprotective role of ERK1/2 and ERK5 in a dopaminergic cell line under basal conditions and in response to oxidative stress. J. Neurosci. Res. 2006, 84, 1367–1375. [Google Scholar] [CrossRef]
- Krawczyk, M.C.; Millan, J.; Blake, M.G.; Feld, M.; Boccia, M.M. Relevance of ERK1/2 Post-retrieval Participation on Memory Processes: Insights in Their Particular Role on Reconsolidation and Persistence of Memories. Front. Mol. Neurosci. 2019, 12, 95. [Google Scholar] [CrossRef] [Green Version]
- Stephan, M.; Volkmann, P.; Rossner, M.J. Assessing behavior and cognition in rodents, nonhuman primates, and humans: Where are the limits of translation? Dialogues Clin. Neurosci. 2022, 21, 249–259. [Google Scholar] [CrossRef]
- Alharbi, I.; Alharbi, H.; Almogbel, Y.; Alalwan, A.; Alhowail, A. Effect of Metformin on Doxorubicin-Induced Memory Dysfunction. Brain Sci. 2020, 10, 152. [Google Scholar] [CrossRef] [Green Version]
- Alhowail, A.H.; Almogbel, Y.S.; Abdellatif, A.A.H.; Alsalehi, N.F.; Alghenaim, F.A.; Aldubayan, M.A.; Felemban, S.G. CMF and MET treatment induce cognitive impairment through upregulation of IL-1alpha in rat brain. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 4385–4393. [Google Scholar] [CrossRef] [PubMed]
- Kozanian, O.O.; Rohac, D.J.; Bavadian, N.; Corches, A.; Korzus, E.; Huffman, K.J. Long-Lasting Effects of Prenatal Ethanol Exposure on Fear Learning and Development of the Amygdala. Front. Behav. Neurosci. 2018, 12, 200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shamim, S.; Khan, K.M.; Ullah, N.; Chigurupati, S.; Wadood, A.; Ur Rehman, A.; Ali, M.; Salar, U.; Alhowail, A.; Taha, M.; et al. Synthesis and screening of (E)-3-(2-benzylidenehydrazinyl)-5,6-diphenyl-1,2,4-triazine analogs as novel dual inhibitors of alpha-amylase and alpha-glucosidase. Bioorg. Chem. 2020, 101, 103979. [Google Scholar] [CrossRef] [PubMed]
- Harvey, R.E.; Berkowitz, L.E.; Hamilton, D.A.; Clark, B.J. The effects of developmental alcohol exposure on the neurobiology of spatial processing. Neurosci. Biobehav. Rev. 2019, 107, 775–794. [Google Scholar] [CrossRef]
- Subbanna, S.; Basavarajappa, B.S. Binge-like Prenatal Ethanol Exposure Causes Impaired Cellular Differentiation in the Embryonic Forebrain and Synaptic and Behavioral Defects in Adult Mice. Brain Sci. 2022, 12, 793. [Google Scholar] [CrossRef]
- Vorhees, C.V.; Williams, M.T. Morris water maze: Procedures for assessing spatial and related forms of learning and memory. Nat. Protoc. 2006, 1, 848–858. [Google Scholar] [CrossRef] [Green Version]
- Chapouthier, G.; Barnhart, C.D.; Yang, D.; Lein, P.J. Using the Morris Water Maze to Assess Spatial Learning and Memory in Weanling Mice. PLoS ONE 2015, 10, e0124521. [Google Scholar] [CrossRef]
- Ieraci, A.; Herrera, D.G. Early Postnatal Ethanol Exposure in Mice Induces Sex-Dependent Memory Impairment and Reduction of Hippocampal NMDA-R2B Expression in Adulthood. Neuroscience 2020, 427, 105–115. [Google Scholar] [CrossRef]
- Christie, B.R.; Swann, S.E.; Fox, C.J.; Froc, D.; Lieblich, S.E.; Redila, V.; Webber, A. Voluntary exercise rescues deficits in spatial memory and long-term potentiation in prenatal ethanol-exposed male rats. Eur. J. Neurosci. 2005, 21, 1719–1726. [Google Scholar] [CrossRef]
- Dodge, N.C.; Thomas, K.G.F.; Meintjes, E.M.; Molteno, C.D.; Jacobson, J.L.; Jacobson, S.W. Reduced Hippocampal Volumes Partially Mediate Effects of Prenatal Alcohol Exposure on Spatial Navigation on a Virtual Water Maze Task in Children. Alcohol. Clin. Exp. Res. 2020, 44, 844–855. [Google Scholar] [CrossRef]
- Hunt, P.S.; Barnet, R.C. An animal model of fetal alcohol spectrum disorder: Trace conditioning as a window to inform memory deficits and intervention tactics. Physiol. Behav. 2015, 148, 36–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes, E.; Wolfe, J.; Savage, D.D. The effects of prenatal alcohol exposure on radial arm maze performance in adult rats. Physiol. Behav. 1989, 46, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Wozniak, D.; Hartman, R.; Boyle, M.; Vogt, S.; Brooks, A.; Tenkova, T.; Young, C.; Olney, J.; Muglia, L. Apoptotic neurodegeneration induced by ethanol in neonatal mice is associated with profound learning/memory deficits in juveniles followed by progressive functional recovery in adults. Neurobiol. Dis. 2004, 17, 403–414. [Google Scholar] [CrossRef] [PubMed]
- du Plooy, C.P.; Malcolm-Smith, S.; Adnams, C.M.; Stein, D.J.; Donald, K.A. The Effects of Prenatal Alcohol Exposure on Episodic Memory Functioning: A Systematic Review: Table 1. Arch. Clin. Neuropsychol. 2016, 31, 710–726. [Google Scholar] [CrossRef] [PubMed]
- Irner, T.B. Substance exposure in utero and developmental consequences in adolescence: A systematic review. Child Neuropsychol. 2012, 18, 521–549. [Google Scholar] [CrossRef] [PubMed]
- Amen, D.G.; Wu, J.; George, N.; Newberg, A. Patterns of Regional Cerebral Blood Flow as a Function of Obesity in Adults. J. Alzheimer’s Dis. 2020, 77, 1331–1337. [Google Scholar] [CrossRef]
- Elabi, O.F.; Cunha, J.P.M.C.M.; Gaceb, A.; Fex, M.; Paul, G. High-fat diet-induced diabetes leads to vascular alterations, pericyte reduction, and perivascular depletion of microglia in a 6-OHDA toxin model of Parkinson disease. J. Neuroinflammation 2021, 18, 175. [Google Scholar] [CrossRef]
- von Frankenberg, A.D.; Marina, A.; Song, X.; Callahan, H.S.; Kratz, M.; Utzschneider, K.M. A high-fat, high-saturated fat diet decreases insulin sensitivity without changing intra-abdominal fat in weight-stable overweight and obese adults. Eur. J. Nutr. 2015, 56, 431–443. [Google Scholar] [CrossRef] [Green Version]
- de Eulate, R.G.; Goñi, I.; Galiano, A.; Vidorreta, M.; Recio, M.; Riverol, M.; Zubieta, J.L.; Fernández-Seara, M.A.; Bondi, M. Reduced Cerebral Blood Flow in Mild Cognitive Impairment Assessed Using Phase-Contrast MRI. J. Alzheimer’s Dis. 2017, 58, 585–595. [Google Scholar] [CrossRef]
- Leeuwis, A.E.; Smith, L.A.; Melbourne, A.; Hughes, A.D.; Richards, M.; Prins, N.D.; Sokolska, M.; Atkinson, D.; Tillin, T.; Jäger, H.R.; et al. Cerebral Blood Flow and Cognitive Functioning in a Community-Based, Multi-Ethnic Cohort: The SABRE Study. Front. Aging Neurosci. 2018, 10, 279. [Google Scholar] [CrossRef] [Green Version]
- Coucha, M.; Abdelsaid, M.; Ward, R.; Abdul, Y.; Ergul, A. Impact of Metabolic Diseases on Cerebral Circulation: Structural and Functional Consequences. Compr. Physiol. 2018, 8, 773–799. [Google Scholar] [CrossRef] [PubMed]
- Knight, S.P.; Laird, E.; Williamson, W.; O’Connor, J.; Newman, L.; Carey, D.; De Looze, C.; Fagan, A.J.; Chappell, M.A.; Meaney, J.F.; et al. Obesity is associated with reduced cerebral blood flow—Modified by physical activity. Neurobiol. Aging 2021, 105, 35–47. [Google Scholar] [CrossRef] [PubMed]
- Kistner, A.; Lhommée, E.; Krack, P. Mechanisms of Body Weight Fluctuations in Parkinson’s Disease. Front. Neurol. 2014, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Royle, N.A.; Booth, T.; Valdés Hernández, M.C.; Penke, L.; Murray, C.; Gow, A.J.; Maniega, S.M.; Starr, J.; Bastin, M.E.; Deary, I.J.; et al. Estimated maximal and current brain volume predict cognitive ability in old age. Neurobiol. Aging 2013, 34, 2726–2733. [Google Scholar] [CrossRef] [Green Version]
- Filley, C.M.; Fields, R.D. White matter and cognition: Making the connection. J. Neurophysiol. 2016, 116, 2093–2104. [Google Scholar] [CrossRef] [Green Version]
- de Ruiter, M.B.; Reneman, L.; Boogerd, W.; Veltman, D.J.; Caan, M.; Douaud, G.; Lavini, C.; Linn, S.C.; Boven, E.; van Dam, F.S.A.M.; et al. Late effects of high-dose adjuvant chemotherapy on white and gray matter in breast cancer survivors: Converging results from multimodal magnetic resonance imaging. Hum. Brain Mapp. 2012, 33, 2971–2983. [Google Scholar] [CrossRef]
- Inagaki, M.; Yoshikawa, E.; Matsuoka, Y.; Sugawara, Y.; Nakano, T.; Akechi, T.; Wada, N.; Imoto, S.; Murakami, K.; Uchitomi, Y. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer 2007, 109, 146–156. [Google Scholar] [CrossRef]
- Hashimoto, K.; Cullen, C.L.; Burne, T.H.J.; Lavidis, N.A.; Moritz, K.M. Low Dose Prenatal Ethanol Exposure Induces Anxiety-Like Behaviour and Alters Dendritic Morphology in the Basolateral Amygdala of Rat Offspring. PLoS ONE 2013, 8, e54924. [Google Scholar] [CrossRef] [Green Version]
- Willoughby, K.A.; Sheard, E.D.; Nash, K.; Rovet, J. Effects of prenatal alcohol exposure on hippocampal volume, verbal learning, and verbal and spatial recall in late childhood. J. Int. Neuropsychol. Soc. 2008, 14, 1022–1033. [Google Scholar] [CrossRef]
- Mayeux, R.; Dupret, D.; Revest, J.-M.; Koehl, M.; Ichas, F.; De Giorgi, F.; Costet, P.; Abrous, D.N.; Piazza, P.V. Spatial Relational Memory Requires Hippocampal Adult Neurogenesis. PLoS ONE 2008, 3, e1959. [Google Scholar] [CrossRef] [Green Version]
- Shivraj Sohur, U.; Emsley, J.G.; Mitchell, B.D.; Macklis, J.D. Adult neurogenesis and cellular brain repair with neural progenitors, precursors and stem cells. Philos. Trans. R. Soc. B Biol. Sci. 2006, 361, 1477–1497. [Google Scholar] [CrossRef] [PubMed]
- Piatti, V.C.; Ewell, L.A.; Leutgeb, J.K. Neurogenesis in the dentate gyrus: Carrying the message or dictating the tone. Front. Neurosci. 2013, 7, 50. [Google Scholar] [CrossRef] [Green Version]
- Alhowail, A.H.; Aldubayan, M. Recent progress in the elucidation of the mechanisms of chemotherapy-induced cognitive impairment. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 5807–5817. [Google Scholar] [CrossRef]
- Motamed, S.; Del Borgo, M.P.; Zhou, K.; Kulkarni, K.; Crack, P.J.; Merson, T.D.; Aguilar, M.-I.; Finkelstein, D.I.; Forsythe, J.S. Migration and Differentiation of Neural Stem Cells Diverted From the Subventricular Zone by an Injectable Self-Assembling β-Peptide Hydrogel. Front. Bioeng. Biotechnol. 2019, 7, 315. [Google Scholar] [CrossRef]
- Tau, G.Z.; Peterson, B.S. Normal Development of Brain Circuits. Neuropsychopharmacology 2009, 35, 147–168. [Google Scholar] [CrossRef] [Green Version]
- Shin, M.; Wang, Y.; Borgus, J.R.; Venton, B.J. Electrochemistry at the Synapse. Annu. Rev. Anal. Chem. 2019, 12, 297–321. [Google Scholar] [CrossRef]
- Toda, T.; Parylak, S.L.; Linker, S.B.; Gage, F.H. The role of adult hippocampal neurogenesis in brain health and disease. Mol. Psychiatry 2018, 24, 67–87. [Google Scholar] [CrossRef] [PubMed]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereda, A.E. Electrical synapses and their functional interactions with chemical synapses. Nat. Rev. Neurosci. 2014, 15, 250–263. [Google Scholar] [CrossRef]
- Gil-Mohapel, J.; Boehme, F.; Kainer, L.; Christie, B.R. Hippocampal cell loss and neurogenesis after fetal alcohol exposure: Insights from different rodent models. Brain Res. Rev. 2010, 64, 283–303. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, L.; Yin, F.; Yu, Y.; Wang, Y.; Shepard, M.J.; Zhuang, Z.; Qin, J. Probing impaired neurogenesis in human brain organoids exposed to alcohol. Integr. Biol. 2017, 9, 968–978. [Google Scholar] [CrossRef]
- Adams, J.W.; Negraes, P.D.; Truong, J.; Tran, T.; Szeto, R.A.; Guerra, B.S.; Herai, R.H.; Teodorof-Diedrich, C.; Spector, S.A.; Del Campo, M.; et al. Impact of alcohol exposure on neural development and network formation in human cortical organoids. Mol. Psychiatry 2022. [Google Scholar] [CrossRef] [PubMed]
- D’Angiulli, A.; Grunau, P.; Maggi, S.; Herdman, A. Electroencephalographic correlates of prenatal exposure to alcohol in infants and children: A review of findings and implications for neurocognitive development. Alcohol 2006, 40, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Hemington, K.S.; Reynolds, J.N. Electroencephalographic correlates of working memory deficits in children with Fetal Alcohol Spectrum Disorder using a single-electrode pair recording device. Clin. Neurophysiol. 2014, 125, 2364–2371. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hebert, S.L.; Lanza, I.R.; Nair, K.S. Mitochondrial DNA alterations and reduced mitochondrial function in aging. Mech. Ageing Dev. 2010, 131, 451–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akbari, M.; Kirkwood, T.B.L.; Bohr, V.A. Mitochondria in the signaling pathways that control longevity and health span. Ageing Res. Rev. 2019, 54, 100940. [Google Scholar] [CrossRef]
- Chu, J.; Tong, M.; de la Monte, S.M. Chronic ethanol exposure causes mitochondrial dysfunction and oxidative stress in immature central nervous system neurons. Acta Neuropathol. 2007, 113, 659–673. [Google Scholar] [CrossRef]
- Ramachandran, V.; Perez, A.; Chen, J.; Senthil, D.; Schenker, S.; Henderson, G.I. In Utero Ethanol Exposure Causes Mitochondrial Dysfunction, Which Can Result in Apoptotic Cell Death in Fetal Brain: A Potential Role for 4-Hydroxynonenal. Alcohol. Clin. Exp. Res. 2001, 25, 862–871. [Google Scholar] [CrossRef]
- Da Lee, R.; Mi An, S.; Sun Kim, S.; Seek Rhee, G.; Jun Kwack, S.; Hyun Seok, J.; Yeong Chae, S.; Hoon Park, C.; Woo Choi, Y.; Sik Kim, H.; et al. Neurotoxic Effects of Alcohol and Acetaldehyde During Embryonic Development. J. Toxicol. Environ. Health Part A 2005, 68, 2147–2162. [Google Scholar] [CrossRef]
- Tong, M.; Longato, L.; Nguyen, Q.-G.; Chen, W.C.; Spaisman, A.; de la Monte, S.M. Acetaldehyde-Mediated Neurotoxicity: Relevance to Fetal Alcohol Spectrum Disorders. Oxidative Med. Cell. Longev. 2011, 2011, 213286. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Liu, P.; Li, Y. Impaired development of mitochondria plays a role in the central nervous system defects of fetal alcohol syndrome. Birth Defects Res. Part A Clin. Mol. Teratol. 2005, 73, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Bukiya, A.N. Fetal Cerebral Artery Mitochondrion as Target of Prenatal Alcohol Exposure. Int. J. Environ. Res. Public Health 2019, 16, 1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, D.; Fujihashi, A.; Majrashi, M.; Bloemer, J.; Bhattacharya, S.; Buabeid, M.; Escobar, M.; Moore, T.; Suppiramaniam, V.; Dhanasekaran, M. Concurrent nicotine exposure to prenatal alcohol consumption alters the hippocampal and cortical neurotoxicity. Heliyon 2020, 6, e03045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behuet, S.; Cremer, J.N.; Cremer, M.; Palomero-Gallagher, N.; Zilles, K.; Amunts, K. Developmental Changes of Glutamate and GABA Receptor Densities in Wistar Rats. Front. Neuroanat. 2019, 13, 100. [Google Scholar] [CrossRef]
- Brickley, S.G.; Mody, I. Extrasynaptic GABAA Receptors: Their Function in the CNS and Implications for Disease. Neuron 2012, 73, 23–34. [Google Scholar] [CrossRef] [Green Version]
- Stephens, D.N.; King, S.L.; Lambert, J.J.; Belelli, D.; Duka, T. GABAAreceptor subtype involvement in addictive behaviour. Genes Brain Behav. 2017, 16, 149–184. [Google Scholar] [CrossRef] [Green Version]
- Sallard, E.; Letourneur, D.; Legendre, P. Electrophysiology of ionotropic GABA receptors. Cell. Mol. Life Sci. 2021, 78, 5341–5370. [Google Scholar] [CrossRef]
- Terunuma, M. Diversity of structure and function of GABAB receptors: A complexity of GABAB-mediated signaling. Proc. Jpn. Acad. Ser. B 2018, 94, 390–411. [Google Scholar] [CrossRef]
- Sigel, E.; Steinmann, M.E. Structure, Function, and Modulation of GABAA Receptors. J. Biol. Chem. 2012, 287, 40224–40231. [Google Scholar] [CrossRef] [Green Version]
- Sakimoto, Y.; Oo, P.M.-T.; Goshima, M.; Kanehisa, I.; Tsukada, Y.; Mitsushima, D. Significance of GABAA Receptor for Cognitive Function and Hippocampal Pathology. Int. J. Mol. Sci. 2021, 22, 12456. [Google Scholar] [CrossRef] [PubMed]
- Mousavi Majd, A.; Ebrahim Tabar, F.; Afghani, A.; Ashrafpour, S.; Dehghan, S.; Gol, M.; Ashrafpour, M.; Pourabdolhossein, F. Inhibition of GABA A receptor improved special memory impairment in the local model of demyelination in rat hippocampus. Behav. Brain Res. 2018, 336, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Woods, K.J.; Thomas, K.G.F.; Molteno, C.D.; Jacobson, J.L.; Jacobson, S.W.; Meintjes, E.M. Prenatal alcohol exposure affects brain function during place learning in a virtual environment differently in boys and girls. Brain Behav. 2018, 8, e01103. [Google Scholar] [CrossRef] [Green Version]
- Marguet, F.; Friocourt, G.; Brosolo, M.; Sauvestre, F.; Marcorelles, P.; Lesueur, C.; Marret, S.; Gonzalez, B.J.; Laquerrière, A. Prenatal alcohol exposure is a leading cause of interneuronopathy in humans. Acta Neuropathol. Commun. 2020, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, T.; Gal-Ben-Ari, S.; Dieterich, D.C.; Kreutz, M.R.; Ziv, N.E.; Gundelfinger, E.D.; Rosenblum, K. The roles of protein expression in synaptic plasticity and memory consolidation. Front. Mol. Neurosci. 2014, 7, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alhowail, A.H.; Bloemer, J.; Majrashi, M.; Pinky, P.D.; Bhattacharya, S.; Yongli, Z.; Bhattacharya, D.; Eggert, M.; Woodie, L.; Buabeid, M.A.; et al. Doxorubicin-induced neurotoxicity is associated with acute alterations in synaptic plasticity, apoptosis, and lipid peroxidation. Toxicol. Mech. Methods 2019, 29, 457–466. [Google Scholar] [CrossRef]
- Giese, K.P.; Mizuno, K. The roles of protein kinases in learning and memory. Learn. Mem. 2013, 20, 540–552. [Google Scholar] [CrossRef] [Green Version]
- Abel, T.; Nguyen, P.V. Regulation of hippocampus-dependent memory by cyclic AMP-dependent protein kinase. Prog. Brain Res. 2008, 169, 97–115. [Google Scholar] [CrossRef] [Green Version]
- Gerber, K.J.; Squires, K.E.; Hepler, J.R. Roles for Regulator of G Protein Signaling Proteins in Synaptic Signaling and Plasticity. Mol. Pharmacol. 2016, 89, 273–286. [Google Scholar] [CrossRef]
- Voglis, G.; Tavernarakis, N. The role of synaptic ion channels in synaptic plasticity. EMBO Rep. 2006, 7, 1104–1110. [Google Scholar] [CrossRef] [Green Version]
- Elgharabawy, R.M.; Alhowail, A.H.; Emara, A.M.; Aldubayan, M.A.; Ahmed, A.S. The impact of chicory (Cichoriumintybus L.) on hemodynamic functions and oxidative stress in cardiac toxicity induced by lead oxide nanoparticles in male rats. Biomed. Pharm. 2021, 137, 111324. [Google Scholar] [CrossRef] [PubMed]
- Alhowail, A.H.; Pinky, P.D.; Eggert, M.; Bloemer, J.; Woodie, L.N.; Buabeid, M.A.; Bhattacharya, S.; Jasper, S.L.; Bhattacharya, D.; Dhanasekaran, M.; et al. Doxorubicin induces dysregulation of AMPA receptor and impairs hippocampal synaptic plasticity leading to learning and memory deficits. Heliyon 2021, 7, e07456. [Google Scholar] [CrossRef]
- Sakata, K.; Martinowich, K.; Woo, N.H.; Schloesser, R.J.; Jimenez, D.V.; Ji, Y.; Shen, L.; Lu, B. Role of activity-dependent BDNF expression in hippocampal–prefrontal cortical regulation of behavioral perseverance. Proc. Natl. Acad. Sci. USA 2013, 110, 15103–15108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bye, C.M.; McDonald, R.J. A Specific Role of Hippocampal NMDA Receptors and Arc Protein in Rapid Encoding of Novel Environmental Representations and a More General Long-Term Consolidation Function. Front. Behav. Neurosci. 2019, 13, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsushima, D.; Ishihara, K.; Sano, A.; Kessels, H.W.; Takahashi, T. Contextual learning requires synaptic AMPA receptor delivery in the hippocampus. Proc. Natl. Acad. Sci. USA 2011, 108, 12503–12508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavis, P.; Dash, P.K.; Johnson, D.; Clark, J.; Orsi, S.A.; Zhang, M.; Zhao, J.; Grill, R.J.; Moore, A.N.; Pati, S. Involvement of the Glycogen Synthase Kinase-3 Signaling Pathway in TBI Pathology and Neurocognitive Outcome. PLoS ONE 2011, 6, e24648. [Google Scholar] [CrossRef] [Green Version]
- Ginsberg, S.D.; Hu, Y.-S.; Long, N.; Pigino, G.; Brady, S.T.; Lazarov, O. Molecular Mechanisms of Environmental Enrichment: Impairments in Akt/GSK3β, Neurotrophin-3 and CREB Signaling. PLoS ONE 2013, 8, e64460. [Google Scholar] [CrossRef] [Green Version]
- Pungsrinont, T.; Kallenbach, J.; Baniahmad, A. Role of PI3K-AKT-mTOR Pathway as a Pro-Survival Signaling and Resistance-Mediating Mechanism to Therapy of Prostate Cancer. Int. J. Mol. Sci. 2021, 22, 11088. [Google Scholar] [CrossRef]
- Asselin, E.; Hossini, A.M.; Quast, A.S.; Plötz, M.; Grauel, K.; Exner, T.; Küchler, J.; Stachelscheid, H.; Eberle, J.; Rabien, A.; et al. PI3K/AKT Signaling Pathway Is Essential for Survival of Induced Pluripotent Stem Cells. PLoS ONE 2016, 11, e0154770. [Google Scholar] [CrossRef]
- Furukawa, H. Structure and function of glutamate receptor amino terminal domains. J. Physiol. 2012, 590, 63–72. [Google Scholar] [CrossRef] [Green Version]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R.; et al. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405–496. [Google Scholar] [CrossRef] [Green Version]
- Purkey, A.M.; Dell’Acqua, M.L. Phosphorylation-Dependent Regulation of Ca2+-Permeable AMPA Receptors During Hippocampal Synaptic Plasticity. Front. Synaptic Neurosci. 2020, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Ma, Y.-Y. Calcium Permeable-AMPA Receptors and Excitotoxicity in Neurological Disorders. Front. Neural Circuits 2021, 15, 711564. [Google Scholar] [CrossRef] [PubMed]
- Gardner, S.M.; Takamiya, K.; Xia, J.; Suh, J.-G.; Johnson, R.; Yu, S.; Huganir, R.L. Calcium-Permeable AMPA Receptor Plasticity Is Mediated by Subunit-Specific Interactions with PICK1 and NSF. Neuron 2005, 45, 903–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wright, A.; Vissel, B. The essential role of AMPA receptor GluR2 subunit RNA editing in the normal and diseased brain. Front. Mol. Neurosci. 2012, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feldmeyer, D.; Kask, K.; Brusa, R.; Kornau, H.C.; Kolhekar, R.; Rozov, A.; Burnashev, N.; Jensen, V.; Hvalby, O.; Sprengel, R.; et al. Neurological dysfunctions in mice expressing different levels of the Q/R site-unedited AMPAR subunit GluR-B. Nat. Neurosci. 1999, 2, 57–64. [Google Scholar] [CrossRef]
- Miranda, M.; Morici, J.F.; Zanoni, M.B.; Bekinschtein, P. Brain-Derived Neurotrophic Factor: A Key Molecule for Memory in the Healthy and the Pathological Brain. Front. Cell. Neurosci. 2019, 13, 363. [Google Scholar] [CrossRef]
- Heldt, S.A.; Stanek, L.; Chhatwal, J.P.; Ressler, K.J. Hippocampus-specific deletion of BDNF in adult mice impairs spatial memory and extinction of aversive memories. Mol. Psychiatry 2007, 12, 656–670. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Arime, Y.; Hall, F.S.; Uhl, G.R.; Sora, I. Impaired spatial working memory and decreased frontal cortex BDNF protein level in dopamine transporter knockout mice. Eur. J. Pharmacol. 2010, 628, 104–107. [Google Scholar] [CrossRef]
- Gaiarsa, J.-L.; Zhang, L.; Fang, Y.; Lian, Y.; Chen, Y.; Wu, T.; Zheng, Y.; Zong, H.; Sun, L.; Zhang, R.; et al. Brain-Derived Neurotrophic Factor Ameliorates Learning Deficits in a Rat Model of Alzheimer’s Disease Induced by Aβ1-42. PLoS ONE 2015, 10, e0122415. [Google Scholar] [CrossRef] [Green Version]
- Cirulli, F.; Berry, A.; Chiarotti, F.; Alleva, E. Intrahippocampal administration of BDNF in adult rats affects short-term behavioral plasticity in the Morris water maze and performance in the elevated plus-maze. Hippocampus 2004, 14, 802–807. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Nie, Z.; Shu, H.; Kuang, Y.; Chen, X.; Cheng, J.; Yu, S.; Liu, H. The Role of BDNF on Neural Plasticity in Depression. Front. Cell. Neurosci. 2020, 14, 82. [Google Scholar] [CrossRef] [PubMed]
- Takasaki, I.; Takarada, S.; Tatsumi, S.; Azegami, A.; Yasuda, M.; Fukuchi, M.; Tabuchi, A.; Kondo, T.; Tabuchi, Y.; Tsuda, M. Extracellular adenosine 5′-triphosphate elicits the expression of brain-derived neurotrophic factor exon IV mRNA in rat astrocytes. Glia 2008, 56, 1369–1379. [Google Scholar] [CrossRef] [PubMed]
- Tecuatl, C.; Herrrera-López, G.; Martín-Ávila, A.; Yin, B.; Weber, S.; Barrionuevo, G.; Galván, E.J. TrkB-mediated activation of the phosphatidylinositol-3-kinase/Akt cascade reduces the damage inflicted by oxygen-glucose deprivation in area CA3 of the rat hippocampus. Eur. J. Neurosci. 2018, 47, 1096–1109. [Google Scholar] [CrossRef] [PubMed]
- Alhowai, A.H. Doxorubicin Attenuates BDNF mRNA Expression in Hippocampal Neuronal Cells. Int. J. Pharmacol. 2021, 17, 414–419. [Google Scholar] [CrossRef]
- Swulius, M.T.; Waxham, M.N. Ca2+/Calmodulin-dependent Protein Kinases. Cell. Mol. Life Sci. 2008, 65, 2637–2657. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zalcman, G.; Federman, N.; Romano, A. CaMKII Isoforms in Learning and Memory: Localization and Function. Front. Mol. Neurosci. 2018, 11, 445. [Google Scholar] [CrossRef] [Green Version]
- Rossetti, T.; Banerjee, S.; Kim, C.; Leubner, M.; Lamar, C.; Gupta, P.; Lee, B.; Neve, R.; Lisman, J. Memory Erasure Experiments Indicate a Critical Role of CaMKII in Memory Storage. Neuron 2017, 96, 207–216.e202. [Google Scholar] [CrossRef] [Green Version]
- Giese, K.P. The role of CaMKII autophosphorylation for NMDA receptor-dependent synaptic potentiation. Neuropharmacology 2021, 193, 108616. [Google Scholar] [CrossRef]
- Yabuki, Y.; Nakagawasai, O.; Moriguchi, S.; Shioda, N.; Onogi, H.; Tan-No, K.; Tadano, T.; Fukunaga, K. Decreased CaMKII and PKC activities in specific brain regions are associated with cognitive impairment in neonatal ventral hippocampus-lesioned rats. Neuroscience 2013, 234, 103–115. [Google Scholar] [CrossRef]
- Moriguchi, S.; Tagashira, H.; Sasaki, Y.; Yeh, J.Z.; Sakagami, H.; Narahashi, T.; Fukunaga, K. CaMKII activity is essential for improvement of memory-related behaviors by chronic rivastigmine treatment. J. Neurochem. 2014, 128, 927–937. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, A.; Giese, K.P. Calcium/calmodulin-dependent kinase II and Alzheimer’s disease. Mol. Brain 2015, 8, 78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakaria, M.; Park, S.-Y.; Haque, M.E.; Karthivashan, G.; Kim, I.-S.; Ganesan, P.; Choi, D.-K. Neurotoxic Agent-Induced Injury in Neurodegenerative Disease Model: Focus on Involvement of Glutamate Receptors. Front. Mol. Neurosci. 2018, 11, 307. [Google Scholar] [CrossRef] [PubMed]
- Lisman, J.; Yasuda, R.; Raghavachari, S. Mechanisms of CaMKII action in long-term potentiation. Nat. Rev. Neurosci. 2012, 13, 169–182. [Google Scholar] [CrossRef] [Green Version]
- Garic, A.; Flentke, G.R.; Amberger, E.; Hernandez, M.; Smith, S.M. CaMKII activation is a novel effector of alcohol’s neurotoxicity in neural crest stem/progenitor cells. J. Neurochem. 2011, 118, 646–657. [Google Scholar] [CrossRef] [Green Version]
- Kong, T.; Liu, M.; Ji, B.; Bai, B.; Cheng, B.; Wang, C. Role of the Extracellular Signal-Regulated Kinase 1/2 Signaling Pathway in Ischemia-Reperfusion Injury. Front. Physiol. 2019, 10, 1038. [Google Scholar] [CrossRef] [Green Version]
- El Gaamouch, F.; Buisson, A.; Moustie, O.; Lemieux, M.; Labrecque, S.; Bontempi, B.; De Koninck, P.; Nicole, O. Interaction between CaMKII and GluN2B Controls ERK-Dependent Plasticity. J. Neurosci. 2012, 32, 10767–10779. [Google Scholar] [CrossRef]
- Mohmmad Abdul, H.; Butterfield, D.A. Involvement of PI3K/PKG/ERK1/2 signaling pathways in cortical neurons to trigger protection by cotreatment of acetyl-L-carnitine and α-lipoic acid against HNE-mediated oxidative stress and neurotoxicity: Implications for Alzheimer’s disease. Free Radic. Biol. Med. 2007, 42, 371–384. [Google Scholar] [CrossRef] [Green Version]
- Schafe, G.E.; Swank, M.W.; Rodrigues, S.M.; Dȩbiec, J.; Doyère, V. Phosphorylation of ERK/MAP kinase is required for long-term potentiation in anatomically restricted regions of the lateral amygdala in vivo. Learn. Mem. 2008, 15, 55–62. [Google Scholar] [CrossRef]
- Trifilieff, P.; Calandreau, L.; Herry, C.; Mons, N.; Micheau, J. Biphasic ERK1/2 activation in both the hippocampus and amygdala may reveal a system consolidation of contextual fear memory. Neurobiol. Learn. Mem. 2007, 88, 424–434. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Lazarovici, P.; Quirion, R.; Zheng, W. cAMP Response Element-Binding Protein (CREB): A Possible Signaling Molecule Link in the Pathophysiology of Schizophrenia. Front. Mol. Neurosci. 2018, 11, 255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bourgognon, J.-M.; Cavanagh, J. The role of cytokines in modulating learning and memory and brain plasticity. Brain Neurosci. Adv. 2020, 4, 2398212820979802. [Google Scholar] [CrossRef] [PubMed]
- Medina, J.H.; Viola, H. ERK1/2: A Key Cellular Component for the Formation, Retrieval, Reconsolidation and Persistence of Memory. Front. Mol. Neurosci. 2018, 11, 361. [Google Scholar] [CrossRef] [PubMed]
- Samudio-Ruiz, S.L.; Allan, A.M.; Valenzuela, C.F.; Perrone-Bizzozero, N.I.; Caldwell, K.K. Prenatal ethanol exposure persistently impairs NMDA receptor-dependent activation of extracellular signal-regulated kinase in the mouse dentate gyrus. J. Neurochem. 2009, 109, 1311–1323. [Google Scholar] [CrossRef]
- Swart, P.C.; Russell, V.A.; Dimatelis, J.J. Maternal separation stress reduced prenatal-ethanol-induced increase in exploratory behaviour and extracellular signal-regulated kinase activity. Behav. Brain Res. 2019, 356, 470–482. [Google Scholar] [CrossRef]
- Wen, A.Y.; Sakamoto, K.M.; Miller, L.S. The Role of the Transcription Factor CREB in Immune Function. J. Immunol. 2010, 185, 6413–6419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daniel, P.; Filiz, G.; Brown, D.V.; Hollande, F.; Gonzales, M.; D’Abaco, G.; Papalexis, N.; Phillips, W.A.; Malaterre, J.; Ramsay, R.G.; et al. Selective CREB-dependent cyclin expression mediated by the PI3K and MAPK pathways supports glioma cell proliferation. Oncogenesis 2014, 3, e108. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.-H.; Quirion, R. Insulin-like growth factor-1 (IGF-1) induces the activation/phosphorylation of Akt kinase and cAMP response element-binding protein (CREB) by activating different signaling pathways in PC12 cells. BMC Neurosci. 2006, 7, 51. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.; Martin, K.J.; Arthur, J.S.C. CREB phosphorylation at Ser133 regulates transcription via distinct mechanisms downstream of cAMP and MAPK signalling. Biochem. J. 2014, 458, 469–479. [Google Scholar] [CrossRef]
- Steven, A.; Friedrich, M.; Jank, P.; Heimer, N.; Budczies, J.; Denkert, C.; Seliger, B. What turns CREB on? And off? And why does it matter? Cell. Mol. Life Sci. 2020, 77, 4049–4067. [Google Scholar] [CrossRef]
- Hardt, O.; Nader, K.; Wang, Y.-T. GluA2-dependent AMPA receptor endocytosis and the decay of early and late long-term potentiation: Possible mechanisms for forgetting of short- and long-term memories. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henley, J.M.; Wilkinson, K.A. AMPA receptor trafficking and the mechanisms underlying synaptic plasticity and cognitive aging. Dialogues Clin. Neurosci. 2022, 15, 11–27. [Google Scholar] [CrossRef] [PubMed]
- Middei, S.; Houeland, G.; Cavallucci, V.; Ammassari-Teule, M.; D’Amelio, M.; Marie, H. CREB is necessary for synaptic maintenance and learning-induced changes of the ampa receptor GluA1 subunit. Hippocampus 2013, 23, 488–499. [Google Scholar] [CrossRef]
- Cai, H.; Guo, W.; Crossey, E.L.; Zhang, L.; Zucca, S.; George, O.L.; Valenzuela, C.F.; Zhao, X. Alcohol Exposure Decreases CREB Binding Protein Expression and Histone Acetylation in the Developing Cerebellum. PLoS ONE 2011, 6, e19351. [Google Scholar] [CrossRef] [Green Version]
- Montagud-Romero, S.; Cantacorps, L.; Fernández-Gómez, F.J.; Núñez, C.; Miñarro, J.; Rodríguez-Arias, M.; Milanés, M.V.; Valverde, O. Unraveling the molecular mechanisms involved in alcohol intake and withdrawal in adolescent mice exposed to alcohol during early life stages. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2021, 104, 110025. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Xu, D.; Cheng, S.; Zhang, L.; Shi, Z.; Qin, J.; Zhang, Z.; Wang, H. Prenatal ethanol exposure enhances the susceptibility to depressive behavior of adult offspring rats fed a high-fat diet by affecting BDNF-associated pathway. Int. J. Mol. Med. 2019, 45, 365–374. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhowail, A. Mechanisms Underlying Cognitive Impairment Induced by Prenatal Alcohol Exposure. Brain Sci. 2022, 12, 1667. https://doi.org/10.3390/brainsci12121667
Alhowail A. Mechanisms Underlying Cognitive Impairment Induced by Prenatal Alcohol Exposure. Brain Sciences. 2022; 12(12):1667. https://doi.org/10.3390/brainsci12121667
Chicago/Turabian StyleAlhowail, Ahmad. 2022. "Mechanisms Underlying Cognitive Impairment Induced by Prenatal Alcohol Exposure" Brain Sciences 12, no. 12: 1667. https://doi.org/10.3390/brainsci12121667