Molecular Survey and Spatial Distribution of Rickettsia spp. in Ticks Infesting Free-Ranging Wild Animals in Pakistan (2017–2021)
Abstract
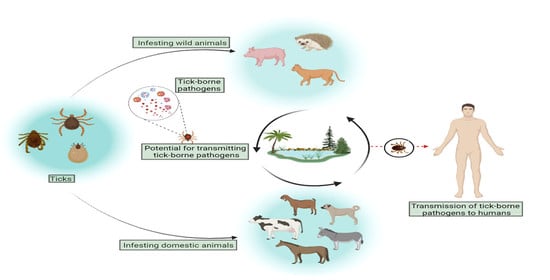
:1. Introduction
2. Results
2.1. Ticks’ Morphological Description
2.2. Tick Infestation and Wild Animals
2.3. Molecular Identification and Phylogeny of Ticks
2.4. Detection of Rickettsia spp. in Ticks
3. Discussion
4. Materials and Methods
4.1. Ethical Approval
4.2. Study Area
4.3. Tick Collection and Morphological Identification
4.4. DNA Extraction and PCR
4.5. Detection of Rickettsia
4.6. DNA Purification and Sequencing
4.7. Phylogenetic Analysis
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Clark, N.J.; Sedden, J.M.; Slapeta, J.; Wells, K. Parasite spread at the domestic animal- wildlife interface: Anthropogenic habitat use, phylogeny and body mass drive risk of cat and dog flea (Ctenocephlides spp.) infestation in wild mammals. Parasit. Vectors 2018, 11, 8. [Google Scholar] [CrossRef] [PubMed]
- Daszak, P.; Cunningham, A.A.; Hyatt, A.D. Emerging infectious diseases of wildlife--threats to biodiversity and human health. Science 2000, 287, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Davies, S.; Abdullah, S.; Helps, C.; Tasker, S.; Newbury, H.; Wall, R. Prevalence of ticks and tick-borne pathogens: Babesia and Borrelia species in ticks infesting cats of Great Britain. Vet. Parasitol. 2017, 244, 129–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, S.E.; Barrett, A.W.; Nagamori, Y.; Herrin, B.H.; Normile, D.; Heaney, K.; Armstrong, R. Ticks from cats in the United States: Patterns of infestation and infection with pathogens. Vet. Parasitol. 2018, 257, 15–20. [Google Scholar] [CrossRef]
- Ghosh, P.; Saleh, M.N.; Sundstrom, K.D.; Ientile, M.; Little, S.E. Ixodes spp. from dogs and cats in the United States: Diversity, seasonality, and prevalence of Borrelia burgdorferi and Anaplasma phagocytophilum. Vector-Borne Zoonotic Dis. 2021, 21, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.N.; Sundstrom, K.D.; Duncan, K.T.; Ientile, M.M.; Jordy, J.; Ghosh, P.; Little, S.E. Show us your ticks: A survey of ticks infesting dogs and cats across the USA. Parasit. Vectors 2019, 12, 595. [Google Scholar] [CrossRef] [PubMed]
- Karim, S.; Budachetri, K.; Mukherjee, N.; Williams, J.; Kausar, A.; Hassan, M.J.; Adamson, S.; Dowd, S.E.; Apanskevich, D.; Arijo, A.; et al. A study of ticks and tick-borne livestock pathogens in Pakistan. PLoS Negl. Trop. Dis. 2017, 11, 0005681. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Inokuma, H.; Beppu, T.; Okuda, M.; Onishi, T. Survey of ixodid tick species on domestic cats in Japan. Vet. Parasitol. 2003, 111, 231–239. [Google Scholar] [CrossRef]
- Iwakami, S.; Ichikawa, Y.; Inokuma, H. A nationwide survey of ixodid tick species recovered from domestic dogs and cats in Japan in 2011. Ticks Tick-Borne Dis. 2014, 5, 771–779. [Google Scholar] [CrossRef]
- Duplan, F.; Davies, S.; Filler, S.; Abdullah, S.; Keyte, S.; Newbury, H.; Helps, C.R.; Wall, R.; Tasker, S. Anaplasma phagocytophilum, Bartonella spp., haemoplasma species and Hepatozoon spp. in ticks infesting cats: A large-scale survey. Parasit. Vectors 2018, 11, 201. [Google Scholar] [CrossRef]
- Nguyen, V.L.; Colella, V.; Greco, G.; Fang, F.; Nurcahyo, W.; Hadi, U.K.; Venturina, V.; Tong, K.B.Y.; Tsai, Y.L.; Taweethavonsawat, P.; et al. Molecular detection of pathogens in ticks and fleas collected from companion dogs and cats in East and Southeast Asia. Parasit. Vectors 2020, 13, 420. [Google Scholar] [CrossRef] [PubMed]
- Földvári, G.; Rigó, K.; Jablonszky, M.; Biró, N.; Majoros, G.; Molnár, V.; Tóth, M. Ticks and the city: Ectoparasites of the Northern white-breasted hedgehog (Erinaceus roumanicus) in an urban park. Ticks Tick-Borne Dis. 2011, 2, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Goz, Y.; Yilmaz, A.B.; Aydin, A.; Dicle, Y. Ticks and fleas infestation on east hedgehogs (Erinaceus concolor) in Van Province, Eastern Region of Turkey. J. Arthropod. Borne Dis. 2016, 10, 50. [Google Scholar] [PubMed]
- Khodadadi, N.; Nabavi, R.; Sarani, A.; Saadati, D.; Ganjali, M.; Mihalca, A.D.; Otranto, D.; Sazmand, A. Identification of Anaplasma marginale in long-eared hedgehogs (Hemiechinus auritus) and their Rhipicephalus turanicus ticks in Iran. Ticks Tick-Borne Dis. 2021, 12, 101641. [Google Scholar] [CrossRef] [PubMed]
- Skuballa, J.; Oehme, R.; Hartelt, K.; Petney, T.; Bucher, T.; Kimmig, P.; Taraschewski, H. European hedgehogs as hosts for Borrelia spp., Germany. Emerg. Infect. Dis. 2007, 13, 952. [Google Scholar] [CrossRef]
- Silaghi, C.; Skuballa, J.; Thiel, C.; Pfister, K.; Petney, T.; Pfaffle, M.; Taraschewski, H.; Passos, L.M. The European hedgehog (Erinaceus europaeus)–A suitable reservoir for variants of Anaplasma phagocytophilum? Ticks Tick-Borne Dis. 2012, 3, 49–54. [Google Scholar] [CrossRef]
- Sgroi, G.; Iatta, R.; Lia, R.P.; D’Alessio, N.; Manoj, R.R.S.; Veneziano, V.; Otranto, D. Spotted fever group Rickettsiae in Dermacentor marginatus from wild boars in Italy. Transbound. Emerg. Dis. 2020, 68, 2111–2120. [Google Scholar] [CrossRef]
- Ortuno, A.; Quesada, M.; Lopez, S.; Miret, J.; Cardenosa, N.; Castella, J.; Anton, E.; Segura, F. Prevalence of Rickettsia slovaca in Dermacentor marginatus ticks removed from wild boar (Sus scrofa) in northeastern Spain. Ann. N. Y. Acad. Sci. 2006, 1078, 324–327. [Google Scholar] [CrossRef]
- Liyanaarachchi, D.R.; Rajakaruna, R.S.; Dikkumbura, A.W.; Rajapakse, R.P.V.J. Ticks infesting wild and domestic animals and humans of Sri Lanka with new host records. Acta Trop. 2015, 142, 64–70. [Google Scholar] [CrossRef]
- Ali, A.; Khan, M.A.; Zahid, H.; Yaseen, P.M.; Khan, M.Q.; Nawab, J.; Rehman, Z.U.; Ateeq, M.; Khan, S.; Ibrahim, M. Seasonal dynamics, record of ticks infesting humans, wild and domestic animals and molecular phylogeny of Rhipicephalus microplus in Khyber Pakhtunkhwa Pakistan. Front. Physiol. 2019, 10, 793. [Google Scholar] [CrossRef] [Green Version]
- Ciebiera, O.; Lopinska, A.; Gabrys, G. Ticks on game animals in the fragmented agricultural landscape of western Poland. Parasitol. Res. 2021, 120, 1781–1788. [Google Scholar] [CrossRef] [PubMed]
- Hrazdilova, K.; Lesiczka, P.M.; Bardon, J.; Vyroubalova, S.; Simek, B.; Zurek, L.; Modry, D. Wild boar as a potential reservoir of zoonotic tick-borne pathogens. Ticks Tick-Borne Dis. 2021, 12, 101558. [Google Scholar] [CrossRef] [PubMed]
- Lim, F.S.; Khoo, J.J.; Tan, K.K.; Zainal, N.; Loong, S.K.; Khor, C.S.; AbuBakar, S. Bacterial communities in Haemaphysalis, Dermacentor and Amblyomma ticks collected from wild boar of an Orang Asli Community in Malaysia. Ticks Tick-Borne Dis. 2020, 11, 101352. [Google Scholar] [CrossRef] [PubMed]
- Ghafar, A.; Cabezas-Cruz, A.; Galon, C.; Obregon, D.; Gasser, R.B.; Moutailler, S.; Jabbar, A. Bovine ticks harbour a diverse array of microorganisms in Pakistan. Parasit. Vectors 2020, 13, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eremeeva, M.E.; Bosserman, E.A.; Demma, L.J.; Zambrano, M.L.; Blau, D.M.; Dasch, G.A. Isolation and identification of Rickettsia massiliae from Rhipicephalus sanguineus ticks collected in Arizona. Appl. Environ. Microbiol. 2006, 72, 5569–5577. [Google Scholar] [CrossRef] [Green Version]
- Keysary, A.; Eremeeva, M.E.; Leitner, M.; Din, A.B.; Wikswo, M.E.; Mumcuoglu, K.Y.; Inbar, M.; Wallach, A.D.; Shanas, U.; King, R.; et al. Spotted fever group rickettsiae in ticks collected from wild animals in Israel. Am. J. Trop. Med. Hyg. 2011, 85, 919. [Google Scholar] [CrossRef] [Green Version]
- Olivieri, E.; Wijnveld, M.; Bonga, M.; Berger, L.; Manfredi, M.T.; Veronesi, F.; Jongejan, F. Transmission of Rickettsia raoultii and Rickettsia massiliae DNA by Dermacentor reticulatus and Rhipicephalus sanguineus (s.l.) ticks during artificial feeding. Parasit. Vectors 2018, 11, 494. [Google Scholar] [CrossRef]
- Fernandez-Soto, P.; Perez-Sanchez, R.; Martin, V.D.; Encinas-Grandes, A.; Sanz, R.A. Rickettsia massiliae in ticks removed from humans in Castilla y León, Spain. Eur. J. Clin. Microbiol. Infect. Dis. 2006, 25, 811–813. [Google Scholar] [CrossRef]
- Garcia-Garcia, J.C.; Portillo, A.; Nunez, M.J.; Santibanez, S.; Castro, B.; Oteo, J.A. A patient from Argentina infected with Rickettsia massiliae. Am. J. Trop. Med. Hyg. 2010, 82, 691–692. [Google Scholar] [CrossRef] [Green Version]
- Ali, A.; Mulenga, A.; Vaz Jr, I. Tick and Tick-Borne Pathogens: Molecular and Immune Targets for Control Strategies. Front. Physiol. 2020, 11, 00744. [Google Scholar] [CrossRef]
- Kamran, K.; Ali, A.; Villagra, C.; Siddiqui, S.; Alouffi, A.S.; Iqbal, A. A cross-sectional study of hard ticks (acari: Ixodidae) on horse farms to assess the risk factors associated with tick-borne diseases. Zoonoses Public Health 2021, 68, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Kamran, K.; Ali, A.; Villagra, C.A.; Bazai, Z.A.; Iqbal, A.; Sajid, M.S. Hyalomma anatolicum resistance against ivermectin and fipronil is associated with indiscriminate use of acaricides in southwestern Balochistan, Pakistan. Parasitol. Res. 2021, 120, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Zahid, H.; Munoz-Leal, S.; Khan, M.Q.; Alouffi, A.S.; Labruna, M.B.; Ali, A. Life Cycle and genetic identification of Argas persicus Infesting Domestic Fowl in Khyber Pakhtunkhwa, Pakistan. Front. Vet. Sci. 2021, 8, 302. [Google Scholar] [CrossRef] [PubMed]
- Ali, A.; Zahid, H.; Zeb, I.; Tufail, M.; Khan, S.; Haroon, M.; Tufail, M.; Bilal, M.; Hussain, M.; Alouffi, S.A.; et al. Risk Factors Associated with Tick Infestations on Equids in Khyber Pakhtunkhwa, Pakistan, with Notes on Rickettsia massiliae Detection. Parasit. Vectors 2021, 14, 363. [Google Scholar] [CrossRef]
- Ali, A.; Parizi, L.F.; Ferreira, B.R.; Vaz, I., Jr. A revision of two distinct species of Rhipicephalus: R. microplus and R. australis. Cienc. Rural 2016, 46, 1240–1248. [Google Scholar] [CrossRef] [Green Version]
- Estrada-Pena, A.; D’Amico, G.; Palomar, A.M.; Dupraz, M.; Fonville, M.; Heylen, D.; Habela, M.A.; Hornok, S.; Lempereur, L.; Madder, M.; et al. A comparative test of ixodid tick identification by a network of European researchers. Ticks Tick-Borne Dis. 2017, 8, 540–546. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Lv, J.; Li, F.; Li, K.; He, B.; Zhang, L.; Han, X.; Wang, H.; Johnson, N.; Lin, X.; et al. Identification and molecular analysis of ixodid ticks (Acari: Ixodidae) infesting domestic animals and tick-borne pathogens at the Tarim Basin of Southern Xinjiang, China. Korean J. Parasitol. 2020, 58, 37. [Google Scholar] [CrossRef] [Green Version]
- Castillo-Contreras, R.; Magen, L.; Birtles, R.; Varela-Castro, L.; Hall, J.L.; Conejero, C.; Aguilar, X.F.; Colom-Cadena, A.; Lavín, S.; Mentaberre, G.; et al. Ticks on wild boar in the metropolitan area of Barcelona (Spain) are infected with spotted fever group rickettsiae. Transbound. Emerg. Dis. 2021, 1–14. [Google Scholar] [CrossRef]
- Eremeeva, M.E.; Stromdahl, E.Y. New spotted fever group Rickettsia in a Rhipicephalus turanicus tick removed from a child in eastern Sicily, Italy. Am. J. Trop. Med. Hyg. 2011, 84, 99–101. [Google Scholar] [CrossRef]
- Jahfari, S.; Ruyts, S.C.; Frazer-Mendelewska, E.; Jaarsma, R.; Verheyen, K.; Sprong, H. Melting pot of tick-borne zoonoses: The European hedgehog contributes to the maintenance of various tick-borne diseases in natural cycles urban and suburban areas. Parasit. Vectors 2017, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Liu, Q.; Wang, D.; Li, W.; Beugnet, F.; Zhou, J. Epidemiological survey of ticks and tick-borne pathogens in pet dogs in south-eastern China. Parasite 2017, 24, 35. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Huang, J.L.; Chien, C.H.; Shih, H.C.; Wang, H.C. First molecular detection of Anaplasma phagocytophilum in the hard tick Rhipicephalus haemaphysaloides in Taiwan. Exp. Appl. Acarol. 2018, 75, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.Q.; Liu, K.; Li, X.L.; Liang, S.; Yang, Y.; Yao, H.W.; Sun, R.X.; Sun, Y.; Chen, W.J.; Zuo, S.Q.; et al. Emerging tick-borne infections in mainland China: An increasing public health threat. Lancet Infect. Dis. 2015, 15, 1467–1479. [Google Scholar] [CrossRef] [Green Version]
- Walker, A. The Genus Rhipicephalus (Acari, Ixodidae): A Guide to the Brown Ticks of the World; Walker, J.B., Keirans, J.E., Horak, I.G., Eds.; Kluwer Academic Publishers: Drodrecht, The Netherlands, 2000; pp. 417–418. [Google Scholar]
- Roy, B.C.; Estrada-Pena, A.; Krucken, J.; Rehman, A.; Nijhof, A.M. Morphological and phylogenetic analyses of Rhipicephalus microplus ticks from Bangladesh, Pakistan and Myanmar. Ticks Tick-Borne Dis. 2018, 9, 1069–1079. [Google Scholar] [CrossRef] [PubMed]
- Sambrook, J.; Russell, D.W. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: Long Island, NY, USA, 2001; pp. 23–44. [Google Scholar]
- Chitimia, L.; Lin, R.Q.; Cosoroaba, I.; Wu, X.Y.; Song, H.Q.; Yuan, Z.G.; Zhu, X.Q. Genetic characterization of ticks from southwestern Romania by sequences of mitochondrial cox 1 and nad 5 genes. Exp. Appl. Acarol. 2010, 52, 305–311. [Google Scholar] [CrossRef]
- Mangold, A.J.; Bargues, M.D.; Mas-Coma, S. Mitochondrial 16S rDNA sequences and phylogenetic relationships of species of Rhipicephalus and other tick genera among Metastriata (Acari: Ixodidae). Parasitol. Res. 1998, 84, 478–484. [Google Scholar] [CrossRef]
- Labruna, M.B.; Whitworth, T.; Horta, M.C.; Bouyer, D.H.; McBride, J.W.; Pinter, A.; Popov, V.; Gennari, S.M.; Walker, D.H. Rickettsia species infecting Amblyomma cooperi ticks from an area in the state of Sao Paulo, Brazil, where Brazilian spotted fever is endemic. J. Clin. Microbiol. 2004, 42, 90–98. [Google Scholar] [CrossRef] [Green Version]
- Roux, V.; Fournier, P.E.; Raoult, D. Differentiation of spotted fever group rickettsiae by sequencing and analysis of restriction fragment length polymorphism of PCR-amplified DNA of the gene encoding the protein rOmpA. J. Clin. Microbiol. 1996, 34, 2058–2065. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Hall, T.; Biosciences, I.; Carlsbad, C. BioEdit: An important software for molecular biology. GERF Bull. Biosci. 2011, 2, 60–61. [Google Scholar]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]





| Districts | Host | Tick Species | Examined Hosts (%) | Infested Hosts (%) | Collected Ticks (%) | Tick Life Stages | Ticks Molecularly Analyzed * | Rickettsia gltA and ompA |
|---|---|---|---|---|---|---|---|---|
| Mardan | Cats | Rh. microplus Rh. haemaphysaloides | 10 (18.8) | 4 (40) | 33 (73.3) 12 (36.6) | 14F, 11M, 5N, 3L | 8F, 2N | 0 |
| 5F, 4M, 3N | 5F, 2M, 3N | 0 | ||||||
| Wild boar | Rh. turanicus Rh. sanguineus | 10 (18.8) | 9 (90) | 90 (57.6) 66 (42.4) | 32F, 28M, 21N, 9L | 6F, 4N | 4 | |
| 27F, 19M, 13N, 7L | 7F, 3N | |||||||
| Peshawar | Cats | Rh. microplus | 11 (20.7) | 6 (54.5) | 55 (36) | 18F, 15M, 12N, 10L | 8F, 2N | 0 |
| Hedgehogs | Rh. turanicus | 4 (7.54) | 2 (50) | 98 (64) | 64F, 34M | 10F | 0 | |
| Charsadda | Wild boar | Rh. turanicus Rh. sanguineus Rh. haemaphysaloides | 10 (18.8) | 5 (50) | 58 (52) 40 (36) 14 (12) | 21F, 17M, 14N, 6L 19F, 17M, 4N | 9F, 1N 8F, 2N | 3 0 |
| 6F, 4M, 2N, 2L | 6F, 2M, 2N | 3 | ||||||
| Swabi | Cats | Rh. microplus | 4 (7.54) | 3 (75) | 35 (54) | 19F, 12M, 4N | 8F, 2N | 0 |
| Rh. haemaphysaloides | 10 (15.3) | 5F, 3M, 2N | 5F,3M,2N | 0 | ||||
| Hedgehogs | Rh. turanicus | 4 (7.54) | 2 (50) | 20 (30.7) | 12F, 8M | 6F, 4M | 0 | |
| Total | 53 (100) | 31 (58.4) | 531 (Mean 44.25) | 242F, 172M, 80N, 37L | 86F, 11M, 23N Total: 120 | 10(8.3%) |
| Organism | Gene | Primer | Sequence | Amplicon bp | References |
|---|---|---|---|---|---|
| Tick | cox 1 | cox1F | GGAACAATATATTTAATTTTTGG | 850 | [47] |
| cox1R | ATCTATCCCTACTGTAAATATATG | ||||
| 16S | 16S+1 | CCGGTCTGAACTCAGATCAAGT | 460 | [48] | |
| 16S-1 | GCTCAATGATTTTTTAAATTGCTGT | ||||
| Rickettsia spp. | gltA | CS-78 | GCAAGTATCGGTGAGGATGTAAT | 401 | [49] |
| CS-323 | GCTTCCTTAAAATTCAATAAATCAGGAT | ||||
| ompA | Rrl9O.70 | ATGGCGAATATTTCTCCAAAA | 631 | [50] | |
| Rr190.701n | GTTCCGTTAATGGCAGCATCT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ali, A.; Shehla, S.; Zahid, H.; Ullah, F.; Zeb, I.; Ahmed, H.; da Silva Vaz, I., Jr.; Tanaka, T. Molecular Survey and Spatial Distribution of Rickettsia spp. in Ticks Infesting Free-Ranging Wild Animals in Pakistan (2017–2021). Pathogens 2022, 11, 162. https://doi.org/10.3390/pathogens11020162
Ali A, Shehla S, Zahid H, Ullah F, Zeb I, Ahmed H, da Silva Vaz I Jr., Tanaka T. Molecular Survey and Spatial Distribution of Rickettsia spp. in Ticks Infesting Free-Ranging Wild Animals in Pakistan (2017–2021). Pathogens. 2022; 11(2):162. https://doi.org/10.3390/pathogens11020162
Chicago/Turabian StyleAli, Abid, Shehla Shehla, Hafsa Zahid, Farman Ullah, Ismail Zeb, Haroon Ahmed, Itabajara da Silva Vaz, Jr., and Tetsuya Tanaka. 2022. "Molecular Survey and Spatial Distribution of Rickettsia spp. in Ticks Infesting Free-Ranging Wild Animals in Pakistan (2017–2021)" Pathogens 11, no. 2: 162. https://doi.org/10.3390/pathogens11020162