Characteristics for Gallium-Based Liquid Alloys of Low Melting Temperature
Abstract
:1. Introduction
2. Experiment
2.1. Preparation of Gallium Alloy
2.2. Melting Point Measurement
2.3. Contact Angle Measurement
2.4. Viscosity Measurement
3. Results and Discussion
3.1. Melting Point Measurement
3.2. Contact Angle Measurement
3.3. Viscosity Measurement
4. Conclusions
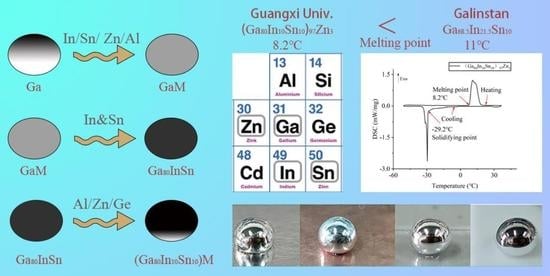
- The (Ga80In10Sn10)97Zn3 alloy produced a low melting point of 8.2 °C in In, Sn, Zn, Ge, and Al of the Ga-based alloy, which was lower than that of Galinstan. This alloy could be utilized as a liquid in a wider temperature range.
- The Ga80In10Sn10 alloy had a larger contact angle of 140–150° on the Cu, Al, SiO2, and Teflon plate compared with other Ga-based alloys, and Teflon, for which the contact angle was 148.6°, was the best container, therefore, the Teflon pipe was convenient to use in the Ga alloy fluid flow application.
- At 25 °C and a 3 1/s shear rate, (Ga80In10Sn10)97Al3 had the largest shear viscosity of 2.98 × 104 mPa·s, and Ga80In7.5Sn12.5 had the lowest viscosity of 1.72 × 104 mPa·s. In addition, the kinetic viscosity of Ga-based alloy was about 3 mPa·s, which was only three times that of water and showed well fluidity.
- For the viscoelasticity of Ga-based (Ga80In10Sn10)97Zn3 alloy, the storage modulus (G’) was much larger than the loss modulus (G”) at a 0.1% strain amplitude, and it showed properties more similar to those of elastic solids that behaved more solidly than water.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Liang, T.; Jusheng, M. Phase diagram calculation on Sn–Zn–Ga solders. J. Non-Cryst. Solids 2004, 336, 153–156. [Google Scholar] [CrossRef]
- Kalantar-Zadeh, K.; Tang, J.; Daeneke, T.; O’Mullane, A.P.; Stewart, L.A.; Liu, J.; Majidi, C.; Ruoff, R.S.; Weiss, P.S.; Dickey, M.D. Emergence of Liquid Metals in Nanotechnology. ACS Nano 2019, 13, 7388–7395. [Google Scholar] [CrossRef] [PubMed]
- Liu, J. Liquid metal machine is evolving to soft robotics. Sci. China Technol. Sci. 2016, 59, 1793–1794. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Sun, X.; Kemme, N.; Damle, V.G.; Schott, C.; Herrmann, M.; Rykaczewski, K. Can liquid metal flow in microchannels made of its own oxide skin? Microfluid. Nanofluid. 2016, 20, 3. [Google Scholar] [CrossRef]
- Kwon, S.H.; Na, S.-M.; Flatau, A.B.; Choi, H.J. Fe–Ga alloy based magnetorheological fluid and its viscoelastic characteristics. J. Ind. Eng. Chem. 2020, 82, 433–438. [Google Scholar] [CrossRef]
- Wang, X.; Li, L.; Yang, X.; Wang, H.; Guo, J.; Wang, Y.; Chen, X.; Hu, L. Electrically Induced Wire-Forming 3D Printing Technology of Gallium-Based Low Melting Point Metals. Adv. Mater. Technol. 2021, 6, 2100228. [Google Scholar] [CrossRef]
- Dickey, M.D. Stretchable and Soft Electronics using Liquid Metals. Adv. Mater. 2017, 29, 1606425. [Google Scholar] [CrossRef]
- Daeneke, T.; Khoshmanesh, K.; Mahmood, N.; de Castro, I.A.; Esrafilzadeh, D.; Barrow, S.J.; Dickey, M.D.; Kalantar-Zadeh, K. Liquid metals: Fundamentals and applications in chemistry. Chem. Soc. Rev. 2018, 47, 4073–4111. [Google Scholar] [CrossRef]
- Fujita, T.; Park, H.-S.; Ono, K.; Matsuo, S.; Okaya, K.; Dodbiba, G. Movement of liquid gallium dispersing low concentration of temperature sensitive magnetic particles under magnetic field. J. Magn. Magn. Mater. 2011, 323, 1207–1210. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, J. Gallium-based thermal interface material with high compliance and wettability. Appl. Phys. A 2012, 107, 701–708. [Google Scholar] [CrossRef]
- Ma, K.-Q.; Liu, J. Heat-driven liquid metal cooling device for the thermal management of a computer chip. J. Phys. D Appl. Phys. 2007, 40, 4722–4729. [Google Scholar] [CrossRef]
- Fumoto, K.; Ikegawa, M.; Kawanami, T. Heat Transfer Characteristics of a Thermo-sensitive Magnetic Fluid in Micro-channel. J. Therm. Sci. Technol. 2009, 4, 332–339. [Google Scholar] [CrossRef] [Green Version]
- Massart, R.; Rasolonjatovo, B.; Neveu, S.; Cabuil, V. Mercury-based cobalt magnetic fluids and cobalt nanoparticles. J. Magn. Magn. Mater. 2007, 308, 10–14. [Google Scholar] [CrossRef]
- Tang, S.-Y.; Khoshmanesh, K.; Sivan, V.; Petersen, P.; O’Mullane, A.P.; Abbott, D.; Mitchell, A.; Kalantar-Zadeh, K. Liquid metal enabled pump. Proc. Natl. Acad. Sci. USA 2014, 111, 3304–3309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Park, H.; Dodbiba, G.; Ono, K.; Tokoro, C.; Fujita, T. Keeping gallium metal to liquid state under the freezing point by using silica nanoparticles. Appl. Phys. Lett. 2011, 99, 143120. [Google Scholar] [CrossRef]
- De Castro, I.A.; Chrimes, A.F.; Zavabeti, A.; Berean, K.J.; Carey, B.J.; Zhuang, J.; Du, Y.; Dou, S.X.; Suzuki, K.; Shanks, R.A.; et al. A Gallium-Based Magnetocaloric Liquid Metal Ferrofluid. Nano Lett. 2017, 17, 7831–7838. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Xue, Z.; Mu, T. Eutectics: Formation, properties, and applications. Chem. Soc. Rev. 2021, 50, 8596–8638. [Google Scholar] [CrossRef]
- Cao, K.B. New Systems Development and Fluidity Research of Low Melting Points Gallium-Based Liquid Metals; Yanshan University: Qinhuangdao, China, 2021. (In Chinese) [Google Scholar]
- Niu, H.; Bonati, L.; Piaggi, P.M.; Parrinello, M. Ab initio phase diagram and nucleation of gallium. Nat. Commun. 2020, 11, 2654. [Google Scholar] [CrossRef]
- Liu, S.; Sweatman, K.; McDonald, S.; Nogita, K. Ga-Based Alloys in Microelectronic Interconnects: A Review. Materials 2018, 11, 1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarfo, D.K.; Taylor, R.R.; O’Mullane, A.P. Investigating Liquid Metal Galinstan as a High Current Carrier and Its Interaction with Collector Electrodes. ACS Appl. Electron. Mater. 2020, 2, 2921–2928. [Google Scholar] [CrossRef]
- Evans, D.S.; Prince, A. Thermal analysis of Ga-In-Sn system. Met. Sci. 1978, 12, 411–414. [Google Scholar] [CrossRef]
- Liu, S.; Qu, D.; McDonald, S.; Gu, Q.; Matsumura, S.; Nogita, K. Intermetallic formation mechanisms and properties in room-temperature Ga soldering. J. Alloy. Compd. 2020, 826, 154221. [Google Scholar] [CrossRef]
- Yang, C.; Liu, Z.; Yu, M.; Bian, X. Liquid metal Ga-Sn alloy based ferrofluids with amorphous nano-sized Fe-Co-B magnetic particles. J. Mater. Sci. 2020, 55, 13303–13313. [Google Scholar] [CrossRef]
- Yu, M.; Bian, X.; Wang, T.; Wang, J. Metal-based magnetic fluids with core–shell structure FeB@SiO2 amorphous particles. Soft Matter 2017, 13, 6340–6348. [Google Scholar] [CrossRef] [PubMed]
- Dobosz, A.; Plevachuk, Y.; Sklyarchuk, V.; Sokoliuk, B.; Tkach, O.; Gancarz, T. Liquid Metals in High-Temperature Cooling Systems: The Effect of Bi Additions for the Physicochemical Properties of Eutectic Ga–Sn–Zn. J. Chem. Eng. Data 2019, 64, 404–411. [Google Scholar] [CrossRef]
- Gancarz, T. Physical, Thermal, Mechanical Properties, and Microstructural Characterization of Sn-9Zn-XGa Alloys. Met. Mater. Trans. A 2016, 47, 326–333. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Sen, P.; Kim, C.-J. Characterization of Nontoxic Liquid-Metal Alloy Galinstan for Applications in Microdevices. J. Microelectromech. Syst. 2012, 21, 443–450. [Google Scholar] [CrossRef] [Green Version]
- Zeng, C.; Shen, J.; Zhang, J. High thermal conductivity in indium-based metal/diamond composites by good wettability of diamond with indium. Diam. Relat. Mater. 2021, 112, 108230. [Google Scholar] [CrossRef]
- Surmann, P.; Zeyat, H. Voltammetric analysis using a self-renewable non-mercury electrode. Anal. Bioanal. Chem. 2005, 383, 1009–1013. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, X.; Qian, Y.; Xue, L.; Dolocan, A.; Yu, G. Room-Temperature All-Liquid-Metal Batteries Based on Fusible Alloys with Regulated Interfacial Chemistry and Wetting. Adv. Mater. 2020, 32, e2002577. [Google Scholar] [CrossRef]
- Ding, Y.; Guo, X.; Yu, G. Next-Generation Liquid Metal Batteries Based on the Chemistry of Fusible Alloys. ACS Cent. Sci. 2020, 6, 1355–1366. [Google Scholar] [CrossRef] [PubMed]
- Ge, H.; Li, H.; Mei, S.; Liu, J. Low melting point liquid metal as a new class of phase change material: An emerging frontier in energy area. Renew. Sustain. Energy Rev. 2013, 21, 331–346. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, J.; Tan, H.; Zhu, S.; Qiao, Z.; Yang, J.; Liu, W. Al-Doped Ga-Based Liquid Metal: Modification Strategy and Controllable High-Temperature Lubricity through Frictional Interface Regulation. Langmuir 2019, 35, 6905–6915. [Google Scholar] [CrossRef] [PubMed]
- Krisnadi, F.; Nguyen, L.L.; Ankit; Ma, J.; Kulkarni, M.R.; Mathews, N.; Dickey, M.D. Directed Assembly of Liquid Metal–Elastomer Conductors for Stretchable and Self-Healing Electronics. Adv. Mater. 2020, 32, 2001642. [Google Scholar] [CrossRef] [PubMed]
- So, J.-H.; Koo, H.-J.; Dickey, M.D.; Velev, O.D. Ionic Current Rectification in Soft-Matter Diodes with Liquid-Metal Electrodes. Adv. Funct. Mater. 2012, 22, 625–631. [Google Scholar] [CrossRef]
- Yi, L.; Ding, Y.; Yuan, B.; Wang, L.; Tian, L.; Chen, C.; Liu, F.; Lu, J.; Song, S.; Liu, J. Breathing to harvest energy as a mechanism towards making a liquid metal beating heart. RSC Adv. 2016, 6, 94692–94698. [Google Scholar] [CrossRef]
- Zhang, J.; Yao, Y.; Sheng, L.; Liu, J. Self-Fueled Biomimetic Liquid Metal Mollusk. Adv. Mater. 2015, 27, 2648–2655. [Google Scholar] [CrossRef]
- Doudrick, K.; Liu, S.; Mutunga, E.M.; Klein, K.L.; Damle, V.; Varanasi, K.K.; Rykaczewski, K. Different Shades of Oxide: From Nanoscale Wetting Mechanisms to Contact Printing of Gallium-Based Liquid Metals. Langmuir 2014, 30, 6867–6877. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.R.; Parekh, D.P.; Dickey, M.D.; Hsiao, L.C. Interfacial Rheology of Gallium-Based Liquid Metals. Langmuir 2019, 35, 11774–11783. [Google Scholar] [CrossRef] [PubMed]
- Hiemen, P.C. Principle of Colloid and Surface Chemistry; CRC: Boca Raton, FL, USA, 1997. [Google Scholar]
- Plevachuk, Y.; Sklyarchuk, V.; Eckert, S.; Gerbeth, G.; Novakovic, R. Thermophysical Properties of the Liquid Ga–In–Sn Eutectic Alloy. J. Chem. Eng. Data 2014, 59, 757–763. [Google Scholar] [CrossRef]
- Lee, J.; Kiyose, A.; Nakatsuka, S.; Nakamoto, M.; Tanaka, T. Improvements in Surface Tension Measurements of Liquid Metals Having Low Capillary Constants by the Constrained Drop Method. ISIJ Int. 2004, 44, 1793–1799. [Google Scholar] [CrossRef] [Green Version]
- An, Q.; Jin, Z.; Li, N.; Wang, H.; Schmierer, J.; Wei, C.; Hu, H.; Gao, Q.; Woodall, J.M. Study on the liquid phase-derived activation mechanism in Al-rich alloy hydrolysis reaction for hydrogen production. Energy 2022, 247, 123489. [Google Scholar] [CrossRef]
- Sunkara, M.K.; Sharma, S.; Miranda, R.; Lian, G.; Dickey, E.C. Bulk synthesis of silicon nanowires using a low-temperature vapor–liquid–solid method. Appl. Phys. Lett. 2001, 79, 1546–1548. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.; Ndao, S. On the Wetting States of Low Melting Point Metal Galinstan® on Silicon Microstructured Surfaces. Adv. Eng. Mater. 2018, 20, 1700829. [Google Scholar] [CrossRef]
- Chen, Y.; Wagner, J.L.; Farias, P.A.; DeMauro, E.P.; Guildenbecher, D.R. Galinstan liquid metal breakup and droplet formation in a shock-induced cross-flow. Int. J. Multiph. Flow 2018, 106, 147–163. [Google Scholar] [CrossRef]
- Hayashi, Y.; Saneie, N.; Yip, G.; Kim, Y.J.; Kim, J.-H. Metallic nanoemulsion with galinstan for high heat-flux thermal management. Int. J. Heat Mass Transf. 2016, 101, 1204–1216. [Google Scholar] [CrossRef]
- Lam, L.S.; Hodes, M.; Enright, R. Analysis of Galinstan-Based Microgap Cooling Enhancement Using Structured Surfaces. J. Heat Transf. 2015, 137, 091003. [Google Scholar] [CrossRef]
- Haynes, W.M. CRC Handbook of Chemistry and Physics; CRC: Boca Raton, FL, USA, 2020; p. 2674. [Google Scholar]
- Yang, Y.; Sun, S.; Tang, S.Y.; Li, W.; Zhang, S. Viscoelastic Properties of Gallium-Indium Alloy. Appl. Rheol. 2018, 28, 42903. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions, and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions, or products referred to in the content. |










| Metal | Melting Point (°C) |
|---|---|
| Pure Ga | 29.76 [20] |
| Galinstan (Ga68.5In21.5Sn10) | 11 [5,20] (solidify point −19 [21,22]) |
| (Ga78.3In14.9Sn6.8) | 13.2 [8] |
| Galinstan + 0.2Gd | 13.2 [16] |
| Galinstan + 1.2Gd | 13.7 [16] |
| Galinstan + 2.3Gd | 16.3 [16] |
| Ga75.5In24.5 | 15.4 [23] |
| Ga90Zn10 | 24.7 [20] |
| Ga62Sn32Bi6 | 128 [20] |
| Ga86.5Sn13.5 | 20.5/21 [20] |
| Ga91.6Sn8.4 | 20.6 [24] |
| Ga85.8In14.2 | Liquid at room temperature [25] |
| Ga91.5Sn8.5 | |
| Ga77.2In14.4Sn8.4 | |
| Ga–In binary eutectic | 15.3 [26] |
| Ga–Sn binary eutectic | 20.5 [26] |
| Al–Ga–Sn eutectic | 19 [26] |
| Al–Ga–In eutectic | 15 [26] |
| Al–Ga–In–Sn eutectic | 10.7 [26] |
| Al–In binary eutectic | 156 [26] |
| Al–Sn binary eutectic | 228 [26] |
| SnZn eutectic | 198 [26] |
| SnZn + 1.8 Ga | 195 [27] |
| SnZn + 2 Ga | 199 [27] |
| SnZn + 3 Ga | 195 [27] |
| SnZn + 4 Ga | 188 [27] |
| SnZn + 6 Ga | 174 [27] |
| SnZn + 8 Ga | 155 [27] |
| Alloy | Melting Point (°C) | Melting Width (°C) |
|---|---|---|
| Ga90In10 | 17.6 | 10.3 |
| Ga80In20 | 17.9 | 9.7 |
| Ga70In30 | 17.7 | 10.2 |
| Ga60In40 | 17.5 | 11.0 |
| Ga50In50 | 17.5 | 9.8 |
| Ga40In60 | 17.7 | 10.6 |
| Ga30In70 | 18.0 | 10.8 |
| Ga20In80 | Solid at room temperature | |
| Ga10In90 | ||
| Ga90Sn10 | 22.5 | 11.4 |
| Ga80Sn20 | 22.1 | 10.5 |
| Ga70Sn30 | 22.4 | 11.5 |
| Ga60Sn40 | 22.3 | 11.0 |
| Ga50Sn50 | 22.3 | 11.2 |
| Ga40Sn60 | Solid at room temperature | |
| Ga30Sn70 | ||
| Ga20Sn80 | ||
| Ga10Sn90 | ||
| Ga90Zn10 | 26.7 | 9.6 |
| Ga80Zn20 | 26.8 | 10.0 |
| Ga70Zn30 | 26.8 | 9.4 |
| Ga60Zn40 | Solid at room temperature | |
| Ga50Zn50 | ||
| Ga90Al10 | ||
| Ga95Al5 | ||
| Ga80In5Sn15 | 19.6 | 10.2 |
| Ga80In10Sn10 | 11.7 | 9.3 |
| Ga80In15Sn5 | 14.9 | 10.1 |
| Ga80In7.5Sn12.5 | 17.5 | 11.2 |
| Ga80In12.5Sn7.5 | 16.2 | 10.5 |
| (Ga80In10Sn10)97Zn3 | 8.2 | 8.9 |
| (Ga80In10Sn10)97Ge3 | 10.1 | 8.3 |
| (Ga80In10Sn10)97Al3 | 9.9 | 8.2 |
| Alloy | Cu | Al | SiO2 | Teflon |
|---|---|---|---|---|
| Average roughness | 7.45 nm | 7.54 nm | 0.0031 nm | 1.73 nm |
| Ga90In10 | 132.8° | 127.2° | 130.7° | 143.2° |
| Ga80In20 | 135.3° | 126.9° | 131.6° | 140.5° |
| Ga90Sn10 | 136.5° | 135.2° | 132.5° | 136.5° |
| Ga80Sn20 | 137.7° | 141.0° | 133.6° | 137.7° |
| Ga80In10Sn10 | 145.4° | 140.2° | 144.7° | 148.6° |
| Ga80In5Sn15 | 134.6° | 132.4° | 142.7° | 144.4° |
| Ga80In15Sn5 | 142.7° | 129.5° | 128.2° | 145.9° |
| Ga80In7.5Sn12.5 | 139.6° | 134.6° | 128.2° | 139.0° |
| Ga80In12.5Sn7.5 | 132.4° | 125.0° | 130.3° | 130.8° |
| Galinstan (Ga68.5In21.5Sn10) | 142.4° | 135.0° | 129.8° | 143.0° |
| (Ga80In10Sn10)97Zn3 | 132.0° | 131.1° | 128.6° | 135.0° |
| (Ga80In10Sn10)97Ge3 | 134.6° | 133.8° | 131.5° | 138.7° |
| (Ga80In10Sn10)97Al3 | 138.3° | 135.3° | 125.9° | 142.4° |
| Alloy | Density (g/cm3) | Viscosity (mPa·s) |
|---|---|---|
| Ga80In10Sn10 | 6.72 | 1.87 × 104 |
| Ga80In15Sn5 | 6.75 | 1.78 × 104 |
| Ga80In5Sn15 | 6.60 | 1.77 × 104 |
| Ga80In12.5Sn7.5 | 6.76 | 1.85 × 104 |
| Ga80In7.5Sn12.5 | 6.68 | 1.72 × 104 |
| Galinstan (Ga68.5In21.5Sn10) | 6.49 | 1.87 × 104 |
| (Ga80In10Sn10)97Zn3 | 6.79 | 2.15 × 104 |
| (Ga80In10Sn10)97Ge3 | 6.67 | 2.51 × 104 |
| (Ga80In10Sn10)97Al3 | 6.59 | 2.98 × 104 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shentu, J.; Pan, J.; Chen, H.; He, C.; Wang, Y.; Dodbiba, G.; Fujita, T. Characteristics for Gallium-Based Liquid Alloys of Low Melting Temperature. Metals 2023, 13, 615. https://doi.org/10.3390/met13030615
Shentu J, Pan J, Chen H, He C, Wang Y, Dodbiba G, Fujita T. Characteristics for Gallium-Based Liquid Alloys of Low Melting Temperature. Metals. 2023; 13(3):615. https://doi.org/10.3390/met13030615
Chicago/Turabian StyleShentu, Jianfei, Jiatong Pan, Hao Chen, Chunlin He, Youbin Wang, Gjergj Dodbiba, and Toyohisa Fujita. 2023. "Characteristics for Gallium-Based Liquid Alloys of Low Melting Temperature" Metals 13, no. 3: 615. https://doi.org/10.3390/met13030615