Importance of ABC Transporters in the Survival of Parasitic Nematodes and the Prospect for the Development of Novel Control Strategies
Abstract
:1. Introduction
2. ABC Transporters in Helminths
3. Physiological Roles of ABC Transporters
4. Methods of Studying ABC Transporters
5. ABC Transporters and Drug Resistance in Nematodes/Evidence for a Role in Resistance
5.1. Are They Substrates?
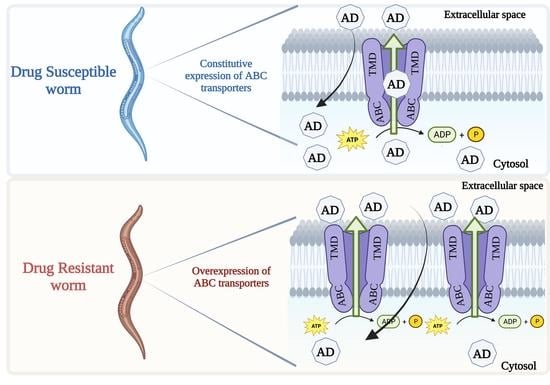
5.2. Constitutive Expression of ABC Transporters and Anthelmintic Resistance
5.3. Effect of Anthelmintics on ABC Transporters Expression
| Parasite/ Helminth | Drug Selection | Significantly Differential Expression of ABC Transporters | Life Stage | References |
|---|---|---|---|---|
| Resistant Isolate vs. Susceptible Isolate | ||||
| B. malayi | IVM | Higher expression of multiple P-gp and MRP genes in response to IVM exposure | Microfilariae and Adults | [97] |
| MOX | Higher expression of multiple ABC transporter genes in response to moxidectin exposure | Adult Worms | [100] | |
| C. oncophora | MLs | Increased transcription of Con pgp-11 in resistant isolate only | L3 | [87] |
| IVM | L3 and adults | |||
| Cyathostomins | IVM | Higher expression of pgp-9 in resistant isolate as compared to the susceptible population | L3 | [101] |
| D. immitis | IVM | Increase in gene expression for Dim-pgp-10 and DIM-pgp-11 | Adults | [102] |
| E. granulosus | Amiodarone Loperamide | Eg-pgp1 and Eg-pgp2 transcripts were up-regulated in response to in vitro drug treatment | Protoscoleses & metascoleces | [31] |
| F. gigantica | Taurocholate | Increased expression of MDR1 and MRP1 | Adult flukes | [33] |
| Triclabendazole | Increased expression of MRP1 | |||
| H. contortus | IVM | In-vivo IVM exposure significantly increased pgp-3 and pgp-9.2 transcription in resistant isolate | Adults | [90] |
| IVM | In-vivo IVM exposure significantly decreased pgp-3 and pgp-9.1 transcription, whereas pgp-2 expression was progressively increased in IVM-resistant isolate | Adult worms | [103] | |
| IVM | Resistant isolate: Increased expression of pgp-1, pgp-2, pgp-9.1, pgp-10, pgp-11 and haf-6. | L3 | [18] | |
| LEV | In resistant isolate: Upregulation of pgp-1, pgp-2, pgp-9.1, pgp-10, pgp-11, abcf-1 and haf-6 genes. No significant changes in susceptible isolate | L3 | ||
| MPL 250 µg/mL | In both MDR and susceptible isolates, expression of multiple ABC transporter genes increased at 3, 6 and 24 hrs. Some pgps were also decreased. | L3 | [24] | |
| MPL 2.5 µg/mL | MDR isolate: Expression of multiple ABC transporter genes decreased. Susceptible isolate: pgp-11 increased at 3 hr, while pgp-9.1 and pgp-11 decreased at 24 hr. | |||
| IVM | No changes in P-gp expression levels in IVM-resistant isolate compared to drug-susceptible parent | L3 | [37] | |
| S. mansoni | PRAZ | Transient increase in transcription levels of SMDR2 in schistosome isolate with reduced praziquantel susceptibility | Adult worms | [93] |
| PRAZ | Transient increase in transcription levels of SmMRP1 following exposure of worms to sub-lethal concentrations of praziquantel | Juvenile adults | [104] |
5.4. Polymorphism in ABC Transporters and Anthelmintic Resistance
| Parasite/Helminth | Anthelmintic Drug | Genetic Polymorphism in ABC Transporters | Life Stage | References |
|---|---|---|---|---|
| Resistant Isolate vs. Susceptible Isolate | ||||
| C. oncophora | Multiple SNPs led to different amino acid sequence variations in resistant isolates compared to susceptible isolates. | L3 | [87] | |
| D. immitis | MLs | The alternate allele frequency of the D. immitis pgp-11 SNP marker ranged from 36% to 40% in resistant isolate and from 0% to 12% in susceptible isolate | MF | [88] |
| 75 SNPs and 89 SNPs in 15 ABC transporter genes of resistant and susceptible isolates, respectively | MF | [30] | ||
| H. contortus | BZs | Multiple allelic polymorphisms in pgp-A were detected against the cambendazole-selected strain of H. contortus, derived from the sensitive strain | L3 | [105] |
| IVM and MOX | Multiple allele variation in P-gp locus between the drug selected and susceptible strains | L3 | [22] | |
| O. volvulus | IVM | Resistant isolate: Reduced polymorphism in OvPLP, OvMDR-1, OvABC-1, OvABC-3 and OvPGP Susceptible isolate: Polymorphism not detected | Adult | [109] |
| P. equorum | MLs | Three SNPs causing missense mutations in the PeqPgp-11 were correlated with reduced sensitivity to MLs | Eggs | [92] |
| T. circumcincta | IVM | Nine non-synonymous SNPs in Tci-pgp-9 in the MDR isolate sequences relative to the susceptible isolate | L3 | [107] |
| IVM | Alternative splicing and four non-synonymous, exonic SNPs in Tci-pgp-9 gene in MDR isolate compared to susceptible “near-isogenic” sister strain. | L3 | [106] |
6. ABC Transporters as Potential Targets to Control Nematodes
6.1. Genetic Manipulation/Gene Silencing
6.2. Modulation of ABC Transporters
6.2.1. First-Generation MDR Inhibitors
6.2.2. Second-Generation MDR Inhibitors
6.2.3. Third-Generation MDR Inhibitors
6.2.4. Nutraceuticals as MDR Inhibitors
7. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ames, G.F.; Mimura, C.S.; Holbrook, S.R.; Shyamala, V. Traffic ATPases: A superfamily of transport proteins operating from Escherichia coli to humans. Adv. Enzymol. Relat. Areas Mol. Biol. 1992, 65, 1–47. [Google Scholar] [PubMed]
- Dean, M.; Allikmets, R. Complete characterization of the human ABC gene family. J. Bioenerg. Biomembr. 2001, 33, 475–479. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.W.; Holm, I.; Graille, M.; Dehoux, P.; Rzhetsky, A.; Wincker, P.; Weissenbach, J.; Brey, P.T. Identification of the Anopheles gambiae ATP-binding cassette transporter superfamily genes. Mol. Cells 2003, 15, 150–158. [Google Scholar]
- Sheps, J.A.; Ralph, S.; Zhao, Z.; Baillie, D.L.; Ling, V. The ABC transporter gene family of Caenorhabditis elegans has implications for the evolutionary dynamics of multidrug resistance in eukaryotes. Genome Biol. 2004, 5, R15. [Google Scholar] [CrossRef] [PubMed]
- Kerr, I.D. Sequence analysis of twin ATP binding cassette proteins involved in translational control, antibiotic resistance, and ribonuclease L inhibition. Biochem. Biophys. Res. Commun. 2004, 315, 166–173. [Google Scholar] [CrossRef]
- Zhao, Z.; Fang, L.L.; Johnsen, R.; Baillie, D.L. ATP-binding cassette protein E is involved in gene transcription and translation in Caenorhabditis elegans. Biochem. Biophys. Res. Commun. 2004, 323, 104–111. [Google Scholar] [CrossRef]
- Holland, I.B.; Cole, S.P.; Kuchler, K.; Higgins, C.F. ABC Proteins: From Bacteria to Man; Academic Press: San Diego, CA, USA, 2003. [Google Scholar]
- Dassa, E.; Bouige, P. The ABC of ABCS: A phylogenetic and functional classification of ABC systems in living organisms. Res. Microbiol. 2001, 152, 211–229. [Google Scholar] [CrossRef]
- Dassa, E. Natural history of ABC systems: Not only transporters. Essays Biochem. 2011, 50, 19–42. [Google Scholar]
- Licht, A.; Schneider, E. ATP binding cassette systems: Structures, mechanisms, and functions. Cent. Eur. J. Biol. 2011, 6, 785. [Google Scholar] [CrossRef]
- Dano, K. Active outward transport of daunomycin in resistant Ehrlich ascites tumor cells. Biochim. Biophys. Acta 1973, 323, 466–483. [Google Scholar] [CrossRef]
- Falasca, M.; Linton, K.J. Investigational ABC transporter inhibitors. Expert Opin. Investig. Drugs 2012, 21, 657–666. [Google Scholar] [CrossRef] [PubMed]
- Ardelli, B.F.; Prichard, R.K. Inhibition of P-glycoprotein enhances sensitivity of Caenorhabditis elegans to ivermectin. Vet. Parasitol. 2013, 191, 264–275. [Google Scholar] [CrossRef]
- Raza, A.; Kopp, S.R.; Jabbar, A.; Kotze, A.C. Effects of third generation P-glycoprotein inhibitors on the sensitivity of drug-resistant and -susceptible isolates of Haemonchus contortus to anthelmintics in vitro. Vet. Parasitol. 2015, 211, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Kopp, S.R.; Kotze, A.C. Synergism between ivermectin and the tyrosine kinase/P-glycoprotein inhibitor crizotinib against Haemonchus contortus larvae in vitro. Vet. Parasitol. 2016, 227, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Kerboeuf, D.; Guegnard, F. Anthelmintics are substrates and activators of nematode P glycoprotein. Antimicrob. Agents Chemother. 2011, 55, 2224–2232. [Google Scholar] [CrossRef] [PubMed]
- De Graef, J.; Demeler, J.; Skuce, P.; Mitreva, M.; Von Samson-Himmelstjerna, G.; Vercruysse, J.; Claerebout, E.; Geldhof, P. Gene expression analysis of ABC transporters in a resistant Cooperia oncophora isolate following in vivo and in vitro exposure to macrocyclic lactones. Parasitology 2013, 140, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Kopp, S.R.; Bagnall, N.H.; Jabbar, A.; Kotze, A.C. Effects of in vitro exposure to ivermectin and levamisole on the expression patterns of ABC transporters in Haemonchus contortus larvae. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 103–115. [Google Scholar] [CrossRef]
- Areskog, M.; Engström, A.; Tallkvist, J.; Samson-Himmelstjerna, G.; Höglund, J. PGP expression in Cooperia oncophora before and after ivermectin selection. Parasitol. Res. 2013, 112, 3005–3012. [Google Scholar] [CrossRef]
- Alvarez, L.; Suarez, G.; Ceballos, L.; Moreno, L.; Canton, C.; Lifschitz, A.; Mate, L.; Ballent, M.; Virkel, G.; Lanusse, C. Integrated assessment of ivermectin pharmacokinetics, efficacy against resistant Haemonchus contortus and P-glycoprotein expression in lambs treated at three different dosage levels. Vet. Parasitol. 2015, 210, 53–63. [Google Scholar] [CrossRef]
- Prichard, R.K.; Roulet, A. ABC transporters and beta-tubulin in macrocyclic lactone resistance: Prospects for marker development. Parasitology 2007, 134, 1123–1132. [Google Scholar] [CrossRef]
- Blackhall, W.J.; Liu, H.Y.; Xu, M.; Prichard, R.K.; Beech, R.N. Selection at a P-glycoprotein gene in ivermectin- and moxidectin-selected strains of Haemonchus contortus. Mol. Biochem. Parasitol. 1998, 95, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Molento, M.; Blackhall, W.; Ribeiro, P.; Beech, R.; Prichard, R. Ivermectin resistance in nematodes may be caused by alteration of P-glycoprotein homolog. Mol. Biochem. Parasitol. 1998, 91, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Bagnall, N.H.; Jabbar, A.; Kopp, S.R.; Kotze, A.C. Increased expression of ATP binding cassette transporter genes following exposure of Haemonchus contortus larvae to a high concentration of monepantel in vitro. Parasit. Vector. 2016, 9, 522. [Google Scholar] [CrossRef] [PubMed]
- Lespine, A.; Ménez, C.; Bourguinat, C.; Prichard, R.K. P-glycoproteins and other multidrug resistance transporters in the pharmacology of anthelmintics: Prospects for reversing transport-dependent anthelmintic resistance. Int. J. Parasitol. Drugs Drug Resist. 2012, 2, 58–75. [Google Scholar] [CrossRef] [PubMed]
- Ardelli, B.F.; Stitt, L.E.; Tompkins, J.B. Inventory and analysis of ATP-binding cassette (ABC) systems in Brugia malayi. Parasitology 2010, 137, 1195–1212. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, T.; Cotton, J.A.; Dalzell, J.J.; Hasegawa, K.; Kanzaki, N.; McVeigh, P.; Takanashi, T.; Tsai, I.J.; Assefa, S.A.; Cock, P.J.; et al. Genomic insights into the origin of parasitism in the emerging plant pathogen Bursaphelenchus xylophilus. PLoS Pathog. 2011, 7, e1002219. [Google Scholar] [CrossRef]
- Drogemuller, M.; Schnieder, T.; von Samson-Himmelstjerna, G. Evidence of p-glycoprotein sequence diversity in cyathostomins. J. Parasitol. 2004, 90, 998–1003. [Google Scholar] [CrossRef]
- Bourguinat, C.; Che, H.; Mani, T.; Keller, K.; Prichard, R.K. ABC-B transporter genes in Dirofilaria immitis. Int. J. Parasitol. Drugs Drug Resist. 2016, 6, 116–124. [Google Scholar] [CrossRef]
- Mani, T.; Bourguinat, C.; Prichard, R.K. Polymorphism in ABC transporter genes of Dirofilaria immitis. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 227–235. [Google Scholar] [CrossRef]
- Nicolao, M.C.; Denegri, G.M.; Cárcamo, J.G.; Cumino, A.C. P-glycoprotein expression and pharmacological modulation in larval stages of Echinococcus granulosus. Parasitol. Int. 2014, 63, 1–8. [Google Scholar] [CrossRef]
- Dicker, A.J.; Nisbet, A.J.; Skuce, P.J. Gene expression changes in a P-glycoprotein (Tci-pgp-9) putatively associated with ivermectin resistance in Teladorsagia circumcincta. Int. J. Parasitol. 2011, 41, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Kumkate, S.; Chunchob, S.; Janvilisri, T. Expression of ATP-binding cassette multidrug transporters in the giant liver fluke Fasciola gigantica and their possible involvement in the transport of bile salts and anthelmintics. Mol. Cell. Biochem. 2008, 317, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kudlacek, O.; Dönmez, Y.; Stockner, T. ABC transporters of the liver fluke Fasciola hepatica. BMC Pharmacol. Toxicol. 2012, 13, A76. [Google Scholar] [CrossRef]
- Wilkinson, R.; Law, C.J.; Hoey, E.M.; Fairweather, I.; Brennan, G.P.; Trudgett, A. An amino acid substitution in Fasciola hepatica P-glycoprotein from triclabendazole-resistant and triclabendazole-susceptible populations. Mol. Biochem. Parasitol. 2012, 186, 69–72. [Google Scholar] [CrossRef]
- Laing, R.; Kikuchi, T.; Martinelli, A.; Tsai, I.J.; Beech, R.N.; Redman, E.; Holroyd, N.; Bartley, D.J.; Beasley, H.; Britton, C.; et al. The genome and transcriptome of Haemonchus contortus, a key model parasite for drug and vaccine discovery. Genome Biol. 2013, 14, R88. [Google Scholar] [CrossRef]
- Williamson, S.M.; Wolstenholme, A.J. P-glycoproteins of Haemonchus contortus: Development of real-time PCR assays for gene expression studies. J. Helminthol. 2012, 86, 202–208. [Google Scholar] [CrossRef]
- Huang, Y.-J.; Prichard, R.K. Identification and stage-specific expression of two putative P-glycoprotein coding genes in Onchocerca volvulus. Mol. Biochem. Parasitol. 1999, 102, 273–281. [Google Scholar] [CrossRef]
- Bourguinat, C.; Ardelli, B.F.; Pion, S.D.; Kamgno, J.; Gardon, J.; Duke, B.O.; Boussinesq, M.; Prichard, R.K. P-glycoprotein-like protein, a possible genetic marker for ivermectin resistance selection in Onchocerca volvulus. Mol. Biochem. Parasitol. 2008, 158, 101–111. [Google Scholar] [CrossRef]
- Mordvinov, V.A.; Ershov, N.I.; Pirozhkova, D.S.; Pakharukov, Y.V.; Pakharukova, M.Y. ABC transporters in the liver fluke Opisthorchis felineus. Mol. Biochem. Parasitol. 2017, 216, 60–68. [Google Scholar] [CrossRef]
- Greenberg, R.M. ABC multidrug transporters in schistosomes and other parasitic flatworms. Parasitol. Int. 2013, 62, 647–653. [Google Scholar] [CrossRef]
- Zhu, X.Q.; Korhonen, P.K.; Cai, H.; Young, N.D.; Nejsum, P.; von Samson-Himmelstjerna, G.; Boag, P.R.; Tan, P.; Li, Q.; Min, J.; et al. Genetic blueprint of the zoonotic pathogen Toxocara canis. Nat. Commun. 2015, 6, 6145. [Google Scholar] [CrossRef] [PubMed]
- Sarai, R.S.; Kopp, S.R.; Coleman, G.T.; Kotze, A.C. Acetylcholine receptor subunit and P-glycoprotein transcription patterns in levamisole-susceptible and -resistant Haemonchus contortus. Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Che, H.; Beech, R.N.; Prichard, R.K. Characterization of Haemonchus contortus P-glycoprotein-16 and its interaction with the macrocyclic lactone anthelmintics. Mol. Biochem. Parasitol. 2015, 204, 11–15. [Google Scholar] [CrossRef] [PubMed]
- Ehrenman, K.; Sehgal, A.; Lige, B.; Stedman, T.T.; Joiner, K.A.; Coppens, I. Novel roles for ATP-binding cassette G transporters in lipid redistribution in Toxoplasma. Mol. Microbiol. 2010, 76, 1232–1249. [Google Scholar] [CrossRef]
- Fath, M.J.; Kolter, R. ABC transporters: Bacterial exporters. Microbiol. Rev. 1993, 57, 995–1017. [Google Scholar] [CrossRef]
- Ardelli, B.F. Transport proteins of the ABC systems superfamily and their role in drug action and resistance in nematodes. Parasitol. Int. 2013, 62, 639–646. [Google Scholar] [CrossRef]
- Hopfner, K.P.; Tainer, J.A. Rad50/SMC proteins and ABC transporters: Unifying concepts from high-resolution structures. Curr. Opin. Struct. Biol. 2003, 13, 249–255. [Google Scholar] [CrossRef]
- Tarling, E.J.; de Aguiar Vallim, T.Q.; Edwards, P.A. Role of ABC transporters in lipid transport and human disease. Trends Endocrinol. Metab. 2013, 24, 342–350. [Google Scholar] [CrossRef]
- Kotlyarov, S.; Kotlyarova, A. Clinical significance of lipid transport function of ABC transporters in the innate immune system. Membranes 2022, 12, 1083. [Google Scholar] [CrossRef]
- Broeks, A.; Janssen, H.W.; Calafat, J.; Plasterk, R.H. A P-glycoprotein protects Caenorhabditis elegans against natural toxins. EMBO J. 1995, 14, 1858–1866. [Google Scholar] [CrossRef]
- Issouf, M.; Guegnard, F.; Koch, C.; Le Vern, Y.; Blanchard-Letort, A.; Che, H.; Beech, R.N.; Kerboeuf, D.; Neveu, C. Haemonchus contortus P-glycoproteins interact with host eosinophil granules: A novel insight into the role of ABC transporters in host-parasite interaction. PLoS ONE 2014, 9, e87802. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Meng, F.; Deng, X.; Wang, X.; Feng, Y.; Zhang, W.; Pan, L.; Zhang, X. Comparative transcriptome analysis of the pinewood nematode Bursaphelenchus xylophilus reveals the molecular mechanism underlying its defense response to host-derived α-pinene. Int. J. Mol. Sci. 2019, 20, 911. [Google Scholar] [CrossRef] [PubMed]
- Bygarski, E.E.; Prichard, R.K.; Ardelli, B.F. Resistance to the macrocyclic lactone moxidectin is mediated in part by membrane transporter P-glycoproteins: Implications for control of drug resistant parasitic nematodes. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.L.; Ma, G.X.; Luo, Y.F.; Kuang, C.Y.; Jiang, A.Y.; Li, G.Q.; Zhou, R.Q. Tissue expression pattern of ABCG transporter indicates functional roles in reproduction of Toxocara canis. Parasitol. Res. 2018, 117, 775–782. [Google Scholar] [CrossRef]
- Dermauw, W.; Van Leeuwen, T. The ABC gene family in arthropods: Comparative genomics and role in insecticide transport and resistance. Insect Biochem. Mol. Biol. 2014, 45, 89–110. [Google Scholar] [CrossRef]
- Broehan, G.; Kroeger, T.; Lorenzen, M.; Merzendorfer, H. Functional analysis of the ATP-binding cassette (ABC) transporter gene family of Tribolium castaneum. BMC Genom. 2013, 14, 6. [Google Scholar] [CrossRef]
- Kasinathan, R.S.; Morgan, W.M.; Greenberg, R.M. Genetic knockdown and pharmacological inhibition of parasite multidrug resistance transporters disrupts egg production in Schistosoma mansoni. PLoS Negl. Trop. Dis. 2011, 5, e1425. [Google Scholar] [CrossRef]
- Kerboeuf, D.; Blackhall, W.; Kaminsky, R.; von Samson-Himmelstjerna, G. P-glycoprotein in helminths: Function and perspectives for anthelmintic treatment and reversal of resistance. Int. J. Antimicrob. Agents 2003, 22, 332–346. [Google Scholar] [CrossRef]
- Sangster, N.C.; Bannan, S.C.; Weiss, A.S.; Nulf, S.C.; Klein, R.D.; Geary, T.G. Haemonchus contortus: Sequence heterogeneity of internucleotide binding domains from P-glycoproteins. Exp. Parasitol. 1999, 91, 250–257. [Google Scholar] [CrossRef]
- Zhao, Z.; Sheps, J.A.; Ling, V.; Fang, L.L.; Baillie, D.L. Expression analysis of ABC transporters reveals differential functions of tandemly duplicated genes in Caenorhabditis elegans. J. Mol. Biol. 2004, 344, 409–417. [Google Scholar] [CrossRef]
- Georges, E.; Tsuruo, T.; Ling, V. Topology of P-glycoprotein as determined by epitope mapping of MRK-16 monoclonal antibody. J. Biol. Chem. 1993, 268, 1792–1798. [Google Scholar] [CrossRef] [PubMed]
- Kerboeuf, D.; Guegnard, F.; Vern, Y.L. Detection of P-glycoprotein-mediated multidrug resistance against anthelmintics in Haemonchus contortus using anti-human mdr1 monoclonal antibodies. Parasitol. Res. 2003, 91, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.M.; Prichard, R.K. Localization of p-glycoprotein mRNA in the tissues of Haemonchus contortus adult worms and its relative abundance in drug-selected and susceptible strains. J. Parasitol. 2002, 88, 612–620. [Google Scholar] [CrossRef]
- Riou, M.; Koch, C.; Delaleu, B.; Berthon, P.; Kerboeuf, D. Immunolocalisation of an ABC transporter, P-glycoprotein, in the eggshells and cuticles of free-living and parasitic stages of Haemonchus contortus. Parasitol. Res. 2005, 96, 142–148. [Google Scholar] [CrossRef]
- David, M.; Lebrun, C.; Duguet, T.; Talmont, F.; Beech, R.; Orlowski, S.; André, F.; Prichard, R.K.; Lespine, A. Structural model, functional modulation by ivermectin and tissue localization of Haemonchus contortus P-glycoprotein-13. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 145–157. [Google Scholar] [CrossRef] [PubMed]
- Chelladurai, J.J.; Brewer, M.T. Detection and quantification of Parascaris P-glycoprotein drug transporter expression with a novel mRNA hybridization technique. Vet. Parasitol. 2019, 267, 75–83. [Google Scholar] [CrossRef]
- Zhang, Y.; Bachmeier, C.; Miller, D.W. In vitro and in vivo models for assessing drug efflux transporter activity. Adv. Drug Deliv. Rev. 2003, 55, 31–51. [Google Scholar] [CrossRef]
- Scala, S.; Akhmed, N.; Rao, U.S.; Paull, K.; Lan, L.B.; Dickstein, B.; Lee, J.S.; Elgemeie, G.H.; Stein, W.D.; Bates, S.E. P-glycoprotein substrates and antagonists cluster into two distinct groups. Mol. Pharmacol. 1997, 51, 1024–1033. [Google Scholar] [CrossRef] [PubMed]
- Beugnet, F.; Gauthey, M.; Kerboeuf, D. Partial in vitro reversal of benzimidazole resistance by the free-living stages of Haemonchus contortus with verapamil. Vet. Rec. 1997, 141, 575–576. [Google Scholar] [CrossRef]
- Kerboeuf, D.; Chambrier, P.; Le Vern, Y.; Aycardi, J. Flow cytometry analysis of drug transport mechanisms in Haemonchus contortus susceptible or resistant to anthelmintics. Parasitol. Res. 1999, 85, 118–123. [Google Scholar] [CrossRef]
- Rosenberg, M.F.; Velarde, G.; Ford, R.C.; Martin, C.; Berridge, G.; Kerr, I.D.; Callaghan, R.; Schmidlin, A.; Wooding, C.; Linton, K.J.; et al. Repacking of the transmembrane domains of P-glycoprotein during the transport ATPase cycle. EMBO J. 2001, 20, 5615–5625. [Google Scholar] [CrossRef] [PubMed]
- Adachi, Y.; Suzuki, H.; Sugiyama, Y. Comparative studies on in vitro methods for evaluating in vivo function of MDR1 P-Glycoprotein. Pharm. Res. 2001, 18, 1660–1668. [Google Scholar] [CrossRef] [PubMed]
- Garrigues, A.; Nugier, J.; Orlowski, S.; Ezan, E. A high-throughput screening microplate test for the interaction of drugs with P-glycoprotein. Anal. Biochem. 2002, 305, 106–114. [Google Scholar] [CrossRef]
- Schinkel, A.H.; Smit, J.J.; van Tellingen, O.; Beijnen, J.H.; Wagenaar, E.; van Deemter, L.; Mol, C.A.; van der Valk, M.A.; Robanus-Maandag, E.C.; te Riele, H.P.; et al. Disruption of the mouse mdr1a P-glycoprotein gene leads to a deficiency in the blood-brain barrier and to increased sensitivity to drugs. Cell 1994, 77, 491–502. [Google Scholar] [CrossRef]
- Kim, R.B.; Fromm, M.F.; Wandel, C.; Leake, B.; Wood, A.J.; Roden, D.M.; Wilkinson, G.R. The drug transporter P-glycoprotein limits oral absorption and brain entry of HIV-1 protease inhibitors. J. Clin. Investig. 1998, 101, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Lespine, A.; Alvinerie, M.; Vercruysse, J.; Prichard, R.K.; Geldhof, P. ABC transporter modulation: A strategy to enhance the activity of macrocyclic lactone anthelmintics. Trends Parasitol. 2008, 24, 293–298. [Google Scholar] [CrossRef] [PubMed]
- Borst, P.; Evers, R.; Kool, M.; Wijnholds, J. The multidrug resistance protein family. Biochim. Biophys. Acta 1999, 1461, 347–357. [Google Scholar] [CrossRef]
- Lespine, A.; Dupuy, J.; Orlowski, S.; Nagy, T.; Glavinas, H.; Krajcsi, P.; Alvinerie, M. Interaction of ivermectin with multidrug resistance proteins (MRP1, 2 and 3). Chem. Biol. Interact. 2006, 159, 169–179. [Google Scholar] [CrossRef]
- Naito, S.; Koike, K.; Ono, M.; Machida, T.; Tasaka, S.; Kiue, A.; Koga, H.; Kumazawa, J. Development of novel reversal agents, imidazothiazole derivatives, targeting MDR1- and MRP-mediated multidrug resistance. Oncol. Res. 1998, 10, 123–132. [Google Scholar]
- Efferth, T.; Volm, M. Reversal of doxorubicin-resistance in sarcoma 180 tumor cells by inhibition of different resistance mechanisms. Cancer Lett. 1993, 70, 197–202. [Google Scholar] [CrossRef]
- Godoy, P.; Che, H.; Beech, R.N.; Prichard, R.K. Characterisation of P-glycoprotein-9.1 in Haemonchus contortus. Parasites Vectors 2016, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.; Lian, J.; Beech, R.N.; Prichard, R.K. Haemonchus contortus P-glycoprotein-2: In situ localisation and characterisation of macrocyclic lactone transport. Int. J. Parasitol. 2015, 45, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Williamson, S.M.; Storey, B.; Howell, S.; Harper, K.M.; Kaplan, R.M.; Wolstenholme, A.J. Candidate anthelmintic resistance-associated gene expression and sequence polymorphisms in a triple-resistant field isolate of Haemonchus contortus. Mol. Biochem. Parasitol. 2011, 180, 99–105. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, L.A.; Reboucas, T.F.; Ferreira, S.R.; Rodrigues-Luiz, G.F.; Miranda, R.C.; Araujo, R.N.; Fujiwara, R.T. Dominance of P-glycoprotein 12 in phenotypic resistance conversion against ivermectin in Caenorhabditis elegans. PLoS ONE 2018, 13, e0192995. [Google Scholar] [CrossRef]
- Yan, R.; Urdaneta-Marquez, L.; Keller, K.; James, C.E.; Davey, M.W.; Prichard, R.K. The role of several ABC transporter genes in ivermectin resistance in Caenorhabditis elegans. Vet. Parasitol. 2012, 190, 519–529. [Google Scholar] [CrossRef]
- Demeler, J.; Krücken, J.; AlGusbi, S.; Ramünke, S.; De Graef, J.; Kerboeuf, D.; Geldhof, P.; Pomroy, W.E.; von Samson-Himmelstjerna, G. Potential contribution of P-glycoproteins to macrocyclic lactone resistance in the cattle parasitic nematode Cooperia oncophora. Mol. Biochem. Parasitol. 2013, 188, 10–19. [Google Scholar] [CrossRef]
- Curry, E.; Prichard, R.; Lespine, A. Genetic polymorphism, constitutive expression and tissue localization of Dirofilaria immitis P-glycoprotein 11: A putative marker of macrocyclic lactone resistance. Parasit. Vector. 2022, 15, 482. [Google Scholar] [CrossRef]
- Tuersong, W.; Zhou, C.; Wu, S.; Qin, P.; Wang, C.; Di, W.; Liu, L.; Liu, H.; Hu, M. Comparative analysis on transcriptomics of ivermectin resistant and susceptible strains of Haemonchus contortus. Parasit. Vector. 2022, 15, 159. [Google Scholar] [CrossRef]
- Mate, L.; Ballent, M.; Cantón, C.; Lanusse, C.; Ceballos, L.; Alvarez L, L.I.; Liron, J.P. ABC-transporter gene expression in ivermectin-susceptible and resistant Haemonchus contortus isolates. Vet. Parasitol. 2022, 302, 109647. [Google Scholar] [CrossRef]
- Reyes-Guerrero, D.E.; Cedillo-Borda, M.; Alonso-Morales, R.A.; Alonso-Díaz, M.A.; Olmedo-Juárez, A.; Mendoza-de-Gives, P.; López-Arellano, M.E. Comparative study of transcription profiles of the P-glycoprotein transporters of two Haemonchus contortus isolates: Susceptible and resistant to ivermectin. Mol. Biochem. Parasitol. 2020, 238, 111281. [Google Scholar] [CrossRef]
- Janssen, I.J.; Krucken, J.; Demeler, J.; Basiaga, M.; Kornas, S.; von Samson-Himmelstjerna, G. Genetic variants and increased expression of Parascaris equorum P-glycoprotein-11 in populations with decreased ivermectin susceptibility. PloS one 2013, 8, e61635. [Google Scholar] [CrossRef] [PubMed]
- Messerli, S.M.; Kasinathan, R.S.; Morgan, W.; Spranger, S.; Greenberg, R.M. Schistosoma mansoni P-glycoprotein levels increase in response to praziquantel exposure and correlate with reduced praziquantel susceptibility. Mol. Biochem. Parasitol. 2009, 167, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Schrenk, D.; Baus, P.R.; Ermel, N.; Klein, C.; Vorderstemann, B.; Kauffmann, H.M. Up-regulation of transporters of the MRP family by drugs and toxins. Toxicol. Lett. 2001, 120, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Tydén, E.; Skarin, M.; Höglund, J. Gene expression of ABC transporters in Cooperia oncophora after field and laboratory selection with macrocyclic lactones. Mol. Biochem. Parasitol. 2014, 198, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Lloberas, M.; Alvarez, L.; Entrocasso, C.; Virkel, G.; Ballent, M.; Mate, L.; Lanusse, C.; Lifschitz, A. Comparative tissue pharmacokinetics and efficacy of moxidectin, abamectin and ivermectin in lambs infected with resistant nematodes: Impact of drug treatments on parasite P-glycoprotein expression. Int. J. Parasitol. Drugs Drug Resist. 2013, 3, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Tompkins, J.B.; Stitt, L.E.; Morrissette, A.M.; Ardelli, B.F. The role of Brugia malayi ATP-binding cassette (ABC) transporters in potentiating drug sensitivity. Parasitol. Res. 2011, 109, 1311–1322. [Google Scholar] [CrossRef]
- Diao, J.; Hao, X.; Ma, W.; Ma, L. Bioinformatics analysis of structure and function in the MRP gene family and its expression in response to various drugs in Bursaphelenchus xylophilus. J. Forest. Res. 2021, 32, 779–787. [Google Scholar] [CrossRef]
- Gerhard, A.P.; Krücken, J.; Heitlinger, E.; Janssen, I.J.I.; Basiaga, M.; Kornaś, S.; Beier, C.; Nielsen, M.K.; Davis, R.E.; Wang, J.; et al. The P-glycoprotein repertoire of the equine parasitic nematode Parascaris univalens. Sci. Report. 2020, 10. [Google Scholar] [CrossRef]
- Stitt, L.E.; Tompkins, J.B.; Dooley, L.A.; Ardelli, B.F. ABC transporters influence sensitivity of Brugia malayi to moxidectin and have potential roles in drug resistance. Exp. Parasitol. 2011, 129, 137–144. [Google Scholar] [CrossRef]
- Peachey, L.E.; Pinchbeck, G.L.; Matthews, J.B.; Burden, F.A.; Lespine, A.; von Samson-Himmelstjerna, G.; Krücken, J.; Hodgkinson, J.E. P-glycoproteins play a role in ivermectin resistance in cyathostomins. Int. J. Parasitol. Drugs Drug Resist. 2017, 7, 388–398. [Google Scholar] [CrossRef]
- Lucchetti, C.; Genchi, M.; Venco, L.; Menozzi, A.; Serventi, P.; Bertini, S.; Bazzocchi, C.; Kramer, L.H.; Vismarra, A. Differential ABC transporter gene expression in adult Dirofilaria immitis males and females following in vitro treatment with ivermectin, doxycycline or a combination of both. Parasit. Vector. 2019, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Maté, L.; Ballent, M.; Cantón, C.; Ceballos, L.; Lifschitz, A.; Lanusse, C.; Alvarez, L.; Liron, J.P. Assessment of P-glycoprotein gene expression in adult stage of Haemonchus contortus in vivo exposed to ivermectin. Vet. Parasitol. 2018, 264, 1–7. [Google Scholar] [CrossRef]
- Kasinathan, R.S.; Morgan, W.M.; Greenberg, R.M. Schistosoma mansoni express higher levels of multidrug resistance-associated protein 1 (SmMRP1) in juvenile worms and in response to praziquantel. Mol. Biochem. Parasitol. 2010, 173, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Blackhall, W.J.; Prichard, R.K.; Beech, R.N. P-glycoprotein selection in strains of Haemonchus contortus resistant to benzimidazoles. Vet. Parasitol. 2008, 152, 101–107. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Bisset, S.A.; Doyle, S.R.; Hallsworth-Pepin, K.; Martin, J.; Grant, W.N.; Mitreva, M. Genomic introgression mapping of field-derived multiple-anthelmintic resistance in Teladorsagia circumcincta. PLoS Genet. 2017, 13, e1006857. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, F.; Jonsson, N.N.; Kenyon, F.; Skuce, P.J.; Bisset, S.A. P-glycoprotein-9 and macrocyclic lactone resistance status in selected strains of the ovine gastrointestinal nematode, Teladorsagia circumcincta. Int. J. Parasitol. Drugs Drug Resist. 2018, 8, 70–80. [Google Scholar] [CrossRef] [PubMed]
- Ardelli, B.F.; Prichard, R.K. Identification of variant ABC-transporter genes among Onchocerca volvulus collected from ivermectin-treated and untreated patients in Ghana, West Africa. Ann. Trop. Med. Parasitol. 2004, 98, 371–384. [Google Scholar] [CrossRef]
- Ardelli, B.F.; Guerriero, S.B.; Prichard, R.K. Ivermectin imposes selection pressure on P-glycoprotein from Onchocerca volvulus: Linkage disequilibrium and genotype diversity. Parasitol. 2006, 132, 375–386. [Google Scholar] [CrossRef]
- Yague, E.; Higgins, C.F.; Raguz, S. Complete reversal of multidrug resistance by stable expression of small interfering RNAs targeting MDR1. Gene Ther. 2004, 11, 1170–1174. [Google Scholar] [CrossRef]
- Huang, H.; Wang, H.; Sinz, M.; Zoeckler, M.; Staudinger, J.; Redinbo, M.R.; Teotico, D.G.; Locker, J.; Kalpana, G.V.; Mani, S. Inhibition of drug metabolism by blocking the activation of nuclear receptors by ketoconazole. Oncogene 2007, 26, 258–268. [Google Scholar] [CrossRef]
- Broeks, A.; Gerrard, B.; Allikmets, R.; Dean, M.; Plasterk, R.H. Homologues of the human multidrug resistance genes MRP and MDR contribute to heavy metal resistance in the soil nematode Caenorhabditis elegans. EMBO J. 1996, 15, 6132–6143. [Google Scholar] [CrossRef] [PubMed]
- Zawadzki, J.L.; Kotze, A.C.; Fritz, J.A.; Johnson, N.M.; Hemsworth, J.E.; Hines, B.M.; Behm, C.A. Silencing of essential genes by RNA interference in Haemonchus contortus. Parasitology 2012, 139, 613–629. [Google Scholar] [CrossRef] [PubMed]
- Samarasinghe, B.; Knox, D.P.; Britton, C. Factors affecting susceptibility to RNA interference in Haemonchus contortus and in vivo silencing of an H11 aminopeptidase gene. Int. J. Parasitol. 2011, 41, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Geldhof, P.; Murray, L.; Couthier, A.; Gilleard, J.S.; McLauchlan, G.; Knox, D.P.; Britton, C. Testing the efficacy of RNA interference in Haemonchus contortus. Int. J. Parasitol. 2006, 36, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Mladineo, I.; Trumbić, Ž.; Hrabar, J.; Vrbatović, A.; Bušelić, I.; Ujević, I.; Roje-Busatto, R.; Babić, I.; Messina, C. Efficiency of target larvicides is conditioned by ABC-mediated transport in the zoonotic nematode Anisakis pegreffii. Antimicrob. Agents Chemother. 2018, 62, e00916–e00918. [Google Scholar] [CrossRef] [PubMed]
- AlGusbi, S.; Krücken, J.; Ramünke, S.; von Samson-Himmelstjerna, G.; Demeler, J. Analysis of putative inhibitors of anthelmintic resistance mechanisms in cattle gastrointestinal nematodes. Int. J. Parasitol. 2014, 44, 647–658. [Google Scholar] [CrossRef]
- Lifschitz, A.; Entrocasso, C.; Alvarez, L.; Lloberas, M.; Ballent, M.; Manazza, G.; Virkel, G.; Borda, B.; Lanusse, C. Interference with P-glycoprotein improves ivermectin activity against adult resistant nematodes in sheep. Vet. Parasitol. 2010, 172, 291–298. [Google Scholar] [CrossRef]
- Pacheco, P.A.; Louvandini, H.; Giglioti, R.; Wedy, B.C.R.; Ribeiro, J.C.; Verissimo, C.J.; Ferreira, J.F.d.S.; Amarante, A.F.T.d.; Katiki, L.M. Phytochemical modulation of P-Glycoprotein and its gene expression in an ivermectin-resistant Haemonchus contortus isolate in vitro. Vet. Parasitol. 2022, 305, 109713. [Google Scholar] [CrossRef]
- Menez, C.; Alberich, M.; Kansoh, D.; Blanchard, A.; Lespine, A. Acquired tolerance to ivermectin and moxidectin after drug selection pressure in the nematode Caenorhabditis elegans. Antimicrob. Agents Chemother. 2016, 60, 4809–4819. [Google Scholar] [CrossRef]
- Bartley, D.J.; McAllister, H.; Bartley, Y.; Dupuy, J.; Menez, C.; Alvinerie, M.; Jackson, F.; Lespine, A. P-glycoprotein interfering agents potentiate ivermectin susceptibility in ivermectin sensitive and resistant isolates of Teladorsagia circumcincta and Haemonchus contortus. Parasitology 2009, 136, 1081–1088. [Google Scholar] [CrossRef]
- Heckler, R.P.; Almeida, G.D.; Santos, L.B.; Borges, D.G.; Neves, J.P.; Onizuka, M.K.; Borges, F.A. P-gp modulating drugs greatly potentiate the in vitro effect of ivermectin against resistant larvae of Haemonchus placei. Vet. Parasitol. 2014, 205, 638–645. [Google Scholar] [CrossRef] [PubMed]
- Kasinathan, R.S.; Sharma, L.K.; Cunningham, C.; Webb, T.R.; Greenberg, R.M. Inhibition or knockdown of ABC transporters enhances susceptibility of adult and juvenile Schistosomes to praziquantel. PLoS Negl. Trop. Dis. 2014, 8, e3265. [Google Scholar] [CrossRef] [PubMed]
- Lifschitz, A.; Suarez, V.H.; Sallovitz, J.; Cristel, S.L.; Imperiale, F.; Ahoussou, S.; Schiavi, C.; Lanusse, C. Cattle nematodes resistant to macrocyclic lactones: Comparative effects of P-glycoprotein modulation on the efficacy and disposition kinetics of ivermectin and moxidectin. Exp. Parasitol. 2010, 125, 172–178. [Google Scholar] [CrossRef]
- Darby, R.A.; Callaghan, R.; McMahon, R.M. P-glycoprotein inhibition: The past, the present and the future. Curr. Drug Metab. 2011, 12, 722–731. [Google Scholar] [CrossRef] [PubMed]
- Molento, M.B.; Lifschitz, A.; Sallovitz, J.; Lanusse, C.; Prichard, R. Influence of verapamil on the pharmacokinetics of the an-tiparasitic drugs ivermectin and moxidectin in sheep. Parasitol. Res. 2004, 92, 121–127. [Google Scholar] [CrossRef]
- Molento, M.B.; Prichard, R.K. Effects of the multidrug-resistance-reversing agents verapamil and CL 347,099 on the efficacy of ivermectin or moxidectin against unselected and drug-selected strains of Haemonchus contortus in jirds (Meriones unguiculatus). Parasitol. Res. 1999, 85, 1007–1011. [Google Scholar] [CrossRef]
- Pérez, R.; Palma, C.; Núñez, M.-J.; Urrutia, P.; Salazar, A.; Morales, L.; Vera, D.; Cox, J. Influence of verapamil on pharmacokinetics and transplacental transfer of ivermectin in sheep. Small Rumin. Res. 2010, 93, 103–109. [Google Scholar] [CrossRef]
- Boesch, D.; Gaveriaux, C.; Jachez, B.; Pourtier-Manzanedo, A.; Bollinger, P.; Loor, F. In vivo circumvention of P-glycoprotein-mediated multidrug resistance of tumor cells with SDZ PSC 833. Cancer Res. 1991, 51, 4226–4233. [Google Scholar]
- Friedenberg, W.R.; Rue, M.; Blood, E.A.; Dalton, W.S.; Shustik, C.; Larson, R.A.; Sonneveld, P.; Greipp, P.R. Phase III study of PSC-833 (valspodar) in combination with vincristine, doxorubicin, and dexamethasone (valspodar/VAD) versus VAD alone in patients with recurring or refractory multiple myeloma (E1A95): A trial of the Eastern Cooperative Oncology Group. Cancer 2006, 106, 830–838. [Google Scholar] [CrossRef]
- Gandhi, L.; Harding, M.W.; Neubauer, M.; Langer, C.J.; Moore, M.; Ross, H.J.; Johnson, B.E.; Lynch, T.J. A phase II study of the safety and efficacy of the multidrug resistance inhibitor VX-710 combined with doxorubicin and vincristine in patients with recurrent small cell lung cancer. Cancer 2007, 109, 924–932. [Google Scholar] [CrossRef]
- James, C.E.; Davey, M.W. Increased expression of ABC transport proteins is associated with ivermectin resistance in the model nematode Caenorhabditis elegans. Int. J. Parasitol. 2009, 39, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Ruff, P.; Vorobiof, D.A.; Jordaan, J.P.; Demetriou, G.S.; Moodley, S.D.; Nosworthy, A.L.; Werner, I.D.; Raats, J.; Burgess, L.J. A randomized, placebo-controlled, double-blind phase 2 study of docetaxel compared to docetaxel plus zosuquidar (LY335979) in women with metastatic or locally recurrent breast cancer who have received one prior chemotherapy regimen. Cancer Chemother. Pharmacol. 2009, 64, 763–768. [Google Scholar] [CrossRef] [PubMed]
- Pusztai, L.; Wagner, P.; Ibrahim, N.; Rivera, E.; Theriault, R.; Booser, D.; Symmans, F.W.; Wong, F.; Blumenschein, G.; Fleming, D.R.; et al. Phase II study of tariquidar, a selective P-glycoprotein inhibitor, in patients with chemotherapy-resistant, advanced breast carcinoma. Cancer 2005, 104, 682–691. [Google Scholar] [CrossRef]
- Hoste, H.; Torres-Acosta, J.F.J.; Sandoval-Castro, C.A.; Mueller-Harvey, I.; Sotiraki, S.; Louvandini, H.; Thamsborg, S.M.; Terrill, T.H. Tannin containing legumes as a model for nutraceuticals against digestive parasites in livestock. Vet. Parasitol. 2015, 212, 5–17. [Google Scholar] [CrossRef]
- Valente, A.H.; de Roode, M.; Ernst, M.; Peña-Espinoza, M.; Bornancin, L.; Bonde, C.S.; Martínez-Valladares, M.; Ramünke, S.; Krücken, J.; Simonsen, H.T.; et al. Identification of compounds responsible for the anthelmintic effects of chicory (Cichorium intybus) by molecular networking and bio-guided fractionation. Int. J. Parasitol. Drugs Drug Resist. 2021, 15, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Lonngren, K.J.; Barone, C.D.; Zajac, A.M.; Brown, R.N.; Reed, J.D.; Krueger, C.G.; Petersson, K.H. Effect of birdsfoot trefoil cultivars on exsheathment of Haemonchus contortus in fistulated sheep. Vet. Parasitol. 2020, 287, 109271. [Google Scholar] [CrossRef]
- Desrues, O.; Peña-Espinoza, M.; Hansen, T.V.A.; Enemark, H.L.; Thamsborg, S.M. Anti-parasitic activity of pelleted sainfoin (Onobrychis viciifolia) against Ostertagia ostertagi and Cooperia oncophora in calves. Parasites Vectors 2016, 9, 329. [Google Scholar] [CrossRef]
- Corrêa, P.S.; Mendes, L.W.; Lemos, L.N.; Crouzoulon, P.; Niderkorn, V.; Hoste, H.; Costa-Júnior, L.M.; Tsai, S.M.; Faciola, A.P.; Abdalla, A.L.; et al. Tannin supplementation modulates the composition and function of ruminal microbiome in lambs infected with gastrointestinal nematodes. FEMS Microbiol. Ecol. 2020, 96, fiaa024. [Google Scholar] [CrossRef]
- Williams, A.R.; Krych, L.; Fauzan Ahmad, H.; Nejsum, P.; Skovgaard, K.; Nielsen, D.S.; Thamsborg, S.M. A polyphenol-enriched diet and Ascaris suum infection modulate mucosal immune responses and gut microbiota composition in pigs. PLoS ONE 2017, 12, e0186546. [Google Scholar] [CrossRef]
- Spanogiannopoulos, P.; Bess, E.N.; Carmody, R.N.; Turnbaugh, P.J. The microbial pharmacists within us: A metagenomic view of xenobiotic metabolism. Nat. Rev. Microbiol. 2016, 14, 273–287. [Google Scholar] [CrossRef]
- Singh, A.; Patel, S.K.; Kumar, P.; Das, K.C.; Verma, D.; Sharma, R.; Tripathi, T.; Giri, R.; Martins, N.; Garg, N. Quercetin acts as a P-gp modulator via impeding signal transduction from nucleotide-binding domain to transmembrane domain. J. Biomol. Struct. Dyn. 2022, 40, 4507–4515. [Google Scholar] [CrossRef]
- El-Readi, M.Z.; Al-Abd, A.M.; Althubiti, M.A.; Almaimani, R.A.; Al-Amoodi, H.S.; Ashour, M.L.; Wink, M.; Eid, S.Y. Multiple molecular mechanisms to overcome multidrug resistance in cancer by natural secondary metabolites. Front. Pharmacol. 2021, 12, 658513. [Google Scholar] [CrossRef] [PubMed]
- Ravisankar, S.; Agah, S.; Kim, H.; Talcott, S.; Wu, C.; Awika, J. Combined cereal and pulse flavonoids show enhanced bioavailability by downregulating phase II metabolism and ABC membrane transporter function in Caco-2 model. Food Chem. 2019, 279, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Chieli, E.; Romiti, N.; Catiana Zampini, I.; Garrido, G.; Inés Isla, M. Effects of Zuccagnia punctata extracts and their flavonoids on the function and expression of ABCB1/P-glycoprotein multidrug transporter. J. Ethnopharmacol. 2012, 144, 797–801. [Google Scholar] [CrossRef] [PubMed]
- Dupuy, J.; Larrieu, G.; Sutra, J.F.; Lespine, A.; Alvinerie, M. Enhancement of moxidectin bioavailability in lamb by a natural flavonoid: Quercetin. Vet. Parasitol. 2003, 112, 337–347. [Google Scholar] [CrossRef]
- Hansen, T.V.A.; Fryganas, C.; Acevedo, N.; Caraballo, L.; Thamsborg, S.M.; Mueller-Harvey, I.; Williams, A.R. Proanthocyanidins inhibit Ascaris suum glutathione-S-transferase activity and increase susceptibility of larvae to levamisole in vitro. Parasitol. Int. 2016, 65, 336–339. [Google Scholar] [CrossRef]
- Armstrong, S.A.; Klein, D.R.; Whitney, T.R.; Scott, C.B.; Muir, J.P.; Lambert, B.D.; Craig, T.M. Effect of using redberry juniper (Juniperus pinchotii) to reduce Haemonchus contortus in vitro motility and increase ivermectin efficacy. Vet. Parasitol. 2013, 197, 271–276. [Google Scholar] [CrossRef]
- Whitney, T.R.; Wildeus, S.; Zajac, A.M. The use of redberry juniper (Juniperus pinchotii) to reduce Haemonchus contortus fecal egg counts and increase ivermectin efficacy. Vet. Parasitol. 2013, 197, 182–188. [Google Scholar] [CrossRef]
- Gaudin, E.; Simon, M.; Quijada, J.; Schelcher, F.; Sutra, J.F.; Lespine, A.; Hoste, H. Efficacy of sainfoin (Onobrychis viciifolia) pellets against multi resistant Haemonchus contortus and interaction with oral ivermectin: Implications for on-farm control. Vet. Parasitol. 2016, 227, 122–129. [Google Scholar] [CrossRef]


| Parasites | ABC Transporter Genes | References |
|---|---|---|
| Brugia malayi | 8 pgps, 5 mrps, 8 haf genes | [26] |
| Bursaphelenchus xylophilus | 106 ABC transporter genes | [27] |
| Cooperia oncophora | 7 pgps, 3 mrps and 5 haf genes | [17] |
| Cyathostomins | 2 pgps genes | [28] |
| Dirofilaria immitis | 3 pgps, 2 ABC-B (Dim-haf-1, and Dim-haf-4) and 2 ABC-C (Dim-haf-5.1 and Dim-haf-5.2), 1 pseudogene | [29] |
| 3 pgps, 2 ABC-A (Dim-abt-2, Dim-abt-4), 5 ABC-B (Dim-haf-1 Dim-haf-4) and 5 ABC-C (Dim-mrp-1, Dim-mrp-5, Dim-mrp-7, Dim-haf-5.1 and Dim-haf-5.2), 2 ABC-G transporters (Dim-wht-4, Dim-wht-4), 1 pseudogene | [30] | |
| Echinococcus granulosus | 5 pgp genes | [31] |
| Teladorsagia circumcincta | 11 pgps genes (partial sequences) | [32] |
| Fasciola gigantica | 4 ABC transporters (MDR1, MRP1, BCRP, and BSEP) genes | [33] |
| Fasciola hepatica | 1 P-gp orthologue gene | [34,35] |
| Haemonchus contortus | 11 pgps, one haf, two mrps and 2 abcf genes | [36,37] |
| Onchocerca volvulus | 2 pgps and 1 haf genes | [38,39] |
| Opisthorcis felis | 4 ABC-A, 8 ABC-B (including 4 pgps), 6 ABC-C, ABC-D, 2 ABC-F, 3 ABC-G transporter genes | [40] |
| Schistosoma mansoni | 20 ABC transporter genes, including pgps and mrps | [41] |
| Toxocara canis | 1 ABC-B and 1 ABC-C transporter genes | [42] |
| Parasite/Helminth | Differential Expression of ABC Transporters | Life Stage | References |
|---|---|---|---|
| C. oncophora | Constitutive overexpression of Con haf-9 and mrp-1 in resistant isolate as compared to susceptible | Eggs | [87] |
| D. immitis | A significant lower level of DimPgp-11 constitutive expression in the ML-resistant JYD-34 isolate compared to the ML-susceptible Missouri isolate | MF | [88] |
| H. contortus | Significantly higher transcription level of P-gp-9.1 in Ivermectin resistant strain as compared to the susceptible isolate | Adults | [89] |
| Higher expression of pgp-16 in resistant isolate | Eggs | [90] | |
| Higher expression of pgp-2 and pgp-10 in resistant isolate | L4 | [91] | |
| Higher expression of pgp-1, pgp-9, pgp-12, pgp-14, and pgp-16 in resistant isolate | Adults | ||
| Constitutive overexpression of pgp-1, pgp-9.1 and pgp-9.2 in multi-drug resistant isolate compared to susceptible isolate. | L3 | [18] | |
| Significant overexpression of Hco-pgp-3, Hco-pgp-9.2, Hco-pgp-11 and Hco-pgp-16 transcripts in L4 and adults as compared to free living stages | L3, L4 and Adults | [52] | |
| Significantly increased transcription of Hco-pgp-2, and Hco-pgp-9 in resistant and Hco-pgp-1 in susceptible cattle | L3 | [84] | |
| P. equorum | No difference | Eggs | [92] |
| Significant overexpression of pgp-11 in resistant worms | L3 | ||
| S. mansoni | Transient increase in transcription levels of SMDR2 in schistosome isolate with reduced praziquantel susceptibility | Adult worms | [93] |
| T. circumcincta | Constitutive overexpression of Tci-pgp-3, Tci-pgp-a and Tci-pgp-9 in multidrug-resistant isolate compared to susceptible isolate. | Eggs | [32] |
| Constitutive overexpression of Tci-pgp-9 and Tci-pgp-e in multidrug-resistant isolate compared to susceptible isolate. | xL3 | ||
| Constitutive overexpression of Tci-pgp-9 and Tci-pgp-d in multidrug-resistant isolate compared to susceptible isolate. | Adults |
| Nematode spp. | Inhibitors Used | Study Design | Effect on Anthelmintic Efficacy | Reference(s) |
|---|---|---|---|---|
| A. pegreffii | Valspodar, MK-571 | In vitro | Increased toxicity of LEV, Nerolidol and Farnesol | [116] |
| B. malayi | Verapamil, cyclosporin A, vinblastine, and daunorubicin | In vitro motility assay | Increased susceptibility of adult and microfilariae to IVM | [90] |
| C. oncophora | Verapamil | In vitro LDA, LMIA | Completely restored sensitivity of IVM-resistant isolate | [97] |
| Cattle nematodes | Verapamil | In vitro EHA, LDA, LMIA | Increased IVM sensitivity | [117] |
| Cattle nematodes | Loperamide | In vivo | Increased IVM and MOX efficacy in terms of reduced FEC | [118] |
| H. contortus | Verapamil, Limonene and other phytochemicals | In vitro LDA, AMT | Verapamil and Limonene completely restored IVM sensitivity in resistant isolate | [119] |
| Verapamil | In vitro LDA | Increased the susceptibility of wild-type and ML-selected isolates to IVM and MOX | [120] | |
| Crizotinib | In vitro LDA and LMA | Increased IVM toxicity to resistant isolate in LMA, and both resistant and susceptible isolates in LDA | [15] | |
| Verapamil. valsopodar, elacridar, zosuquidar, tariquidar | In vitro LDA and LMA | Increased the sensitivity of both resistant and susceptible isolates with comparatively marked effects in resistant isolate. | [14] | |
| Verapamil | In vitro (EHA) | Increased sensitivity of resistant isolate to thiabendazole | [71] | |
| Verapamil | In vitro (EHA) | Increased BZ sensitivity of resistant and susceptible isolates | [70] | |
| H. contortus, T. circumcincta | Valspodar, verapamil, quercetin, ketoconazole, pluronic acid P85 | In vitro (LFIA) | Significantly increased IVM sensitivity of susceptible and resistant isolates | [121] |
| H. placei | Cyclosporin A, ceftriaxone, dexamethasone, diminazene aceturate, quercetin, trifluoperazine, verapamil, vinblastin | In vitro (LMIT) | All inhibitors increased IVM sensitivity of resistant isolate, except diminazene aceturate | [122] |
| S. mansoni | Elacridar, tariquidar, zosuquidar, verapamil | In vitro (worm motility) | Significantly increased sensitivity of adult worms to praziquantel | [123] |
| S. mansoni | Cyclosporin A, dexverapamil, curcumin derivative (C-4), tariquidar, MK-571 | In vitro and In vivo in mice | In vitro and in vivo disruption of egg production in resistant isolate | [58] |
| Sheep nematodes | Loperamide | In vivo in sheep | Increased IVM efficacy in terms of reduced FEC, increased plasma and availability. | [124] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raza, A.; Williams, A.R.; Abeer, M.M. Importance of ABC Transporters in the Survival of Parasitic Nematodes and the Prospect for the Development of Novel Control Strategies. Pathogens 2023, 12, 755. https://doi.org/10.3390/pathogens12060755
Raza A, Williams AR, Abeer MM. Importance of ABC Transporters in the Survival of Parasitic Nematodes and the Prospect for the Development of Novel Control Strategies. Pathogens. 2023; 12(6):755. https://doi.org/10.3390/pathogens12060755
Chicago/Turabian StyleRaza, Ali, Andrew R. Williams, and Muhammad Mustafa Abeer. 2023. "Importance of ABC Transporters in the Survival of Parasitic Nematodes and the Prospect for the Development of Novel Control Strategies" Pathogens 12, no. 6: 755. https://doi.org/10.3390/pathogens12060755