Immunization Trials with Recombinant Major Sperm Protein of the Bovine Lungworm Dictyocaulus viviparus
Abstract
:1. Introduction
2. Results
2.1. Condition of the Calves
2.2. Parasitological Parameters (Worm Burden, Larvae Shedding and Worm Size)
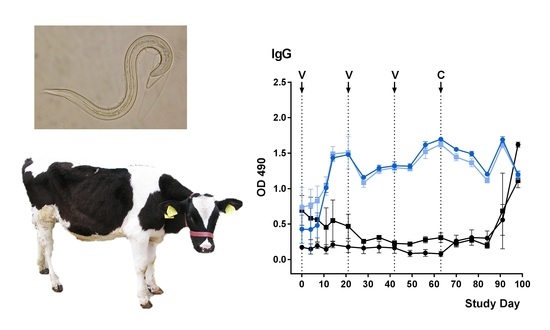
2.3. Development of rMSP-Specific Antibodies
2.4. Binding of Anti-rMSP Antibodies to Native MSP
3. Discussion
4. Materials and Methods
4.1. Recombinant Expression in Escherichia coli
4.2. Immunization Trials and Challenge Infection
4.3. Determination of Larval Shedding
4.4. Determination of Worm Burden and Worm Size
4.5. Determination of Antibody Development
4.6. Binding of Anti-rMSP Antibodies to Native MSP
4.7. Statistical Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holzhauer, M.; van Schaik, G.; Saatkamp, H.W.; Ploeger, H.W. Lungworm outbreaks in adult dairy cows: Estimating economic losses and lessons to be learned. Vet. Rec. 2011, 169, 494:1–494:5. [Google Scholar] [CrossRef] [PubMed]
- Dank, M.; Holzhauer, M.; Veldhuis, A.; Frankena, K. Association between Dictyocaulus viviparus status and milk production parameters in Dutch dairy herds. J. Dairy Sci. 2015, 98, 7741–7747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Charlier, J.; Ghebretinsae, A.; Meyns, T.; Czaplicki, G.; Vercruysse, J.; Claerebout, E. Antibodies against Dictyocaulus viviparus major sperm protein in bulk tank milk: Association with clinical appearance, herd management and milk production. Vet. Parasitol. 2016, 232, 36–42. [Google Scholar] [CrossRef] [PubMed]
- May, K.; Brügemann, K.; König, S.; Strube, C. The effect of patent Dictyocaulus viviparus (re)infections on individual milk yield and milk quality in pastured dairy cows and correlation with clinical signs. Parasit. Vectors 2018, 11, 24. [Google Scholar] [CrossRef]
- Eysker, M.; Kooyman, F.N.J.; Ploeger, H.W. Immunity in calves against Dictyocaulus viviparus following a low primary infection. Parasitology 2001, 123, 591–597. [Google Scholar] [CrossRef]
- Jarrett, W.F.H.; Jennings, F.W.; Mcintyre, W.I.M.; Mulligan, W.; Thomas, B.A.C.; Urquhart, G.M. Immunological studies on Dictyocaulus viviparus infection: The immunity resulting from experimental infection. Immunology 1959, 2, 252–261. [Google Scholar]
- Michel, J. Studies on resistance to Dictyocaulus infection: IV. The rate of acquisition of protective immunity in infection of D. viviparus. J. Comp. Pathol. 1962, 72, 281–285. [Google Scholar] [CrossRef]
- Jarrett, W.F.H.; Jennings, F.; McIntyre, W.; Mulligan, W.; Urquhart, G. Immunological studies on Dictyocaulus viviparus infection. Passive immunisation. Vet. Rec. 1955, 67, 291–296. [Google Scholar]
- Claerebout, E.; Geldhof, P. Helminth vaccines in ruminants: From development to application. Vet. Clin. Food Anim. 2020, 36, 159–171. [Google Scholar] [CrossRef]
- Strube, C.; Daugschies, A. Vaccines against livestock parasites: Expectations and reality. Berl. Munch. Tierarztl. Wochenschr. 2015, 128, 437–450. [Google Scholar] [CrossRef]
- Fawzi, E.M.; Gonzalez-Sanchez, M.E.; Corral, M.J.; Alunda, J.M.; Cuquerella, M. Vaccination of lambs with the recombinant protein rHc23 elicits significant protection against Haemonchus contortus challenge. Vet. Parasitol. 2015, 211, 54–59. [Google Scholar] [CrossRef] [PubMed]
- Nisbet, A.J.; McNeilly, T.N.; Greer, A.W.; Bartley, Y.; Oliver, E.M.; Smith, S.; Palarea-Albaladejo, J.; Matthews, J.B. Protection of ewes against Teladorsagia circumcincta infection in the periparturient period by vaccination with recombinant antigens. Vet. Parasitol. 2016, 228, 130–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nisbet, A.J.; McNeilly, T.N.; Wildblood, L.A.; Morrison, A.A.; Bartley, D.J.; Bartley, Y.; Longhi, C.; McKendrick, I.J.; Palarea-Albaladejo, J.; Matthews, J.B. Successful immunization against a parasitic nematode by vaccination with recombinant proteins. Vaccine 2013, 31, 4017–4023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strube, C.; Haake, C.; Sager, H.; Schorderet Weber, S.; Kaminsky, R.; Buschbaum, S.; Joekel, D.; Schicht, S.; Kremmer, E.; Korrell, J.; et al. Vaccination with recombinant paramyosin against the bovine lungworm Dictyocaulus viviparus considerably reduces worm burden and larvae shedding. Parasit. Vectors 2015, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Joekel, D.; Hinse, P.; Raulf, M.K.; Schicht, S.; Baumer, W.; Werling, D.; Kremmer, E.; Strube, C. Vaccination of calves with yeast- and bacterial-expressed paramyosin from the bovine lungworm Dictyocaulus viviparus. Parasite Immunol. 2015, 37, 614–623. [Google Scholar] [CrossRef] [Green Version]
- Hofmann, J.D.I.; Schmid, K.D. Dictyocaulus viviparus Antigen for Diagnosis of Lungworm Affection and for Vaccination. European Patent EP0785253A1, 23 July 1997. [Google Scholar]
- Strube, C.; Buschbaum, S.; Schnieder, T. Molecular characterization and real-time PCR transcriptional analysis of Dictyocaulus viviparus major sperm proteins. Parasitol. Res. 2009, 104, 543–551. [Google Scholar] [CrossRef]
- Sepsenwol, S.; Ris, H.; Roberts, T.M. A unique cytoskeleton associated with crawling in the amoeboid sperm of the nematode, Ascaris suum. J. Cell Biol. 1989, 108, 55–66. [Google Scholar] [CrossRef]
- Miller, M.A.; Nguyen, V.Q.; Lee, M.-H.; Kosinski, M.; Schedl, T.; Caprioli, R.M.; Greenstein, D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science 2001, 291, 2144–2147. [Google Scholar] [CrossRef] [Green Version]
- Schnieder, T. Use of a recombinant Dictyocaulus viviparus antigen in an enzyme-linked immunosorbent assay for immunodiagnosis of bovine dictyocaulosis. Parasitol. Res. 1992, 78, 298–302. [Google Scholar] [CrossRef]
- von Holtum, C.; Strube, C.; Schnieder, T.; von Samson-Himmelstjerna, G. Development and evaluation of a recombinant antigen-based ELISA for serodiagnosis of cattle lungworm. Vet. Parasitol. 2008, 151, 218–226. [Google Scholar] [CrossRef]
- Fiedor, C.; Strube, C.; Forbes, A.; Buschbaum, S.; Klewer, A.-M.; von Samson-Himmelstjerna, G.; Schnieder, T. Evaluation of a milk ELISA for the serodiagnosis of Dictyocaulus viviparus in dairy cows. Vet. Parasitol. 2009, 166, 255–261. [Google Scholar] [CrossRef]
- Schunn, A.-M.; Forbes, A.; Schnieder, T.; Strube, C. Validation of a Dictyocaulus viviparus MSP-ELISA and cut-off adjustment in a one-year longitudinal field study in dairy cattle herds. Vet. Parasitol. 2012, 189, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Jarrett, W.F.; Jennings, F.W.; McIntyre, W.I.; Mulligan, W. The natural history of parasitic bronchitis with notes on prophylaxis and treatment. Vet. Rec. 1957, 69, 1329–1339. [Google Scholar]
- McKeand, J.B. Vaccine development and diagnostics of Dictyocaulus viviparus. Parasitology 2000, 120, S17–S23. [Google Scholar] [CrossRef]
- Urwin, P.E.; Lilley, C.J.; Atkinson, H.J. Ingestion of double-stranded RNA by preparasitic juvenile cyst nematodes leads to RNA interference. Mol. Plant Microbe Interact. 2002, 15, 747–752. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, R.T.; Zhan, B.; Mendez, S.; Loukas, A.; Bueno, L.L.; Wang, Y.; Plieskatt, J.; Oksov, Y.; Lustigman, S.; Bottazzi, M.E.; et al. Reduction of Worm Fecundity and Canine Host Blood Loss Mediates Protection against Hookworm Infection Elicited by Vaccination with Recombinant Ac16. Clin. Vaccine Immunol. 2007, 14, 281–287. [Google Scholar] [CrossRef] [Green Version]
- Loukas, A.; Bethony, J.M.; Williamson, A.L.; Goud, G.N.; Mendez, S.; Zhan, B.; Hawdon, J.M.; Bottazzi, M.E.; Brindley, P.J.; Hotez, P.J. Vaccination of Dogs with a Recombinant Cysteine Protease from the Intestine of Canine Hookworms Diminishes the Fecundity and Growth of Worms. J. Infect. Dis. 2004, 189, 1952–1961. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, S.E.; Meeusen, E.N. Progress and new technologies for developing vaccines against gastrointestinal nematode parasites of sheep. Parasite Immunol. 2003, 25, 283–296. [Google Scholar] [CrossRef] [PubMed]
- Redmond, D.L.; Knox, D.P. Further protection studies using recombinant forms of Haemonchus contortus cysteine proteinases. Parasite Immunol. 2006, 28, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Smith, W.D.; Newlands, G.F.; Smith, S.K.; Pettit, D.; Skuce, P.J. Metalloendopeptidases from the intestinal brush border of Haemonchus contortus as protective antigens for sheep. Parasite Immunol. 2003, 25, 313–323. [Google Scholar] [CrossRef]
- Bendtsen, J.D.; Jensen, L.J.; Blom, N.; Von Heijne, G.; Brunak, S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng. Des. Sel. 2004, 17, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woollard, D.J.; Gauci, C.G.; Heath, D.D.; Lightowlers, M.W. Protection against hydatid disease induced with the EG95 vaccine is associated with conformational epitopes. Vaccine 2000, 19, 498–507. [Google Scholar] [CrossRef]
- Davies, G. Vaccine Adjuvants: Methods and Protocols; Humana Press: New York, NY, USA, 2010. [Google Scholar]
- Holzhausen, J.; Haake, C.; Schicht, S.; Hinse, P.; Jordan, D.; Kremmer, E.; Strube, C. Biological function of Dictyocaulus viviparus asparaginyl peptidase legumain-1 and its suitability as a vaccine target. Parasitology 2017, 145, 378–392. [Google Scholar] [CrossRef] [PubMed]
- Kooyman, F.N.J.; Yatsuda, A.P.; Ploeger, H.W.; Eysker, M. Serum immunoglobulin E response in calves infected with the lungworm Dictyocaulus viviparus and its correlation with protection. Parasite Immunol. 2002, 24, 47–56. [Google Scholar] [CrossRef]
- Foster, N.; Elsheikha, H.M. The immune response to parasitic helminths of veterinary importance and its potential manipulation for future vaccine control strategies. Parasitol. Res. 2012, 110, 1587–1599. [Google Scholar] [CrossRef] [PubMed]
- Piedrafita, D.; Preston, S.; Kemp, J.; de Veer, M.; Sherrard, J.; Kraska, T.; Elhay, M.; Meeusen, E. The effect of different adjuvants on immune parameters and protection following vaccination of sheep with a larval-specific antigen of the gastrointestinal nematode, Haemonchus contortus. PLoS ONE 2013, 8, e78357. [Google Scholar] [CrossRef]
- Jacobs, H.J.; Wiltshire, C.; Ashman, K.; Meeusen, E.N. Vaccination against the gastrointestinal nematode, Haemonchus contortus, using a purified larval surface antigen. Vaccine 1999, 17, 362–368. [Google Scholar] [CrossRef]
- Scott, C.A.; McKeand, J.B.; Devaney, E. A longitudinal study of local and peripheral isotype/subclass antibodies in Dictyocaulus viviparus-infected calves. Vet. Immunol. Immunopathol. 1996, 53, 235–247. [Google Scholar] [CrossRef]





| rMSP Quil A (N = 4) | Quil-A-Only (N = 4) | rMSP Al(OH)3 (N = 4) | Al(OH)3-Only (N = 4) | |
|---|---|---|---|---|
| Total worm count | ||||
| GM | 320.56 | 155.56 | 203.68 | 518.67 |
| AM | 407.50 | 188.75 | 348.50 | 563.50 |
| Female worm count | ||||
| GM | 202.61 | 93.38 | 121.87 | 302.79 |
| AM | 244.00 | 117.00 | 195.75 | 327.50 |
| Male worm count | ||||
| GM | 115.50 | 61.55 | 81.35 | 208.65 |
| AM | 163.50 | 71.75 | 152.75 | |
| Larvae/female worm | ||||
| GM | 15.04 | 32.44 | 30.31 | 21.07 |
| AM | 16.23 | 35.62 | 36.83 | 22.69 |
| Sum of daily LPG 1 | ||||
| GM | 3029.81 | 3012.51 | 3653.46 | 6359.34 |
| AM | 3200.25 | 4272.75 | 4407.25 | 8179.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Springer, A.; von Holtum, C.; von Samson-Himmelstjerna, G.; Strube, C. Immunization Trials with Recombinant Major Sperm Protein of the Bovine Lungworm Dictyocaulus viviparus. Pathogens 2022, 11, 55. https://doi.org/10.3390/pathogens11010055
Springer A, von Holtum C, von Samson-Himmelstjerna G, Strube C. Immunization Trials with Recombinant Major Sperm Protein of the Bovine Lungworm Dictyocaulus viviparus. Pathogens. 2022; 11(1):55. https://doi.org/10.3390/pathogens11010055
Chicago/Turabian StyleSpringer, Andrea, Christian von Holtum, Georg von Samson-Himmelstjerna, and Christina Strube. 2022. "Immunization Trials with Recombinant Major Sperm Protein of the Bovine Lungworm Dictyocaulus viviparus" Pathogens 11, no. 1: 55. https://doi.org/10.3390/pathogens11010055