Risk Factors for Exposure of Wild Birds to West Nile Virus in A Gradient of Wildlife-Livestock Interaction
Abstract
:1. Introduction
2. Materials and Methods
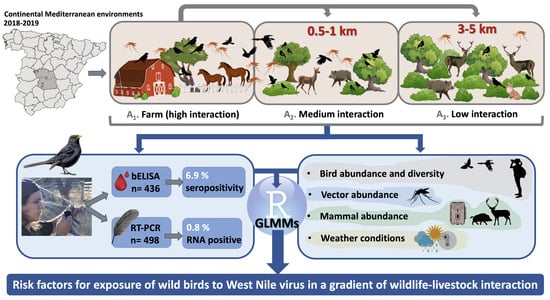
2.1. Sampling Area
2.2. Sample Collection
2.3. Serological Analyses
2.4. Real-Time RT-PCR Analyses
2.5. Predictors of Flavivirus Exposure Risk
2.5.1. Bird Population Diversity and Abundance
2.5.2. Mammal Population Diversity and Abundance
2.5.3. Mosquito Vector Abundance
2.5.4. Weather Determinants
2.5.5. Risk Analysis
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríguez-Alarcón, L.G.S.M.; Fernández-Martínez, B.; Moros, M.J.S.; Vázquez, A.; Pachés, P.J.; Villacieros, E.G.; Gómez Martín, M.B.; Figuerola Borras, J.; Lorusso, N.; Ramos Aceitero, J.M.; et al. Unprecedented increase of West Nile virus neuroinvasive disease, Spain, summer 2020. Euro Surveill. 2021, 26, 19. [Google Scholar] [CrossRef]
- Beck, C.; Jiménez-Clavero, M.A.; Leblond, A.; Durand, B.; Nowotny, N.; Leparc-Goffart, I.; Zientara, S.; Jourdain, E.; Lecollinet, S. Flaviviruses in Europe: Complex circulation patterns and their consequences for the diagnosis and control of West Nile disease. Int. J. Environ. Res. Public Health 2013, 10, 6049–6083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruiz-Fons, F.; Balseiro, A.; Willoughby, K.; Oleaga, Á.; Dagleish, M.P.; Pérez-Ramírez, E.; Havlíková, S.; Klempa, B.; Llorente, F.; Martín-Hernando, M.P. Clinical infection of Cantabrian chamois (Rupicapra pyrenaica parva) by louping ill virus: New concern for mountain ungulate conservation? Eur. J. Wildl. Res. 2014, 60, 4. [Google Scholar] [CrossRef] [Green Version]
- Gould, E.A.; de Lamballerie, X.; Zanotto, P.M.; Holmes, E.C. Origins, evolution, and vector/host coadaptations within the Genus Flavivirus. Adv. Virus Res. 2003, 59, 277–314. [Google Scholar] [PubMed]
- Guerrero-Carvajal, F.; Bravo-Barriga, D.; Martín-Cuervo, M.; Aguilera-Sepúlveda, P.; Ferraguti, M.; Jiménez-Clavero, M.A.; Llorente, F.; Alonso, J.M.; Frontera, E. Serological evidence of co-circulation of West Nile and Usutu viruses in equids from western Spain. Transbound. Emerg. Dis. 2020, 68, 1432–1444. [Google Scholar] [CrossRef] [PubMed]
- Platonov, A.E.; Rossi, G.; Karan, L.S.; Mironov, K.O.; Busani, L.; Rezza, G. Does the Japanese encephalitis virus (JEV) represent a threat for human health in Europe? Detection of JEV RNA sequences in birds collected in Italy. Euro Surveill. 2012, 17, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agüero, M.; Fernández-Pinero, J.; Buitrago, D.; Sánchez, A.; Elizalde, M.; San Miguel, E.; Villalba, R.; Llorente, F.; Jiménez-Clavero, M.A. Bagaza virus in partridges and pheasants, Spain 2010. Emerg. Infect. Dis. 2011, 17, 8. [Google Scholar] [CrossRef] [PubMed]
- Calzolari, M.; Zé-Zé, L.; Vázquez, A.; Sánchez-Seco, M.P.; Amaro, F.; Dottori, M. Insect-specific flaviviruses, a worlwide widespread group of viruses only detected in insects. Infect. Genet. Evol. 2016, 40, 381–388. [Google Scholar] [CrossRef]
- Mackenzie, J.S.; Barrett, A.D.T.; Deubel, V. The Japanese Encephalitis Serological Group of Flaviviruses: A Brief Introduction to the Group. Japanese Encephalitis and West Nile Viruses: Current Topics in Microbiology and Immunology; Springer: Berlin/Heidelberg, Germany, 2002; Volume 267. [Google Scholar]
- López, G.; Jiménez-Clavero, M.A.; Gómez-Tejedor, C.; Soriguer, R.; Figuerola, J. Prevalence of West Nile Virus neutralizing antibodies in Spain is related to the behavior of migratory birds. Vector-Borne Zoonotic Dis. 2008, 8, 5. [Google Scholar] [CrossRef] [Green Version]
- Brugueras, S.; Fernández-Martínez, B.; Martínez-de la Puente, J.; Figuerola, J.; Porro, T.M.; Rius, C.; Larrauri, A.; Gómez-Barroso, D. Environmental drivers, climate change and emergent diseases transmitted by mosquitoes and their vectors in southern Europe: A systematic review. Environ. Res. 2020, 191, 110038. [Google Scholar] [CrossRef]
- Verdonschot, P.F.M.; Besse-Lototskaya, A.A. Flight distance of mosquitoes (Culicidae): A metadata analysis to support the management of barrier zones around rewetted and newly constructed wetlands. Limnologica 2014, 45, 69–79. [Google Scholar] [CrossRef]
- Pérez-Ramirez, E.; Llorente, F.; Jimenez-Clavero, M.A. Experimental infections of wild birds with West Nile virus. Viruses 2014, 6, 752–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeth, N.M.; Oesterle, P.T.; Bowen, R.A. Humoral immunity to West Nile virus is long-lasting and protective in the house sparrow (Passer domesticus). Am. J. Trop. Med. Hyg. 2009, 80, 864–869. [Google Scholar] [CrossRef] [Green Version]
- Becker, N.; Petrić, D.; Zgomba, M.; Boase, C.; Madon, M.; Dahl, C.; Kaiser, A. Mosquitoes and Their Control, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Jiménez-Clavero, M.A.; Sotelo, E.; Fernández-Pinero, J.; Llorente, F.; Blanco, J.M.; Rodríguez-Ramos, J.; Pérez-Ramírez, E.; Höfle, U. West Nile virus in golden eagles, Spain, 2007. Emerg. Infect. Dis. 2008, 14, 1489–1491. [Google Scholar] [CrossRef] [Green Version]
- CCAES, Centro de Coordinación de Alertas y Emergencias Sanitarias, Ministerio de Sanidad, Servicios Sociales e Igualdad (2017) Informe de Situación y Evaluación del Riesgo de la Fiebre por Virus del Nilo Occidental en España. Available online: https://www.sanidad.gob.es/ca/profesionales/saludPublica/ccayes/analisisituacion/doc/Evaluacion_de_riesgo-VNO-2017.pdf (accessed on 4 May 2022).
- Boadella, M.; Díez-Delgado, I.; Gutiérrez-Guzmán, A.V.; Höfle, U.; Gortázar, C. Do wild ungulates allow improved monitoring of Flavivirus circulation in Spain? Vector-Borne Zoonotic Dis. 2012, 12, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busquets, N.; Alba, A.; Allepuz, A.; Aranda, C.; Nuñez, J.I. Usutu virus sequences in Culex pipiens (Dipetara: Culicidae), Spain. Emerg. Infect. Dis. 2008, 14, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Höfle, U.; Gamino, V.; Fernández de la Mera, I.G.; Mangold, A.J.; Ortíz, J.A.; de la Fuente, J. Usutu virus in migratory song thrushes, Spain. Emerg. Infect. Dis. 2013, 19, 7. [Google Scholar] [CrossRef] [Green Version]
- Durán-Martínez, M. Distribución, Abundancia y Composición de la Comunidad de Dípteros Hematófagos Vectores de Enfermedades en Castilla-La Mancha: Riesgos para la Salud Pública y la Sanidad Animal. Ph.D. Thesis, University of Castilla-La Mancha, Ciudad Real, Spain, 2012. Available online: https://digital.csic.es/handle/10261/147173 (accessed on 11 December 2022).
- Sotelo, E.; Llorente, F.; Rebollo, B.; Camuñas, A.; Venteo, A.; Gallardo, C.; Lubisi, A.; Rodríguez, M.J.; Sanz, A.J.; Figuerola, J.; et al. Development and evaluation of a new epitope-blocking ELISA for universal detection of antibodies to West Nile virus. J. Virol. Methods 2011, 174, 35–41. [Google Scholar] [CrossRef]
- Llorente, F.; García-Irazábal, A.; Pérez-Ramírez, E.; Cano-Gómez, C.; Sarasa, M.; Vázquez, A.; Jiménez-Clavero, M.A. Influence of flavivirus co-circulation in serological diagnostics and surveillance: A model of study using West Nile, Usutu and Bagaza viruses. Transbound. Emerg. Dis. 2019, 66, 2100–2106. [Google Scholar] [CrossRef]
- Elizalde, M.; Cano-Gómez, C.; Llorente, F.; Pérez-Ramírez, E.; Casades-Martí, L.; Aguilera-Sepúlveda, P.; Ruiz-Fons, F.; Jiménez-Clavero, M.A.; Fernández-Pinero, J. A Duplex quantitative real-time reverse transcription-PCR for simultaneous detection and differentiation of flaviviruses of the Japanese encephalitis and Ntaya serocomplexes in birds. Front. Vet. Sci. 2020, 7, 203. [Google Scholar] [CrossRef]
- Del Amo, J.; Sotelo, E.; Fernández-Pinero, J.; Gallardo, C.; Llorente, F.; Agüero, M.; Jiménez-Clavero, M.A. A novel quantitative multiplex real-time RT-PCR for the simultaneous detection and differentiation of West Nile virus lineages 1 and 2, and of Usutu virus. J. Virol. Methods 2013, 189, 321–327. [Google Scholar] [CrossRef]
- Loss, S.R.; Hamer, G.L.; Walker, E.D.; Ruiz, M.O.; Goldberg, T.L.; Kitron, U.D.; Brawn, J.D. Avian host community structure and prevalence of West Nile virus in Chicago, Illinois. Oecologia 2009, 159, 415–424. [Google Scholar] [CrossRef]
- Roiz, D.; Vázquez, A.; Ruiz, S.; Tenorio, A.; Soriguer, R.; Figuerola, J. Evidence that passerine birds act as amplifying hosts for Usutu virus circulation. EcoHealth 2019, 16, 734–742. [Google Scholar] [CrossRef] [PubMed]
- Bibby, C.J.; Burguess, N.D.; Hill, D.A.; Mustoe, S.H. Bird Census Techniques, 2nd ed.; Elsevier: London, UK, 2000. [Google Scholar]
- Bunning, M.L.; Bowen, R.A.; Bruce Cropp, C.; Sullivan, K.G.; Davis, B.S.; Komar, N.; Godsey, M.S.; Baker, D.; Hettler, D.L.; Holmes, D.A.; et al. Experimental infection of horses with West Nile virus. Emerg. Infect. Dis. 2002, 8, 380–386. [Google Scholar] [CrossRef]
- Hofmeester, T.R.; Rowcliffe, J.M.; Jansen, P.A. Quantifying the availability of vertebrate hosts to ticks: A camera-trapping approach. Front. Vet. Sci. 2017, 4, 115. [Google Scholar] [CrossRef] [Green Version]
- Palencia, P.; Rowcliffe, J.M.; Vicente, J.; Acevedo, P. Assessing the camera trap methodologies used to estimate density of unmarked populations. J. Appl. Ecol. 2021, 58, 1583–1592. [Google Scholar] [CrossRef]
- Fay, R.L.; Ngo, K.A.; Kuo, L.; Willsey, G.G.; Kramer, L.D.; Ciota, A.T. Experimental evolution of West Nile Virus at higher temperatures facilitates broad adaptation and increased genetic diversity. Viruses 2021, 13, 1889. [Google Scholar] [CrossRef] [PubMed]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A protocol for data exploration to avoid common statistical problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Del Amo, J.; Llorente, F.; Figuerola, J.; Soriguer, R.C.; Moreno, A.M.; Cordioli, P.; Weissenböck, H.; Jiménez-Clavero, M.A. Experimental Infection of house sparrows (Passer domesticus) with West Nile virus isolates of Euro-Mediterranean and North American origins. Vet. Res. 2014, 45, 33. [Google Scholar] [CrossRef] [Green Version]
- Snoeck, C.J.; Sausy, A.; Losch, S.; Wildschutz, F.; Bourg, M.; Hübschen, J.M. Usutu virus Africa 3 lineage, Luxembourg, 2020. Emerg. Infect. Dis. 2022, 28, 1076–1079. [Google Scholar] [CrossRef]
- Bravo-Barriga, D.; Ferraguti, M.; Magallanes, S.; Aguilera-Sepúlveda, P.; Llorente, F.; Pérez-Ramírez, E.; Vázquez, A.; Guerrero-Carvajal, F.; Sánchez-Seco, M.P.; Jiménez-Clavero, M.A.; et al. Identification of Usutu virus Africa 3 lineage in a survey of mosquitoes and birds from urban areas of western Spain. Transboun. Emerg. Dis. 2023, in press. [Google Scholar]
- Bravo-Barriga, D.; Aguilera-Sepúlveda, P.; Guerrero-Carvajal, F.; Llorente, F.; Reina, D.; Pérez-Martín, J.E.; Jiménez-Clavero, M.A. West Nile and Usutu virus infections in wild birds admitted to rehabilitation centres in Extremadura, western Spain, 2017–2019. Vet. Microbiol. 2021, 255, 109020. [Google Scholar] [CrossRef]
- Marzal, A.; Ferraguti, M.; Muriel, J.; Magallanes, S.; Ortiz, J.A.; García-Longoria, L.; Bravo-Barriga, D.; Guerrero-Carvajal, F.; Aguilera-Sepúlveda, P.; Llorente, F.; et al. Circulation of zoonotic flaviviruses in wild passerine birds in Western Spain. Vet. Microbiol. 2022, 268, 109399. [Google Scholar] [CrossRef]
- López, G.; Jiménez-Clavero, M.A.; Vázquez, A.; Soriguer, R.; Gómez-Tejedor, C.; Tenorio, A.; Figuerola, J. Incidence of West Nile virus in birds arriving in wildlife rehabilitation centers in southern Spain. Vector-Borne Zoonotic Dis. 2011, 11, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Figuerola, J.; Soriguer, R.; Rojo, G.; Gómez-Tejedor, C.; Jiménez-Clavero, M.A. Seroconversion in wild birds and local circulation of West Nile virus, Spain. Emerg. Infect. Dis. 2007, 13, 1915–1917. [Google Scholar] [CrossRef]
- Ferraguti, M.; Heesterbeek, H.; Martínez-de la Puente, J.; Jiménez-Clavero, M.A.; Vázquez, A.; Ruiz, S.; Llorente, F.; Roiz, D.; Vernooij, H.; Soriguer, R.; et al. The role of different Culex mosquito species in the transmission of West Nile virus and malaria parasites in Mediterranean areas. Transbound. Emerg. Dis. 2020, 68, 920–930. [Google Scholar] [CrossRef]
- Adelman, J.S.; Tokarz, R.E.; Euken, A.E.; Field, E.N.; Russell, M.C.; Smith, R.C. Relative influence of land use, mosquito abundance, and bird communities in defining West Nile virus infection rates in Culex mosquito populations. Insects. 2022, 13, 758. [Google Scholar] [CrossRef] [PubMed]
- Cano-Terriza, D.; Guerra, R.; Lecollinet, S.; Cerdà-Cuéllar, M.; Cabezón, O.; Almería, S.; García-Bocanegra, N. Epidemiological survey of zoonotic pathogens in feral pigeons (Columba livia var domestica) and sympatric zoo species in southern Spain. Microbiol. Infect. Dis. 2015, 43, 22–27. [Google Scholar] [CrossRef]
- Niczyporuk, J.S.; Samorek-Salamonowicz, E.; Lecollinet, S.; Andrzej Pancewicz, S.; Kozdruń, W.; Czekaj, H. Occurrence of West Nile virus antibodies in wild birds, horses, and humans in Poland. BioMed Res. Int. 2015, 2015, 234181. [Google Scholar] [CrossRef] [Green Version]
- Ferraguti, M.; Martínez-de La Puente, J.; Soriguer, R.; Llorente, F.; Jiménez-Clavero, M.A.; Figuerola, J. West Nile virus-neutralizing antibodies in wild birds from southern Spain. Epidemiol. Infect. 2016, 144, 1907–1911. [Google Scholar] [CrossRef] [Green Version]
- García-Bocanegra, I.; Paniagua, J.; Gutiérrez-Guzmán, A.V.; Lecollinet, S.; Boadella, M.; Arenas-Montes, A.; Cano-Terriza, D.; Lowenski, S.; Gortázar, C.; Höfle, U. Spatio-temporal trends and risk factors affecting West Nile virus and related flavivirus exposure in Spanish wild ruminants. BMC Vet. Res. 2016, 12, 249. [Google Scholar] [CrossRef] [PubMed]
- Jurado-Tarifa, E.; Napp, S.; Lecollinet, S.; Arenas, A.; Beck, C.; Cerdà-Cuéllar, M.; Fernández-Morente, M.; García-Bocanegra, I. Monitoring of West Nile virus, Usutu virus and Meaban virus in waterfowl used as decoys and wild raptors in southern Spain. Microbiol. Infect. Dis. 2016, 49, 58–64. [Google Scholar] [CrossRef] [PubMed]
- García-Bocanegra, I.; Jurado-Tarifa, E.; Cano-Terriza, D.; Martínez, R.; Pérez-Marín, J.E.; Lecollinet, S. Exposure to West Nile virus and tick-borne encephalitis virus in dogs in Spain. Transbound. Emerg. Dis. 2018, 65, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Martínez-de la Puente, J.; Ferraguti, M.; Ruiz, S.; Roiz, D.; Llorente, F.; Pérez-Ramírez, E.; Jiménez-Clavero, M.A.; Soriguer, R.; Figuerola, J. Mosquito community influences West Nile virus seroprevalence in wild birds: Implications for the risk of spillover into human populations. Sci. Rep. 2018, 8, 2599. [Google Scholar] [CrossRef] [Green Version]
- Caballero-Gómez, J.; Cano-Terriza, D.; Lecollinet, S.; Carbonell, M.D.; Martínez-Valverde, R.; Martínez-Nevado, E.; García-Párraga, D.; Lowenski, S.; García-Bocanegra, I. Evidence of exposure to zoonotic flaviviruses in zoo mammals in Spain and their potential role as sentinel species. Vet. Microbiol. 2020, 247, 108763. [Google Scholar] [CrossRef] [PubMed]



| Factor | Predictor | Description | Type & Values |
|---|---|---|---|
| Spatial | Site | Survey location | Categorical (1–5) |
| igrad | Interaction gradient | Categorical (1–3) | |
| Bird host population | ab.tot | Bird average abundance index | Numerical (4.2–170) |
| ab.pass | Abundance of passerine bird index | Numerical (3.6–167) | |
| ab.col | Abundance of columbiform bird index | Numerical (0.08–3.02) | |
| ab.bucer | Abundance of bucerotiform bird index | Numerical (0–0.65) | |
| ab.picif | Abundance of piciform bird index | Numerical (0–0.27) | |
| ab.accip | Abundance of accipitriform bird index | Numerical (0–0.31) | |
| ab.corac | Abundance of coraciiform bird index | Numerical (0–5.25) | |
| ab.sulif | Abundance of suliform bird index | Numerical (0–0.06) | |
| ab.cicon | Abundance of ciconiform bird index | Numerical (0–0.15) | |
| rich | Bird richness | Numerical (16–36) | |
| ish | Shannon diversity index | Numerical (0.18–2.77) | |
| ismp | Simpson diversity index | Numerical (0.07–2.01) | |
| Ungulate population | ab.ung | Ungulate abundance index | Numerical (0.00–0.02) |
| Vector population | cx | Culex spp. abundance index | Numerical (2–103) |
| Weather | ar | Annual accumulated rainfall | Numerical (361.1–739.4 mm) |
| swr | Rainfall accumulated over Dec–May | Numerical (185.0– 553.6 mm) | |
| sr | Rainfall accumulated in spring (March–May) | Numerical (127.0– 355.6 mm) | |
| smr | Summer (July–Sept) accumulated rainfall | Numerical (16.7– 166.5 mm) | |
| wt | Average winter (Dec–Feb) temperature | Numerical (9.8–15.9 °C) | |
| st | Average spring (March–May) temperature | Numerical (9.8–15.9 °C) | |
| smt | Average summer (July–Sept) temperature | Numerical (18.6–27.9 °C) | |
| ar1 | Annual cumulative rainfall of year t-1 | Numerical (362.1–856.5 mm) | |
| swr1 | Rainfall accumulated over Dec-May year t-1 | Numerical (208.4–502.4 mm) | |
| sr1 | Rainfall accumulated in spring year t-1 | Numerical (100.2–249.1 mm) | |
| smr1 | Summer accumulated rainfall year t-1 | Numerical (2.0–60.4 mm) | |
| wt1 | Average winter temperature year t-1 | Numerical (3.4–8.5 °C) | |
| st1 | Average spring temperature year t-1 | Numerical (9.0–17.1 °C) | |
| smt1 | Average summer temperature year t-1 | Numerical (18.7–27.9 °C) | |
| ar2 | Annual cumulative rainfall of year t-2 | Numerical (353.4–497.2 mm) | |
| swr2 | Rainfall accumulated over Dec-May year t-2 | Numerical (175.8–372.1 mm) | |
| sr2 | Rainfall accumulated in spring year t-2 | Numerical (69.0–249.1 mm) | |
| smr2 | Summer accumulated rainfall year t-2 | Numerical (2.0–90.6 mm) | |
| wt2 | Average winter temperature year t-2 | Numerical (5.2–9.3 °C) | |
| st2 | Average spring temperature year t-2 | Numerical (11.2–15.2 °C) | |
| smt2 | Average summer temperature year t-2 | Numerical (19.7–28.1 °C) |
| Area | Site | No. Captures | bELISA No. Positive/No. Tested(Prevalence) | bELISAlow No. Positive/No. Tested(Prevalence) | bELISAhigh No. Positive/No. Tested(Prevalence) | rRT-PCR No. Positive/No. Tested (Prevalence) |
|---|---|---|---|---|---|---|
| A1 | S1 | 36 | 4/34 (11.8%) | 2/34 (5.9%) | 4/34 (11.8%) | 0/32 (0.0%) |
| S2 | 56 | 2/48 (4.2%) | 1/48 (2.1%) | 2/48 (4.2%) | 0/43 (0.0%) | |
| S3 | 24 | 3/18 (16.7%) | 1/18 (5.6%) | 3/18 (16.7%) | 1/22 (4.5%) | |
| S4 | 24 | 3/23 (13.0%) | 2/23 (8.7%) | 3/23 (13.0%) | 0/22 (0.0%) | |
| S5 | 34 | 2/32 (6.3%) | 1/32 (3.1%) | 1/32 (3.1%) | 0/32 (0.0%) | |
| Subtotal A1 | 174 | 14/155 (9.0%) | 7/155 (4.5%) | 13/155 (8.4%) | 1/151 (0.7%) | |
| A2 | S1 | 28 | 0/7 (0.0%) | 0/7 (0.0%) | 0/7 (0.0%) | 0/28 (0.0%) |
| S2 | 67 | 2/44 (4.5%) | 1/44 (2.3%) | 2/44 (4.5%) | 1/58 (1.7%) | |
| S3 | 45 | 1/32 (3.1%) | 1/32 (3.1%) | 1/32 (3.1%) | 1/37 (2.7%) | |
| S4 | 25 | 1/25 (4.0%) | 0/25 (0.0%) | 1/25 (4.0%) | 0/24 (0.0%) | |
| S5 | 38 | 4/35 (11.4%) | 2/35 (5.7%) | 4/35 (11.4%) | 1/30 (3.3%) | |
| Subtotal A2 | 203 | 8/143 (5.6%) | 4/143 (2.8%) | 8/143 (5.6%) | 3/177 (1.7%) | |
| A3 | S1 | 34 | 2/20 (10.0%) | 1/20 (5.0%) | 2/20 (10.0%) | 0/33 (0.0%) |
| S2 | 38 | 0/27 (0.0%) | 0/27 (0.0%) | 0/27 (0.0%) | 0/34 (0.0%) | |
| S3 | 38 | 0/32 (0.0%) | 0/32 (0.0%) | 0/32 (0.0%) | 0/32 (0.0%) | |
| S4 | 45 | 1/34 (2.9%) | 1/34 (2.9%) | 1/34 (2.9%) | 0/45 (0.0%) | |
| S5 | 29 | 5/25 (20.0%) | 2/25 (8.0%) | 5/25 (20.0%) | 0/26 (0.0%) | |
| Subtotal A3 | 184 | 8/138 (5.8%) | 4/138 (2.9%) | 8/138 (5.8%) | 0/170 (0.0%) | |
| All areas | S1 | 98 | 6/61 (9.8%) | 3/61 (4.9%) | 6/61 (9.8%) | 0/93 (0.0%) |
| S2 | 161 | 4/119 (3.4%) | 2/119 (1.7%) | 4/119 (3.4%) | 1/135 (0.7%) | |
| S3 | 107 | 4/82 (4.9%) | 2/82 (2.4%) | 4/82 (4.9%) | 2/91 (2.2%) | |
| S4 | 94 | 5/82 (6.1%) | 3/82 (3.7%) | 5/82 (6.1%) | 0/91 (0.0%) | |
| S5 | 101 | 11/92 (12.0%) | 5/92 (5.4%) | 10/92 (10.9%) | 1/88 (1.1%) | |
| Total | 561 | 30/436 (6.9%) | 15/436 (3.4%) | 29/436 (6.7%) | 4/498 (0.8%) |
| Bird Family | bELISA No. Positive /No. Tested (Seroprevalence) | rRT-PCR No. Positive /No. Tested (Prevalence) |
|---|---|---|
| Corvidae | 1/29 (3.4%) | 0/28 (0.0%) |
| Emberezidae | 1/13 (7.7%) | 1/19 (5.3%) |
| Fringilidae | 3/27 (11.1%) | 0/25 (0.0%) |
| Muscicapidae | 0/15 (0.0%) | 1/14 (7.1%) |
| Paridae | 14/124 (11.3%) | 0/167 (0.0%) |
| Passeridae | 5/82 (6.1%) | 0/77 (0.0%) |
| Turdidae | 6/40 (15.0%) | 2/32 (6.3%) |
| TOTAL | 30/330 (9.1%) | 4/362 (1.1%) |
| Model Set | Model Reference | ΔAICc/ Weight | Predictors | |||||
|---|---|---|---|---|---|---|---|---|
| igrad | ab.col | ab.pass | cx | ish | ar1 | |||
| Flavivirus spp. | model 1 | 0.00/0.088 | ns | ns | ns | ns | 0.6765 | ns |
| model 2 | 0.07/0.085 | ns | ns | ns | ns | 0.5703 | 0.2433 | |
| model 3 | 0.53/0.068 | ns | ns | ns | 0.2211 | 0.5498 | ns | |
| model 4 | 1.36/0.045 | + | −0.5336 | ns | ns | 0.7767 | 0.5429 | |
| model 5 | 1.39/0.044 | ns | ns | −0.1983 | ns | 0.5159 | 0.3965 | |
| model 6 | 1.41/0.043 | ns | −0.1938 | ns | ns | 0.6571 | 0.3313 | |
| model 7 | 1.82/0.036 | ns | ns | 0.07899 | ns | 0.6691 | ns | |
| model 8 | 1.98/0.033 | ns | ns | ns | 0.09002 | 0.5452 | 0.1825 | |
| WNVlow | model 1 | 0.00/0.122 | ns | ns | ns | ns | 0.5262 | ns |
| model 2 | 0.84/0.080 | ns | ns | ns | ns | ns | ns | |
| model 3 | 1.67/0.053 | ns | ns | ns | ns | ns | 0.2632 | |
| model 4 | 1.80/0.050 | ns | ns | ns | ns | 0.4735 | 0.1223 | |
| model 5 | 1.93/0.046 | ns | ns | ns | 0.2370 | ns | ns | |
| model 6 | 1.95/0.046 | ns | ns | 0.06779 | ns | 0.5170 | ns | |
| model 7 | 1.97/0.045 | ns | ns | ns | 0.0664 | 0.4904 | ns | |
| WNVhigh | model 1 | 0.00/0.145 | ns | ns | ns | ns | 0.6818 | ns |
| model 2 | 1.06/0.085 | ns | ns | ns | 0.1838 | 0.5746 | ns | |
| model 3 | 1.52/0.068 | ns | ns | ns | ns | 0.6282 | 0.1291 | |
| model 4 | 1.79/0.059 | ns | −0.1041 | ns | ns | 0.7494 | ns | |
| Model Set | Predictor | Estimate | SE | z | p |
|---|---|---|---|---|---|
| Flavivirus spp. | Intercept | −2.77232 | 0.27952 | 9.899 | *** |
| ish | 0.61848 | 0.29538 | 2.089 | * | |
| ar1 | 0.18747 | 0.24631 | 0.760 | 0.447 | |
| cx | 0.04062 | 0.12610 | 0.322 | 0.747 | |
| igrad | 0.147 | ||||
| A1 | Ref. | ||||
| A2 | −0.11159 | 0.37507 | 0.297 | 0.766 | |
| A3 | −0.07787 | 0.29499 | 0.264 | 0.792 | |
| ab.col | −0.07307 | 0.20228 | 0.361 | 0.718 | |
| ab.pass | −0.01337 | 0.10871 | 0.123 | 0.902 | |
| WNVlow | Intercept | −3.40845 | 0.28796 | 11.804 | *** |
| ish | 0.30226 | 0.36776 | 0.821 | 0.412 | |
| ar1 | 0.04525 | 0.14548 | 0.311 | 0.756 | |
| cx | 0.03166 | 0.13447 | 0.235 | 0.814 | |
| ab.pass | 0.00703 | 0.07749 | 0.090 | 0.928 | |
| WNVhigh | Intercept | −2.81347 | 0.22789 | 13.311 | *** |
| ish | 0.65721 | 0.28526 | 2.298 | * | |
| ar1 | 0.02446 | 0.09261 | 0.264 | 0.792 | |
| cx | 0.04395 | 0.11972 | 0.366 | 0.714 | |
| ab.col | −0.01728 | 0.09418 | 0.183 | 0.855 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casades-Martí, L.; Holgado-Martín, R.; Aguilera-Sepúlveda, P.; Llorente, F.; Pérez-Ramírez, E.; Jiménez-Clavero, M.Á.; Ruiz-Fons, F. Risk Factors for Exposure of Wild Birds to West Nile Virus in A Gradient of Wildlife-Livestock Interaction. Pathogens 2023, 12, 83. https://doi.org/10.3390/pathogens12010083
Casades-Martí L, Holgado-Martín R, Aguilera-Sepúlveda P, Llorente F, Pérez-Ramírez E, Jiménez-Clavero MÁ, Ruiz-Fons F. Risk Factors for Exposure of Wild Birds to West Nile Virus in A Gradient of Wildlife-Livestock Interaction. Pathogens. 2023; 12(1):83. https://doi.org/10.3390/pathogens12010083
Chicago/Turabian StyleCasades-Martí, Laia, Rocío Holgado-Martín, Pilar Aguilera-Sepúlveda, Francisco Llorente, Elisa Pérez-Ramírez, Miguel Ángel Jiménez-Clavero, and Francisco Ruiz-Fons. 2023. "Risk Factors for Exposure of Wild Birds to West Nile Virus in A Gradient of Wildlife-Livestock Interaction" Pathogens 12, no. 1: 83. https://doi.org/10.3390/pathogens12010083