Progress on Electrodeposition of Metals and Alloys Using Ionic Liquids as Electrolytes
Abstract
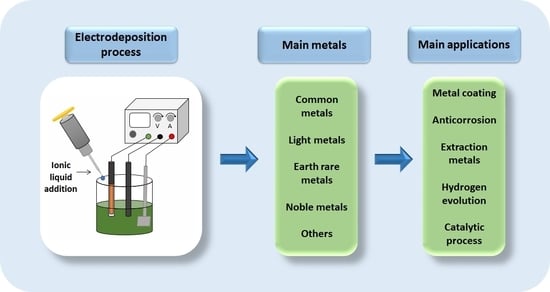
:1. Introduction
2. Electrodeposition of Common Metals
2.1. Aluminum
2.2. Iron
2.3. Nickel
2.4. Cobalt
2.5. Copper
2.6. Zinc
3. Electrodeposition of Light Metals (Li, Na, and Mg)
4. Electrodeposition of Noble Metals
4.1. Silver
4.2. Gold
4.3. Platinum
4.4. Palladium
4.5. Rhodium, Ruthenium, and Iridium
5. Electrodeposition of Rare Earth Metals
5.1. Light Rare Earth Metals
5.2. Medium and Heavy Rare Earth Metals
6. Electrodeposition with Other Metals
7. Patents and Future Perspectives
8. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Djokic, S.S. Electrodeposition—Theory and Practice, 1st ed.; Springer: New York, NY, USA, 2010; p. XVII-295. [Google Scholar]
- Gamburg, Y.D.; Zangari, G. Theory and Practice of Metal Electrodeposition, 1st ed.; Springer: New York, NY, USA, 2011; p. XVII-378. [Google Scholar]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Lebedeva, O.; Kultin, D.; Zakharov, A.; Kustov, L. Advances in application of ionic liquids: Fabrication of surface nanoscale oxide structures by anodization of metals and alloys. Surf. Interfaces 2022, 34, 102345. [Google Scholar] [CrossRef]
- Maniam, K.K.; Paul, S. A Review on the Electrodeposition of Aluminum and Aluminum Alloys in Ionic Liquids. Coatings 2021, 11, 80. [Google Scholar] [CrossRef]
- Tiago, G.A.O.; Matias, I.A.S.; Ribeiro, A.P.C.; Martins, L.M.D.R.S. Application of Ionic Liquids in Electrochemistry—Recent Advances. Molecules 2020, 25, 5812. [Google Scholar] [CrossRef] [PubMed]
- Welton, T. Ionic liquids: A brief history. Biophys. Rev. 2018, 10, 691–706. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Sui, H.; Jia, Z.; Yang, Z.; He, L.; Li, X. Recovery and purification of ionic liquids from solutions: A review. RSC Adv. 2018, 8, 32832–32864. [Google Scholar] [CrossRef] [Green Version]
- Dołżonek, J.; Kowalska, D.; Maculewicz, J.; Stepnowski, P. Regeneration, Recovery, and Removal of Ionic Liquids. In Encyclopedia of Ionic Liquids; Zhang, S., Ed.; Springer: Singapore, 2020; pp. 1–9. [Google Scholar]
- Quijada-Maldonado, E.; Olea, F.; Sepúlveda, R.; Castillo, J.; Cabezas, R.; Merlet, G.; Romero, J. Possibilities and challenges for ionic liquids in hydrometallurgy. Sep. Purif. Technol. 2020, 251, 117289. [Google Scholar] [CrossRef]
- Zhang, M.; Peng, D.; Peng, F.; Huang, A.; Song, K.; He, Q.; Yin, C.; Rao, J.; Zhang, Y.; Chen, H.; et al. Effects of Additives Containing Cyanopyridine on Electrodeposition of Bright Al Coatings from AlCl3-EMIC Ionic Liquids. Coatings 2021, 11, 1396. [Google Scholar] [CrossRef]
- Yavuz, A.; Ozdemir, N.; Yilmaz Erdogan, P.; Zengin, H.; Zengin, G.; Bedir, M. Effect of electrodeposition potential and time for nickel film generation from ionic liquid electrolytes for asymmetric supercapacitor production. Thin Solid Film. 2020, 711, 138309. [Google Scholar] [CrossRef]
- Li, W.; Hao, J.; Liu, W.; Mu, S. Electrodeposition of nano Ni–Co alloy with (220) preferred orientation from choline chloride-urea: Electrochemical behavior and nucleation mechanism. J. Alloy. Compd. 2021, 853, 157158. [Google Scholar] [CrossRef]
- Sides, W.D.; Huang, Q. Electrodeposition of manganese thin films on a rotating disk electrode from choline chloride/urea based ionic liquids. Electrochim. Acta 2018, 266, 185–192. [Google Scholar] [CrossRef]
- Li, W.; Hao, J.; Mu, S.; Liu, W. Electrochemical behavior and electrodeposition of Ni-Co alloy from choline chloride-ethylene glycol deep eutectic solvent. Appl. Surf. Sci. 2020, 507, 144889. [Google Scholar] [CrossRef]
- Alesary, H.F.; Cihangir, S.; Ballantyne, A.D.; Harris, R.C.; Weston, D.P.; Abbott, A.P.; Ryder, K.S. Influence of additives on the electrodeposition of zinc from a deep eutectic solvent. Electrochim. Acta 2019, 304, 118–130. [Google Scholar] [CrossRef] [Green Version]
- Bakkar, A.; Neubert, V. Recycling of cupola furnace dust: Extraction and electrodeposition of zinc in deep eutectic solvents. J. Alloys Compd. 2019, 771, 424–432. [Google Scholar] [CrossRef]
- Wang, S.; Zou, X.; Lu, Y.; Rao, S.; Xie, X.; Pang, Z.; Lu, X.; Xu, Q.; Zhou, Z. Electrodeposition of nano-nickel in deep eutectic solvents for hydrogen evolution reaction in alkaline solution. Int. J. Hydrog. Energy 2018, 43, 15673–15686. [Google Scholar] [CrossRef]
- Omar, I.M.A.; Aziz, M.; Emran, K.M. Part I: Ni-Co alloy foils electrodeposited using ionic liquids. Arab. J. Chem. 2020, 13, 7707–7719. [Google Scholar] [CrossRef]
- Omar, I.M.A.; Al-Fakih, A.M.; Aziz, M.; Emran, K.M. Part II: Impact of ionic liquids as anticorrosives and additives on Ni-Co alloy electrodeposition: Experimental and DFT study. Arab. J. Chem. 2021, 14, 102909. [Google Scholar] [CrossRef]
- Omar, I.M.A.; Aziz, M.; Emran, K.M. Impact of ionic liquid [FPIM]Br on the electrodeposition of Ni and Co from an aqueous sulfate bath. J. Mater. Res. Technol. 2021, 12, 170–185. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Z.; Pan, J.; Yan, F. Ionic Liquid Electrolyte-Based Switchable Mirror with Fast Response and Improved Durability. ACS Appl. Mater. Interfaces 2021, 13, 37339–37349. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, A.; Liu, F.; Shi, Z.; Zhang, B.; Wang, X. Electrodeposition of aluminum–magnesium alloys from an aluminum-containing solvate ionic liquid at room temperature. Electrochem. Commun. 2021, 133, 107160. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, Y.; Shi, Z.; Xu, J.; Liu, Y.; Wang, Z. Communication—Direct Room-Temperature Electrodeposition of La from LaCl3 in an Organic Solvent Supported by LiNO3. J. Electrochem. Soc. 2019, 166, D218. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, L.; Pan, K.; Zhang, W.; Liu, Y.; Zhang, Y.; Zhang, L.; Shi, Z. LiNO3-Supported Electrodeposition of Metallic Nd from Nd-Containing Solvate Ionic Liquid. J. Phys. Chem. C 2021, 125, 20798–20805. [Google Scholar] [CrossRef]
- Liu, A.-M.; Yao, Y.; Guo, M.-X.; Liu, Y.-B.; Shi, Z.-N.; Liu, F.-G.; Hu, X.-W.; He, W.-C.; Wang, Z.-W. Physicochemical properties of DMI–LiNO3 solvated ionic liquid and its application in electrodeposition of neodymium at room temperature. Trans. Nonferrous Met. Soc. China 2021, 31, 2522–2531. [Google Scholar] [CrossRef]
- Liu, A.-M.; Guo, M.-X.; Shi, Z.-N.; Liu, Y.-B.; Liu, F.-G.; Hu, X.-W.; Yang, Y.-J.; Tao, W.-J.; Wang, Z.-W. Physicochemical properties of 1,3-dimethyl-2-imidazolinone−ZnCl2 solvated ionic liquid and its application in zinc electrodeposition. Trans. Nonferrous Met. Soc. China 2021, 31, 832–841. [Google Scholar] [CrossRef]
- Yang, X.; Miao, C.; Sun, Y.; Lei, T.; Xie, Q.; Wang, S. Efficient extraction of gold(I) from alkaline aurocyanide solution using green ionic liquid-based aqueous biphasic systems. J. Taiwan Inst. Chem. Eng. 2018, 91, 176–185. [Google Scholar] [CrossRef]
- Hou, X.; Wang, Z.; Zheng, Z.; Guo, J.; Sun, Z.; Yan, F. Poly(ionic liquid) Electrolytes for a Switchable Silver Mirror. ACS Appl. Mater. Interfaces 2019, 11, 20417–20424. [Google Scholar] [CrossRef]
- Chunyan, L.; Nishikawa, K.; Moon, J.; Kanamura, K. Electrodeposition of Zn from 1--allyl--3--methylimidazolium bromide containing ZnBr2. J. Electroanal. Chem. 2019, 832, 467–474. [Google Scholar] [CrossRef]
- Pham-Truong, T.-N.; Mebarki, O.; Ranjan, C.; Randriamahazaka, H.; Ghilane, J. Electrochemical Growth of Metallic Nanoparticles onto Immobilized Polymer Brush Ionic Liquid as a Hybrid Electrocatalyst for the Hydrogen Evolution Reaction. ACS Appl. Mater. Interfaces 2019, 11, 38265–38275. [Google Scholar] [CrossRef]
- Ezawa, K.; Nishi, N.; Sakka, T. In-situ electrochemical SPR study of gold surface smoothing by repetitive cathodic deposition and anodic dissolution of copper in an ionic liquid. J. Electroanal. Chem. 2020, 877, 114611. [Google Scholar] [CrossRef]
- Manjum, M.; Serizawa, N.; Katayama, Y. Electrodeposition of Co in an Amide-Type Ionic Liquid under an External Magnetic Field. J. Electrochem. Soc. 2021, 168, 042504. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Li, C.-H.; Chen, P.-Y. Galvanic displacement on electrodeposited tangled Zn nanowire sacrificial template for preparing porous and hollow Ni electrodes in ionic liquid. J. Mol. Liq. 2020, 298, 112050. [Google Scholar] [CrossRef]
- Ismail, A.S. Electrodeposition of aluminium–copper alloy from 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl) imide ionic liquid. Egypt. J. Pet. 2017, 26, 61–65. [Google Scholar] [CrossRef] [Green Version]
- Jürjo, S.; Oll, O.; Paiste, P.; Külaviir, M.; Zhao, J.; Lust, E. Electrochemical co-reduction of praseodymium and bismuth from 1-butyl-1-methylpyrrolidinium bis(fluorosulfonyl)imide ionic liquid. Electrochem. Commun. 2022, 138, 107285. [Google Scholar] [CrossRef]
- Wu, Q.; Pulletikurthi, G.; Carstens, T.; Endres, F. On the Electrodeposition of Titanium from TiCl4 in 1-butyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl)amide: In Situ AFM and Spectroscopic Investigations. J. Electrochem. Soc. 2018, 165, D223. [Google Scholar] [CrossRef]
- Simunkova, H.; Lednický, T.; Whitehead, A.H.; Kalina, L.; Simunek, P.; Hubalek, J. Tantalum-based nanotube arrays via porous-alumina-assisted electrodeposition from ionic liquid: Formation and electrical characterization. Appl. Surf. Sci. 2021, 548, 149264. [Google Scholar] [CrossRef]
- Andrew, C.; Murugesan, C.; Jayakumar, M. Electrochemical Behavior of Sm(III) and Electrodeposition of Samarium from 1-Butyl-1-Methylpyrrolidinium Dicyanamide Ionic Liquid. J. Electrochem. Soc. 2022, 169, 022503. [Google Scholar] [CrossRef]
- Hsieh, Y.-T.; Liu, Y.-C.; Lo, N.-C.; Lin, W.-J.; Sun, I.W. Electrochemical co-deposition of gallium and antimonide from the 1-butyl-1-methylpyrrolidinium dicyanamide room temperature ionic liquid. J. Electroanal. Chem. 2019, 832, 48–54. [Google Scholar] [CrossRef]
- Molodkina, E.B.; Ehrenburg, M.R.; Broekmann, P.; Rudnev, A.V. Initial stages of silver electrodeposition on single crystal electrodes from ionic liquids. Electrochim. Acta 2019, 299, 320–329. [Google Scholar] [CrossRef]
- Ehrenburg, M.R.; Molodkina, E.B.; Mishchenko, A.; Rudnev, A.V. The promoting effect of water on the electrodeposition of Eu in a dicyanamide ionic liquid. Electrochim. Acta 2021, 379, 138169. [Google Scholar] [CrossRef]
- Molodkina, E.B.; Ehrenburg, M.R.; Broekmann, P.; Rudnev, A.V. Electrodeposition of chromium on single-crystal electrodes from solutions of Cr(II) and Cr(III) salts in ionic liquids. J. Electroanal. Chem. 2020, 860, 113892. [Google Scholar] [CrossRef]
- Ehrenburg, M.R.; Molodkina, E.B.; Broekmann, P.; Rudnev, A.V. Underpotential Deposition of Silver on Au(111) from an Air- and Water-Stable Ionic Liquid Visualized by In-Situ STM. ChemElectroChem 2019, 6, 1149–1156. [Google Scholar] [CrossRef]
- Rahali, S.; Zarrougui, R.; Marzouki, M.; Ghodbane, O. Electrodeposition of silver from the ionic liquid Butylpyridinium dicyanamide. J. Electroanal. Chem. 2020, 871, 114289. [Google Scholar] [CrossRef]
- Atifi, A.; Baek, D.L.; Fox, R.V. Electrodeposition of Dysprosium in pyrrolidinium triflate ionic liquid at ambient temperature: Unraveling system efficiency and impact of solvation interplays on the reduction process. Electrochim. Acta 2021, 378, 138140. [Google Scholar] [CrossRef]
- Lambri, O.A.; Weidenfeller, B.; Bonifacich, F.G.; Pulletikurthi, G.; Xu, J.; Weidenfeller, L.; Endres, F. Damping of Fe-Al alloy electrodeposited in an ionic liquid. Mat. Res. 2018, 21, e20170805. [Google Scholar] [CrossRef]
- Liu, W.; Wang, Q.; Zheng, Y.; Wang, S.; Yan, Y.; Yang, Y. Extraction behaviour and mechanism of Pt(iv) and Pd(ii) by liquid–liquid extraction with an ionic liquid [HBBIm]Br. Dalton Trans. 2017, 46, 7210–7218. [Google Scholar] [CrossRef]
- He, X.; Zhang, C.; Zhu, Q.; Lu, H.; Cai, Y.; Wu, L. Electrodeposition of Nanocrystalline Ni-Fe Alloy Coatings Based on 1-Butyl-3-Methylimidazolium-Hydrogen Sulfate Ionic Liquid. J. Nanosci. Nanotechnol. 2017, 17, 1108–1115. [Google Scholar] [CrossRef]
- Waldiya, M.; Bhagat, D.; Mukhopadhyay, I. Electrodeposition of CdTe thin film from acetate-based ionic liquid bath. AIP Conf. Proc. 2018, 1961, 030048. [Google Scholar] [CrossRef]
- Orme, K.; Baek, D.L.; Fox, R.V.; Atifi, A. Water Interplays during Dysprosium Electrodeposition in Pyrrolidinium Ionic Liquid: Deconvoluting the Pros and Cons for Rare Earth Metallization. ACS Sustain. Chem. Eng. 2021, 9, 14631–14643. [Google Scholar] [CrossRef]
- Maizi, R.; Meddour, A.; Rousse, C. Structural and electrochemical properties of thin layers of binary Ni-Fe alloys electrodeposited by two different baths: Acid and ionic liquid. Surf. Rev. Lett. 2017, 25, 1950025. [Google Scholar] [CrossRef]
- Yin, Y.; Zhu, H.; Wu, T.; Liao, P.; Lan, C.; Li, C. Bistable Silver Electrodeposition-Based Electrochromic Device with Reversible Three-State Optical Transformation By Using WO3 Nanoislands Modified ITO Electrode. Adv. Mater. Interfaces 2022, 9, 2102566. [Google Scholar] [CrossRef]
- Peng, Y.; Shinde, P.S.; Reddy, R.G. Diffusion coefficient and nucleation density studies on electrochemical deposition of aluminum from chloroaluminate ionic liquid electrolytes. J. Electroanal. Chem. 2021, 895, 115363. [Google Scholar] [CrossRef]
- Zhang, D.; Mu, Y.; Li, H.; Yang, Z.; Yang, Y. A new method for electrodeposition of Al coatings from ionic liquids on AZ91D Mg alloy in air. RSC Adv. 2018, 8, 39170–39176. [Google Scholar] [CrossRef] [Green Version]
- Caporali, S.; Martinuzzi, S.M.; Von Czarnecki, P.; Schubert, T.J.S.; Bardi, U. Effects of Metal Ions on the Aluminum Electrodeposition from Ionic Liquids. J. Mater. Eng. Perform. 2017, 26, 685–691. [Google Scholar] [CrossRef]
- Peng, Y.; Shinde, P.; Reddy, R.G. Nucleation Study on Deposition of Aluminum from 1-Butyl-3-Methylimidazolium Chloride and Aluminum Chloride Ionic Liquid Electrolyte. ECS Trans. 2020, 98, 199. [Google Scholar] [CrossRef]
- Wang, Y.; Reddy, R.G.; Wang, R. Dendrite-free Al recycling via electrodeposition using ionic liquid electrolytes: The effects of deposition temperature and cathode surface roughness. J. Clean. Prod. 2021, 287, 125043. [Google Scholar] [CrossRef]
- Lang, H.; Wang, Q.; Tu, X.; Chen, S. Template-free preparation of spherical Al particles in aluminum chloride and 1-butyl-3-methylimidazolium chloride ionic liquid. Ionics 2018, 24, 1781–1788. [Google Scholar] [CrossRef]
- Zhou, J.; Zheng, X.; Li, J.; Li, X.; Lu, K. Electrodeposited Al mesocrystal with high thermal stability and high hardness. Scr. Mater. 2022, 221, 114964. [Google Scholar] [CrossRef]
- Tian, G.-C.; Yuan, Q.-X. Effect of dichloromethane and toluene on the structure, property, and Al electrodeposition in 1-butyl-3-methylimidazolium chloroaluminate ionic liquid. Chin. J. Eng. 2021, 43, 1037. [Google Scholar] [CrossRef]
- Xu, C.; Hua, Y.; Zhang, Q.; Li, J.; Lei, Z.; Lu, D. Electrodeposition of Al-Ti alloy on mild steel from AlCl3-BMIC ionic liquid. J. Solid State Electrochem. 2017, 21, 1349–1356. [Google Scholar] [CrossRef]
- Shinde, P.S.; Peng, Y.; Reddy, R.G. Potentiostatic Electrodeposition of Ti–Al Alloy with 40% Titanium from the Lewis Acidic 1-Butyl-3-Methylimidazolium Chloride-Aluminum Chloride Ionic Liquid Electrolyte. In TMS 2022 151st Annual Meeting & Exhibition Supplemental Proceedings; Springer International Publishing: Cham, Switzerland, 2022; pp. 74–86. [Google Scholar]
- Sriram, P.; Kumar, M.K.; Selvi, G.T.; Jha, N.S.; Mohanapriya, N.; Jha, S.K. Elucidation of ultrasonic wave-assisted electrodeposited AgPd nanoalloy from ionic liquid as an efficient bifunctional electrocatalyst for methanol oxidation and hydrogen peroxide reduction. Electrochim. Acta 2019, 323, 134809. [Google Scholar] [CrossRef]
- Sun, J.; Ming, T.-Y.; Qian, H.-X.; Li, Q.-S. Preparation of black Cu-Sn alloy with single phase composition by electrodeposition method in 1-butyl-3-methylimidazolium chloride ionic liquids. Mater. Chem. Phys. 2018, 219, 421–424. [Google Scholar] [CrossRef]
- Sun, J.; Ming, T.Y.; Qian, H.X.; Li, Q.S. Electrochemical behaviors and electrodeposition of single-phase Cu-Sn alloy coating in [BMIM]Cl. Electrochim. Acta 2019, 297, 87–93. [Google Scholar] [CrossRef]
- Sun, J.; Ming, T.-Y.; Qian, H.-X.; Li, Q.-S. Preparation of copper–silver alloy with different morphologies by a electrodeposition method in 1-butyl-3-methylimidazolium chloride ionic liquid. Bull. Mater. Sci. 2019, 42, 227. [Google Scholar] [CrossRef] [Green Version]
- Jayanthi, E.; Murugesan, N.; Suneesh, A.S.; Ramesh, C.; Anthonysamy, S. Sensing Behavior of Room Temperature Amperometric H2 Sensor with Pd Electrodeposited from Ionic Liquid Electrolyte as Sensing Electrode. J. Electrochem. Soc. 2017, 164, H5210. [Google Scholar] [CrossRef]
- Hallaj, R.; Mohammadian, N.; Ghaderi, S.; Navaee, A. Nonenzymatic and low potential glucose sensor based on electrodeposited Ru-nanofilm from ionic liquid electrolyte. Mater. Sci. Eng. B 2020, 261, 114666. [Google Scholar] [CrossRef]
- Fan, M.; Li, S.; Deng, H.; Zhang, X.; Luo, G.; Huang, Z.; Chen, M. Separation and recovery of iridium(IV) from simulated secondary resource leachate by extraction—Electrodeposition. Sep. Purif. Technol. 2022, 289, 120765. [Google Scholar] [CrossRef]
- Matsumiya, M.; Kinoshita, R.; Tsuchida, Y.; Sasaki, Y. Recovery of Iridium by Solvent Extraction and Direct Electrodeposition Using Phosphonium-Based Ionic Liquids. J. Electrochem. Soc. 2021, 168, 056501. [Google Scholar] [CrossRef]
- Ries, L.A.d.S.; de Brito, H.A.; Gasparin, F.P.; Muller, I.L. Additive-free electrodeposition of cobalt on silicon from 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid. J. Mol. Liq. 2021, 325, 114787. [Google Scholar] [CrossRef]
- Qian, J.; Li, X.; Luan, H.; Li, T.; Yin, Y. Electrodeposition of Iridium in 1-Butyl-3-Methylimidazolium Tetrafluoroborate Ionic Liquid. Rare Met. Mater. Eng. 2017, 46, 1756–1761. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Li, Y.; Shi, X.; Wang, D.; Wang, G.; Jiao, C.; Zhang, M. Electrochemical properties of Ln(III) (Ln = Ce, Gd) in 1-butyl-3-methylimidazolium tetrafluoroborate ionic liquid. J. Radioanal. Nucl. Chem. 2021, 329, 1269–1276. [Google Scholar] [CrossRef]
- Keist, J.S.; Hammons, J.A.; Wright, P.K.; Evans, J.W.; Orme, C.A. Coupling in situ atomic force microscopy (AFM) and ultra-small-angle X-ray scattering (USAXS) to study the evolution of zinc morphology during electrodeposition within an imidazolium based ionic liquid electrolyte. Electrochim. Acta 2020, 342, 136073. [Google Scholar] [CrossRef]
- Elbasiony, A.M.; Prowald, A.; Abedin, S.Z.E.; Endres, F. Electrochemical synthesis of nanowires and macroporous CuSn alloy from ionic liquids. J. Solid State Electrochem. 2022, 26, 783–789. [Google Scholar] [CrossRef]
- Mardani, R.; Shahmirzaee, H.; Ershadifar, H.; Vahdani, M.R. Electrodeposition of Ni32Fe48Mo20 and Ni52Fe33W15 alloy film on Cu microwire from ionic liquid containing plating bath. Surf. Coat. Technol. 2017, 324, 281–287. [Google Scholar] [CrossRef]
- Jesmani, S.M.; Amini, R.; Abdollah-Pour, H.; Mohammadian-Semnani, H. Effect of current density on Ni-Mo electrodeposition using EMIM [Br]. Surf. Eng. 2019, 35, 1088–1096. [Google Scholar] [CrossRef]
- Jesmani, S.M.; Mohammadian-Semnani, H.; Abdollah-Pour, H.; Amini, R. The effect of pH on electrocatalytic properties of electrodeposited Ni–Mo/Ni coating using 1-ethyl-3-methylimidazolium bromide. Mater. Res. Express 2019, 6, 1065e1062. [Google Scholar] [CrossRef]
- Yang, J.-M.; Hsieh, Y.-T.; Chu-Tien, T.-T.; Sun, I.W. Electrodeposition of Distinct One-Dimensional Zn Biaxial Microbelt from the Zinc Chloride-1-Ethyl-3-methylidazolium Chloride Ionic Liquid. J. Electrochem. Soc. 2011, 158, D235. [Google Scholar] [CrossRef]
- Molodkina, E.B.; Ehrenburg, M.R.; Arkhipushkin, I.A.; Rudnev, A.V. Interfacial effects in the electro(co)deposition of Nd, Fe, and Nd-Fe from an ionic liquid with controlled amount of water. Electrochim. Acta 2021, 398, 139342. [Google Scholar] [CrossRef]
- Xu, D.; Li, J.; Liang, C.; Liu, J.; Wang, H.; Ling, G. Increasing anhydrous chromium chloride concentration in AlCl3–EMIC ionic liquid: A step towards non-hydrogen-embrittlement chromium electroplating. RSC Adv. 2022, 12, 1855–1861. [Google Scholar] [CrossRef]
- Farisi, M.S.A.; Hertel, S.; Wiemer, M.; Otto, T. Investigation of aluminum patterned electrodeposition process from AlCl3-[EMIm]Cl ionic liquid for microsystems application. In Proceedings of the 2018 International Conference on Electronics Packaging and iMAPS All Asia Conference (ICEP-IAAC), Mie, Japan, 17–21 April 2018; pp. 415–418. [Google Scholar]
- Rodríguez González, P.; Ling, G. Anodic behavior in AlCl3-EMIC ionic liquid of Ti6Al4V alloy and its application for enhancing the adhesion strength of aluminum coatings. Surf. Coat. Technol. 2017, 331, 57–65. [Google Scholar] [CrossRef]
- Yamagami, M.; Higashino, S.; Yamamoto, T.; Ikenoue, T.; Miyake, M.; Hirato, T. Aluminum Electrodeposition in Dry Air Atmosphere: Comparative Study of an Acetamide–AlCl3 Deep Eutectic Solvent and a 1-Ethyl-3-Methylimidazolium Chloride–AlCl3 Ionic Liquid. J. Electrochem. Soc. 2022, 169, 062502. [Google Scholar] [CrossRef]
- Elterman, V.A.; Shevelin, P.Y.; Yolshina, L.A.; Borozdin, A.V. Electrodeposition of aluminium from the chloroaluminate ionic liquid 1-ethyl-3-methylimidazolium chloride. Electrochim. Acta 2021, 389, 138715. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, S.; Chi, C.; Hao, J.; Zhao, J.; Xu, Y.; Li, Y. Electrodeposition of a continuous, dendrite-free aluminum film from an ionic liquid and its electrochemical properties. J. Mater. Sci. Mater. Electron. 2020, 31, 9937–9945. [Google Scholar] [CrossRef]
- Jiang, Y.; Ding, J.; Luo, L.; Shi, P.; Wang, X. Electrodepositing aluminum coating on uranium from aluminum chloride-1-ethyl-3-methylimidazolium chloride ionic liquid. Surf. Coat. Technol. 2017, 309, 980–985. [Google Scholar] [CrossRef]
- Wang, J.; Liu, X.; Xie, H.; Yin, H.; Song, Q.; Ning, Z. Effect of a Magnetic Field on the Electrode Process of Al Electrodeposition in a [Emim]Cl-AlCl3 Ionic Liquid. J. Phys. Chem. B 2021, 125, 13744–13751. [Google Scholar] [CrossRef]
- Takahashi, H.; Matsushima, H.; Ueda, M. Al Film Electrodeposition from the AlCl3-EMIC Electrolyte under a Magnetic Field. J. Electrochem. Soc. 2017, 164, H5165. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, Z.; Shen, L.; Liu, A.; Xu, J.; Hu, X. Electrodeposition of Al, Al-Li Alloy, and Li from an Al-Containing Solvate Ionic Liquid under Ambient Conditions. J. Electrochem. Soc. 2018, 165, D321. [Google Scholar] [CrossRef]
- Yang, Y.; Hao, J.; Xue, J.; Liu, S.; Chi, C.; Zhao, J.; Xu, Y.; Li, Y. Co-electrodeposited Al-Ga composite electrode from ionic liquid with volume expansion adaptability in energy storage. Mater. Lett. 2021, 303, 130484. [Google Scholar] [CrossRef]
- Yang, J.; Chang, L.; Jiang, L.; Wang, K.; Huang, L.; He, Z.; Shao, H.; Wang, J.; Cao, C.-N. Electrodeposition of Al-Mn-Zr ternary alloy films from the Lewis acidic aluminum chloride-1-ethyl-3-methylimidazolium chloride ionic liquid and their corrosion properties. Surf. Coat. Technol. 2017, 321, 45–51. [Google Scholar] [CrossRef]
- Hibino, Y.; Azumi, K. Enhancement of Al-Ti Alloy Electrodeposition from AlCl3-EMIC Ionic Liquid Based Bath by Mg Additive. J. Electrochem. Soc. 2019, 166, D776. [Google Scholar] [CrossRef]
- Higashino, S.; Takeuchi, Y.; Miyake, M.; Ikenoue, T.; Tane, M.; Hirato, T. Tungsten(II) chloride hydrates with high solubility in chloroaluminate ionic liquids for the electrodeposition of Al–W alloy films. J. Electroanal. Chem. 2022, 912, 116238. [Google Scholar] [CrossRef]
- Higashino, S.; Miyake, M.; Takahashi, A.; Matamura, Y.; Fujii, H.; Kasada, R.; Hirato, T. Evaluation of the hardness and Young’s modulus of electrodeposited Al–W alloy films by nano-indentation. Surf. Coat. Technol. 2017, 325, 346–351. [Google Scholar] [CrossRef] [Green Version]
- Höhlich, D.; Wachner, D.; Müller, M.; Scharf, I.; Lampke, T. Electrodeposition and characterisation of Al-W alloy films from ionic liquid. IOP Conf. Ser. Mater. Sci. Eng. 2018, 373, 012007. [Google Scholar] [CrossRef]
- Peng, D.; Cong, D.; Song, K.; Ding, X.; Wang, X.; Bai, Y.; Yang, X.; Yin, C.; Zhang, Y.; Rao, J.; et al. Mirror-like Bright Al-Mn Coatings Electrodeposition from 1-Ethyl-3 Methylimidazolium Chloride-AlCl3-MnCl2 Ionic Liquids with Pyridine Derivatives. Materials 2021, 14, 6226. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-C.; Sun, I.W. Template-Free Fabrication of Diameter-Modulated Co-Zn/Oxide Wires from a Chlorozincate Ionic Liquid by Using Pulse Potential Electrodeposition. J. Electrochem. Soc. 2017, 164, D425. [Google Scholar] [CrossRef]
- He, W.; Shi, Z.; Liu, A.; Guan, J.; Yang, S. Electro-reduction of Cu2O to Cu in urea/1-ethyl-3-methylimidazolium chloride. J. Appl. Electrochem. 2021, 51, 1145–1156. [Google Scholar] [CrossRef]
- Lehmann, L.; Höhlich, D.; Mehner, T.; Lampke, T. Irregular Electrodeposition of Cu-Sn Alloy Coatings in [EMIM]Cl Outside the Glove Box with Large Layer Thickness. Coatings 2021, 11, 310. [Google Scholar] [CrossRef]
- Bakkar, A.; Neubert, V. Electrodeposition of photovoltaic thin films from ionic liquids in ambient atmosphere: Gallium from a chloroaluminate ionic liquid. J. Electroanal. Chem. 2020, 856, 113656. [Google Scholar] [CrossRef]
- Zhang, W.; Pesic, B. Electrochemical Behavior of PdCl2 in 1-Ethyl-3-Methylimidazolium Chloride Ionic Liquid at Pt-Ir Electrode. J. Electrochem. Soc. 2021, 168, 072506. [Google Scholar] [CrossRef]
- Zhang, W.; Pesic, B. Electrochemistry of PdCl2 at glassy carbon electrode in 1-Ethyl-3-methylimidazolium chloride ionic liquid. Electrochim. Acta 2021, 370, 137818. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, C.; Hua, Y.; Wang, M.; Su, Z. Electrochemical preparation of Ni-La alloys from the EMIC-EG eutectic-based ionic liquid. Ionics 2017, 23, 1703–1710. [Google Scholar] [CrossRef]
- Xu, X.; Sturm, S.; Zavasnik, J.; Rozman, K.Z. Electrodeposition of a Rare-Earth Iron Alloy from an Ionic-Liquid Electrolyte. ChemElectroChem 2019, 6, 2860–2869. [Google Scholar] [CrossRef]
- Xi, X.; Song, S.; Nie, Z.; Ma, L. Electrodeposition and behavior of palladium in a room temperature ionic liquid. Int. J. Electrochem. Sci. 2017, 12, 1130–1145. [Google Scholar] [CrossRef]
- Zhang, J.; Ma, X.; Zhang, J.; Yang, P.; An, M.; Li, Q. Electrodeposition of Cu-Zn alloy from EMImTfO ionic liquid/ethanol mixtures for replacing the cyanide zincate layer on Al alloy. J. Alloy. Compd. 2019, 806, 79–88. [Google Scholar] [CrossRef]
- Pham, T.A.; Horwood, C.; Maiti, A.; Peters, V.; Bunn, T.; Stadermann, M. Solvation Properties of Silver and Copper Ions in a Room Temperature Ionic Liquid: A First-Principles Study. J. Phys. Chem. B 2018, 122, 12139–12146. [Google Scholar] [CrossRef]
- Shao, M.; Li, S.; Jin, C.; Chen, M.; Huang, Z. Recovery of Pd(II) from Hydrochloric Acid Medium by Solvent Extraction–Direct Electrodeposition Using Hydrophilic/Hydrophobic ILs. ACS Omega 2020, 5, 27188–27196. [Google Scholar] [CrossRef]
- Rani, M.A.A.; Hwang, J.; Matsumoto, K.; Hagiwara, R. Poly(vinyl chloride) Ionic Liquid Polymer Electrolyte Based on Bis(fluorosulfonyl)Amide for Sodium Secondary Batteries. J. Electrochem. Soc. 2017, 164, H5031. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Shi, Z.; Liu, F.; Yang, S. Electrodeposition of Copper Metal from the 1-Ethyl-3-methylimidazolium Fluoride ([EMIM]F)-urea-H2O System Containing Cu2O. Electrochemistry 2020, 88, 253–255. [Google Scholar] [CrossRef]
- Sato, Y.; Maruyama, S.; Matsumoto, Y. Electrodeposition of metallic Cu from CuCl gas source transported into ionic liquid in a vacuum. J. Vac. Sci. Technol. A 2018, 36, 031516. [Google Scholar] [CrossRef]
- Soulmi, N.; Porras-Gutierrez, A.-G.; Mordvinova, N.E.; Lebedev, O.I.; Rizzi, C.; Sirieix-Plénet, J.; Groult, H.; Dambournet, D.; Gaillon, L. Sn(TFSI)2 as a suitable salt for the electrodeposition of nanostructured Cu6Sn5–Sn composites obtained on a Cu electrode in an ionic liquid. Inorg. Chem. Front. 2019, 6, 248–256. [Google Scholar] [CrossRef]
- Shimizu, M.; Sugiyama, Y.; Horita, M.; Yoshii, K.; Arai, S. Cation-Structure Effects on Zinc Electrodeposition and Crystallographic Orientation in Ionic Liquids. ChemElectroChem 2022, 9, e202200016. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Chen, P.-Y.; Ju, S.-P. Preparation of Ni nanotube-modified electrodes via galvanic displacement on sacrificial Zn templates: Solvent effects and attempts for non-enzymatic electrochemical detection of urea. Microchem. J. 2020, 158, 105172. [Google Scholar] [CrossRef]
- Sousa, N.G.; Salgueira, J.F.S.; Sousa, C.P.; Campos, O.S.; Salazar-Banda, G.R.; Eguiluz, K.I.B.; de Lima-Neto, P.; Correia, A.N. Silver electrodeposition at room temperature protic ionic liquid 1-H-methylimidazolium hydrogen sulfate. J. Mol. Liq. 2020, 313, 113487. [Google Scholar] [CrossRef]
- Kuang, Y.; Jiang, F.; Zhu, T.; Wu, H.; Yang, X.; Li, S.; Hu, C. One-step electrodeposition of superhydrophobic copper coating from ionic liquid. Mater. Lett. 2021, 303, 130579. [Google Scholar] [CrossRef]
- Li, M.; Li, Y. Electrodeposition of Zinc from Zinc Oxide and Zinc Chloride in 1-Methylimidazolium Trifluoromethylsulfonate Ionic Liquid. Prot. Met. Phys. Chem. Surf. 2020, 56, 180–188. [Google Scholar] [CrossRef]
- Motobayashi, K.; Shibamura, Y.; Ikeda, K. Origin of a High Overpotential of Co Electrodeposition in a Room-Temperature Ionic Liquid. J. Phys. Chem. Lett. 2020, 11, 8697–8702. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Li, S.; Jin, C.; Shao, M.; Huang, Z. Selective recovery of platinum by combining a novel reusable ionic liquid with electrodeposition. Sep. Purif. Technol. 2021, 259, 118204. [Google Scholar] [CrossRef]
- Wang, N.; Wang, Q.; Lu, W.; Ru, M.; Yang, Y. Extraction and stripping of platinum (IV) from acidic chloride media using guanidinium ionic liquid. J. Mol. Liq. 2019, 293, 111040. [Google Scholar] [CrossRef]
- Guinea, E.; Salicio-Paz, A.; Iriarte, A.; Grande, H.-J.; Medina, E.; García-Lecina, E. Robust Aluminum Electrodeposition from Ionic Liquid Electrolytes Containing Light Aromatic Naphta as Additive. ChemistryOpen 2019, 8, 1094–1099. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.-A.; Chen, P.-Y.; Sun, I.W. An Assessment of Aluminum Electrodeposition from Aluminum Chloride/4-ethylpyridine Ionic Liquid at Ambient Temperature. J. Electrochem. Soc. 2022, 169, 052505. [Google Scholar] [CrossRef]
- Suneesh, P.V.; Ramachandran, T.; Satheesh Babu, T.G. Electrodeposition of Al-Zr-Cu Ternary Alloy from AlCl3-Et3NHCl Ionic Liquid containing Acetylacetonates of Copper and Zirconium. Mater. Today Proc. 2018, 5, 16640–16645. [Google Scholar] [CrossRef]
- Fong, J.-D.; Chen, P.-Y.; Sun, I.W. Template-Free Electrodeposition of Net-Like Co-Al/Oxide Structures from a Lewis Acidic Chloroaluminate Room Temperature Ionic Liquid Using a Potential Step Method. J. Electrochem. Soc. 2018, 165, D716. [Google Scholar] [CrossRef]
- Shao, Y.-A.; Chen, Y.-T.; Chen, P.-Y. Cu and CuPb electrodes prepared via potentiostatic electrodeposition from metal oxides in hydrophobic protic amide-type ionic liquid/water mixture under ambient air for nonenzymatic nitrate reduction. Electrochim. Acta 2019, 313, 488–496. [Google Scholar] [CrossRef]
- Shao, Y.-A.; Chen, Y.-T.; Chen, P.-Y. Cu and CuPb Electrodes Electrodeposited from Metal Oxides in Hydrophobic Protic Amide-Type Ionic Liquid/Water Mixture for Nonenzymatic Glucose Oxidation. J. Electrochem. Soc. 2019, 166, D221. [Google Scholar] [CrossRef]
- Chen, P.; Richter, J.; Wang, G.; Li, D.; Pietsch, T.; Ruck, M. Ionometallurgical Step-Electrodeposition of Zinc and Lead and its Application in a Cycling-Stable High-Voltage Zinc-Graphite Battery. Small 2021, 17, 2102058. [Google Scholar] [CrossRef]
- Yeh, H.-W.; Tang, Y.-H.; Chen, P.-Y. Electrochemical study and extraction of Pb metal from Pb oxides and Pb sulfate using hydrophobic Brønsted acidic amide-type ionic liquid: A feasibility demonstration. J. Electroanal. Chem. 2018, 811, 68–77. [Google Scholar] [CrossRef]
- Kono, S.; Takao, K.; Arai, T. Direct and Selective Electrodeposition of Palladium from Betainium Bis(trifluoromethanesulfonyl)imide Ionic Liquid Phase after Solvent Extraction together with Other Platinum Group Metals. Trans. At. Energy Soc. Jpn. 2020, 19, 76–84. [Google Scholar] [CrossRef]
- Wang, Z.; Wu, T.; Geng, X.; Ru, J.; Hua, Y.; Bu, J.; Xue, Y.; Wang, D. The role of electrolyte ratio in electrodeposition of nanoscale FeCr alloy from choline chloride-ethylene glycol ionic liquid: A suitable layer for corrosion resistance. J. Mol. Liq. 2022, 346, 117059. [Google Scholar] [CrossRef]
- Danilov, F.I.; Bogdanov, D.A.; Smyrnova, O.V.; Korniy, S.A.; Protsenko, V.S. Electrodeposition of Ni–Fe alloy from a choline chloride-containing ionic liquid. J. Solid State Electrochem. 2022, 26, 939–957. [Google Scholar] [CrossRef]
- Rosoiu, S.P.; Pantazi, A.G.; Petica, A.; Cojocaru, A.; Costovici, S.; Zanella, C.; Visan, T.; Anicai, L.; Enachescu, M. Comparative Study of Ni-Sn Alloys Electrodeposited from Choline Chloride-Based Ionic Liquids in Direct and Pulsed Current. Coatings 2019, 9, 801. [Google Scholar] [CrossRef] [Green Version]
- Protsenko, V.S.; Bogdanov, D.A.; Korniy, S.A.; Kityk, A.A.; Baskevich, A.S.; Danilov, F.I. Application of a deep eutectic solvent to prepare nanocrystalline Ni and Ni/TiO2 coatings as electrocatalysts for the hydrogen evolution reaction. Int. J. Hydrog. Energy 2019, 44, 24604–24616. [Google Scholar] [CrossRef]
- Alesary, H.F.; Khudhair, A.F.; Rfaish, S.Y.; Ismail, H.K. Effect of Sodium Bromide on the Electrodeposition of Sn, Cu, Ag and Ni from a Deep Eutectic Solvent-Based Ionic Liquid. Int. J. Electrochem. Sci. 2019, 14, 7116–7132. [Google Scholar] [CrossRef] [Green Version]
- Fashu, S.; Mudzingwa, L.; Khan, R.; Tozvireva, M. Electrodeposition of high corrosion resistant Ni–Sn–P alloy coatings from an ionic liquid based on choline chloride. Trans. IMF 2018, 96, 20–26. [Google Scholar] [CrossRef]
- Panzeri, G.; Pedrazzetti, L.; Rinaldi, C.; Nobili, L.; Magagnin, L. Electrodeposition of Nanostructured Cobalt Films from Choline Chloride-Ethylene Glycol Deep Eutectic Solvent. J. Electrochem. Soc. 2018, 165, D580. [Google Scholar] [CrossRef]
- Pereira, N.M.; Brincoveanu, O.; Pantazi, A.G.; Pereira, C.M.; Araújo, J.P.; Fernando Silva, A.; Enachescu, M.; Anicai, L. Electrodeposition of Co and Co composites with carbon nanotubes using choline chloride-based ionic liquids. Surf. Coat. Technol. 2017, 324, 451–462. [Google Scholar] [CrossRef]
- Alesary, H.F.; Ismail, H.K.; Hameid Odda, A.; Watkins, M.J.; Arkan Majhool, A.; Ballantyne, A.D.; Ryder, K.S. Influence of different concentrations of nicotinic acid on the electrochemical fabrication of copper film from an ionic liquid based on the complexation of choline chloride-ethylene glycol. J. Electroanal. Chem. 2021, 897, 115581. [Google Scholar] [CrossRef]
- Yavuz, A.; Yilmaz Erdogan, P.; Zengin, H.; Zengin, G. Electrodeposition and Characterisation of Zn-Co Alloys from Ionic Liquids on Copper. J. Electron. Mater. 2022, 51, 5253–5261. [Google Scholar] [CrossRef]
- Marín-Sánchez, M.; Gracia-Escosa, E.; Conde, A.; Palacio, C.; García, I. Deposition of Zinc–Cerium Coatings from Deep Eutectic Ionic Liquids. Materials 2018, 11, 2035. [Google Scholar] [CrossRef] [Green Version]
- Fashu, S.; Khan, R.; Zulfiqar, S. Ternary Zn–Mn–Sn alloy electrodeposition from an ionic liquid based on choline chloride. Trans. IMF 2017, 95, 217–225. [Google Scholar] [CrossRef]
- Popescu, A.M.; Soare, V.; Demidenko, O.; Moreno, J.M.C.; Neacsu, E.I.; Donath, C.; Constantin, V. Recovery of Silver and Gold from Electronic Waste by Electrodeposition in Ethaline Ionic Liquid. Rev. Chim. 2020, 71, 122–132. [Google Scholar] [CrossRef]
- Du, C.; Yang, H.; Chen, X.-B.; Wang, L.; Dong, H.; Ning, Y.; Lai, Y.; Jia, J.; Zhao, B. Effect of coordinated water of hexahydrate on nickel platings from choline–urea ionic liquid. J. Mater. Sci. 2018, 53, 10758–10771. [Google Scholar] [CrossRef]
- Li, M.; Chen, B.Q.; Xiong, T.T.; Gao, L.X.; Du, C.; Zhu, Y.N.; Zhang, S.M. Electrodeposition of Pr-Mg-Co ternary alloy films from the choline chloride-Urea ionic liquids and their corrosion properties. J. Dispers. Sci. Technol. 2020, 41, 941–947. [Google Scholar] [CrossRef]
- Li, M.; Chen, B.Q.; He, M.; Xiong, T.; Gao, L. Electrodeposition of Pr-Mg-Ni ternary alloy films from the choline chloride-urea ionic liquid and their corrosion properties. Anti-Corros. Methods Mater. 2018, 65, 437–443. [Google Scholar] [CrossRef]
- Shi, T.; Zou, X.; Wang, S.; Pang, Z.; Tang, W.; Li, G.; Xu, Q.; Lu, X. Electrodeposition of Sn-Co-Ni and Sn-Co-Zn Alloy Coatings on Copper Substrate in a Deep Eutectic Solvent and Their Characterization. Int. J. Electrochem. Sci. 2020, 15, 7493–7507. [Google Scholar] [CrossRef]
- Golgovici, F.; Ionascu, F.G.; Prodana, M.; Demetrescu, I. Simultaneously Embedding Indomethacin and Electrodeposition of Polypyrrole on Various CoCr Alloys from Ionic Liquids. Materials 2022, 15, 4714. [Google Scholar] [CrossRef]
- Zhang, B.; Yao, Y.; Shi, Z.; Xu, J.; Wang, Z. Direct Electrochemical Deposition of Lithium from Lithium Oxide in a Highly Stable Aluminium-Containing Solvate Ionic Liquid. ChemElectroChem 2018, 5, 3368–3372. [Google Scholar] [CrossRef]
- Tachikawa, N.; Kasai, R.; Yoshii, K.; Watanabe, M.; Katayama, Y. Electrochemical Deposition and Dissolution of Lithium on a Carbon Fiber Composite Electrode in a Solvate Ionic Liquid. Electrochemistry 2017, 85, 667–670. [Google Scholar] [CrossRef] [Green Version]
- Berger, C.A.; Ceblin, M.U.; Jacob, T. Lithium Deposition from a Piperidinium–based Ionic Liquid: Rapping Dendrites on the Knuckles. ChemElectroChem 2017, 4, 261–265. [Google Scholar] [CrossRef]
- He, J.-W.; Gu, Y.; Wang, W.-W.; Wang, J.-H.; Chen, Z.-B.; He, H.-Y.; Wu, Q.-H.; Yan, J.-W.; Mao, B.-W. Structures of Solid-Electrolyte Interphases and Impacts on Initial-Stage Lithium Deposition in Pyrrolidinium-Based Ionic Liquids. ChemElectroChem 2021, 8, 62–69. [Google Scholar] [CrossRef]
- Galindo, M.; Sebastián, P.; Cojocaru, P.; Gómez, E. Electrodeposition of aluminium from hydrophobic perfluoro-3-oxa-4,5 dichloro-pentan-sulphonate based ionic liquids. J. Electroanal. Chem. 2018, 820, 41–50. [Google Scholar] [CrossRef]
- Oriani, A.V.; Cojocaru, P.; Monzani, C.; Vallés, E.; Gómez, E. Aluminium electrodeposition from a novel hydrophobic ionic liquid tetramethyl guanidinium-perfluoro-3-oxa-4,5 dichloro-pentan-sulphonate. J. Electroanal. Chem. 2017, 793, 85–92. [Google Scholar] [CrossRef]
- Yu, X.; Cui, J.; Liu, C.; Yuan, F.; Guo, Y.; Deng, T. Separation of magnesium from high Mg/Li ratio brine by extraction with an organic system containing ionic liquid. Chem. Eng. Sci. 2021, 229, 116019. [Google Scholar] [CrossRef]
- Rajagopal, V.; Velayutham, D.; Suryanarayanan, V.; Kathiresan, M.; Ho, K.C. Electrochemical fabrication of dendritic silver–copper bimetallic nanomaterials in protic ionic liquid for electrocarboxylation. J. Taiwan Inst. Chem. Eng. 2018, 87, 158–164. [Google Scholar] [CrossRef]
- Song, Y.; Tsuchida, Y.; Matsumiya, M.; Tsunashima, K. Recovery of ruthenium by solvent extraction and direct electrodeposition using ionic liquid solution. Hydrometallurgy 2018, 181, 164–168. [Google Scholar] [CrossRef]
- Matsumiya, M.; Song, Y.; Tsuchida, Y.; Ota, H.; Tsunashima, K. Recovery of platinum by solvent extraction and direct electrodeposition using ionic liquid. Sep. Purif. Technol. 2019, 214, 162–167. [Google Scholar] [CrossRef]
- García-Montoya, M.F.; Gutiérrez-Granados, S.; Méndez-Quezada, J.Y.; Cholico-González, D.F.; Ponce de León, C.; Hernández-Perales, L.; Ávila-Rodríguez, M. Electrochemistry of Rhodium (III) in Trihexil(tetradecyl) Phosphonium Bis(2,4,4-trimethylpentyl) Phosphinate Ionic Liquid (Cyphos IL 104 ®) and Its Deposition. ECS Trans. 2018, 84, 1. [Google Scholar] [CrossRef]
- Sanchez-Cupido, L.; Pringle, J.M.; Siriwardana, A.L.; Unzurrunzaga, A.; Hilder, M.; Forsyth, M.; Pozo-Gonzalo, C. Water-Facilitated Electrodeposition of Neodymium in a Phosphonium-Based Ionic Liquid. J. Phys. Chem. Lett. 2019, 10, 289–294. [Google Scholar] [CrossRef]
- Deferm, C.; Malaquias, J.C.; Onghena, B.; Banerjee, D.; Luyten, J.; Oosterhof, H.; Fransaer, J.; Binnemans, K. Electrodeposition of indium from the ionic liquid trihexyl(tetradecyl)phosphonium chloride. Green Chem. 2019, 21, 1517–1530. [Google Scholar] [CrossRef] [Green Version]
- Bourbos, E.; Giannopoulou, I.; Karantonis, A.; Paspaliaris, I.; Panias, D. Reduction of Light Rare Earths and a Proposed Process for Nd Electrorecovery Based on Ionic Liquids. J. Sustain. Metall. 2018, 4, 395–406. [Google Scholar] [CrossRef]
- Gao, M.Y.; Yang, C.; Zhang, Q.B.; Zeng, J.R.; Li, X.T.; Hua, Y.X.; Xu, C.Y.; Li, Y. Electrochemical Preparation of Ni-La Alloy Films from N-butyl-N-Methyl Pyrrolidinium Dicyanamide Ionic Liquid as Electrocatalysts for Hydrogen Evolution Reaction. J. Electrochem. Soc. 2017, 164, D778. [Google Scholar] [CrossRef]
- Periyapperuma, K.; Pringle, J.M.; Sanchez-Cupido, L.; Forsyth, M.; Pozo-Gonzalo, C. Fluorine-free ionic liquid electrolytes for sustainable neodymium recovery using an electrochemical approach. Green Chem. 2021, 23, 3410–3419. [Google Scholar] [CrossRef]
- Sano, H.; Kitta, M.; Shikano, M.; Matsumoto, H. Effect of Temperature on Li Electrodeposition Behavior in Room-Temperature Ionic Liquids Comprising Quaternary Ammonium Cation. J. Electrochem. Soc. 2019, 166, A2973. [Google Scholar] [CrossRef]
- Schuett, F.M.; Heubach, M.-K.; Mayer, J.; Ceblin, M.U.; Kibler, L.A.; Jacob, T. Electrodeposition of Zinc onto Au(111) and Au(100) from the Ionic Liquid [MPPip][TFSI]. Angew. Chem. Int. Ed. 2021, 60, 20461–20468. [Google Scholar] [CrossRef] [PubMed]
- Nishi, N.; Ezawa, K.; Sakka, T. In Situ Surface Roughness Analysis of Electrodeposited Co Films in an Ionic Liquid Using Electrochemical Surface Plasmon Resonance: Effect of Leveling Additives. J. Electrochem. Soc. 2021, 168, 072505. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Chen, P.-Y. Electrochemical Study and Electrodeposition of Zn-Ni Alloys in an Imide-Type Hydrophobic Room-Temperature Ionic Liquid: Feasibility of Using Metal Chlorides as the Metal Sources. J. Electrochem. Soc. 2018, 165, D76. [Google Scholar] [CrossRef]
- Yavuz, A.; Yilmaz, N.F.; Artan, M. Fe-Cu Alloy-Based Flexible Electrodes from Ethaline Ionic Liquid. J. Electron. Mater. 2021, 50, 3478–3487. [Google Scholar] [CrossRef]
- Li, M.; Li, Y. Aluminum Electrodeposition using AlCl3/urea Ionic Liquid. Int. J. Electrochem. Sci. 2020, 15, 8498–8505. [Google Scholar] [CrossRef]
- Lambri, O.A.; Weidenfeller, B.; Bonifacich, F.G.; Mohr-Weidenfeller, L.; Lambri, F.D.; Xu, J.; Zelada, G.I.; Endres, F. Study of the damping behaviour in samples consisting of iron electro-deposited on copper in an ionic liquid. J. Alloy. Compd. 2022, 918, 165462. [Google Scholar] [CrossRef]
- Protsenko, V.S.; Butyrina, T.E.; Bobrova, L.S.; Korniy, S.A.; Danilov, F.I. Electrochemical Corrosion Behavior of Ni–TiO2 Composite Coatings Electrodeposited from a Deep Eutectic Solvent-Based Electrolyte. Coatings 2022, 12, 800. [Google Scholar]
- Ramírez, C.; Bozzini, B.; Calderón, J.A. Electrodeposition of copper from triethanolamine as a complexing agent in alkaline solution. Electrochim. Acta 2022, 425, 140654. [Google Scholar] [CrossRef]
- Bhujbal, A.V.; Rout, A.; Venkatesan, K.A.; Bhanage, B.M. Electrochemical Fabrication of Copper and Tin Micro-Crystals from a Protic Ionic Liquid Medium. ChemistrySelect 2020, 5, 3694–3699. [Google Scholar] [CrossRef]
- Maniam, K.K.; Paul, S. Progress in Electrodeposition of Zinc and Zinc Nickel Alloys Using Ionic Liquids. Appl. Sci. 2020, 10, 5321. [Google Scholar] [CrossRef]
- Lahiri, A.; Endres, F. Review—Electrodeposition of Nanostructured Materials from Aqueous, Organic and Ionic Liquid Electrolytes for Li-Ion and Na-Ion Batteries: A Comparative Review. J. Electrochem. Soc. 2017, 164, D597. [Google Scholar] [CrossRef]
- Mittal, N.; Tien, S.; Lizundia, E.; Niederberger, M. Hierarchical Nanocellulose-Based Gel Polymer Electrolytes for Stable Na Electrodeposition in Sodium Ion Batteries. Small 2022, 18, 2107183. [Google Scholar] [CrossRef]
- Miki, A.; Nishikawa, K.; Kamesui, G.; Matsushima, H.; Ueda, M.; Rosso, M. In situ interferometry study of ionic mass transfer phenomenon during the electrodeposition and dissolution of Li metal in solvate ionic liquids. J. Mater. Chem. A 2021, 9, 14700–14709. [Google Scholar] [CrossRef]
- Zhang, S.; Yamazawa, T.; Sakka, T.; Nishi, N. In Situ Electrochemical Surface Plasmon Resonance Study on Lithium Underpotential Deposition and Stripping in Bis(fluorosulfonyl)amide-Based Ionic Liquids. J. Phys. Chem. C 2022, 126, 9551–9558. [Google Scholar] [CrossRef]
- Khosravi, R.; Azizi, A.; Ghaedrahmati, R.; Gupta, V.K.; Agarwal, S. Adsorption of gold from cyanide leaching solution onto activated carbon originating from coconut shell—Optimization, kinetics and equilibrium studies. J. Ind. Eng. Chem. 2017, 54, 464–471. [Google Scholar] [CrossRef]
- Meng, Q.; Yan, X.; Li, G. Eco-friendly and reagent recyclable gold extraction by iodination leaching-electrodeposition recovery. J. Clean. Prod. 2021, 323, 129115. [Google Scholar] [CrossRef]
- Zhang, Y.; Nishi, N.; Sakka, T. One-step fabrication of Au@Pd core-shell bimetallic nanofibers at the interface between water and redox-active ionic liquid. Electrochim. Acta 2019, 325, 134919. [Google Scholar] [CrossRef]
- Sobek, D.; Bhattacharyya, S. Dispensing of Alkali Metals via Electrodeposition Using Alkali Metal Salts in Ionic Liquids. US20200109481A1, 9 April 2020. [Google Scholar]
- Jiang, F.; Shi, W.; Wu, H.; Zhao, X.; Li, Y.; Li, S. A kind of Method of Simple System Electrodepositing Zinc Coating. CN108754556A, 6 November 2018. [Google Scholar]
- Zhou, G.; Wang, W.; Zhang, R.; Wang, S.; Wu, M.; Nível, Q.; Mitsuzaki, S.; Chen, Z. Method for Preparing Super-Hydrophobic Zn-Fe Alloy Coating in Eutectic Ionic Liquid through Electrodeposition. CN113174617A, 27 July 2021. [Google Scholar]
- Fan, J.; Li, J.; Yang, X.; Chen, Y.; Shi, W.; Liu, H. The Method that High Current Density Electrochemistry Prepares Spelter Coating in Ionic Liquid. CN108754557A, 6 November 2018. [Google Scholar]
- Xue, X.; Wang, Y.; Yue, H.; Zhou, S. The Method that the Hydrated Chromium Trichloride Ionic Liquid Electrodeposition of Choline Chloride Six Prepares Crome Metal. CN107254698A, 17 October 2017. [Google Scholar]
- Zhang, Q.; Yang, C.; Hua, Y.; Xu, C.; Li, Y. The Method that Electro-Deposition Prepares Self-Cradling Type Nanometer Cobalt Bimetallic Phosphide Catalytic Hydrogen Evolution Electrode Material in Eutectic Type Ionic Liquid. CN108360030A, 23 January 2018. [Google Scholar]
- Zhang, Q.; Gao, M.; Yang, C.; Hua, Y.; Xu, C.; Li, J.; Li, Y. Method for Preparing High-Catalytic Oxygen Evolution Performance Nano Porous Nickel-Iron-Sulfur Alloy by Electrodeposition in Eutectic Ionic Liquid. CN107335450B, 7 February 2020. [Google Scholar]
- Um, M.; Song, Y.; Yang, P.; Zhang, J.; Feng, Z. Ionic Liquid Gold Plating Solution Containing Coordination Agent and Additive and Gold Plating Method Adopting Ionic Liquid Gold Plating Solution. CN106676595A, 17 May 2017. [Google Scholar]
- Li, Y.; Li, M. A Method of Preparing Metallic Zinc Using Ionic Liquid Electrolytic Oxidation Zinc. CN108315763A, 24 July 2018. [Google Scholar]
- Shi, Z.; Liu, A.; Wang, Z.; Gao, B.; Hu, X.; Liu, F.; Tao, W.; Yang, Y.; Yu, J. A Kind of Method that Electrodeposition Process Prepares Metallic Lead. CN109536994A, 29 March 2019. [Google Scholar]





| Nomenclature | Abbreviation | Metal or Alloy | Reference |
|---|---|---|---|
| (1-methyl-3-(2-oxo-2-((2,4,5 trifluorophenyl)amino)ethyle)-1H-imidazol-3-ium iodide) | [MOFIM]I | Ni–Co | [19,20] |
| 1-(4-fluorobenzyl)-3-(4-phenoxybutyl)imidazole-3-ium bromide | [FPIM]Br | Co | [19,21] |
| Ni | |||
| Ni–Co | [20] | ||
| 1,3-dibutylpyrazolium bromide | DBPz–Br | Ag | [22] |
| 1,3-dibutylmorpholinium bromide | DBMp–Br | Ag | [22] |
| 1,3-dimethyl-2-imidazolidinone–AlCl3 | DMI−AlCl3 | Al–Mg | [23] |
| 1,3-dimethyl-2-imidazolidinone–LiNO3 | DMI–LiNO3 | La | [24] |
| Nd | [25,26] | ||
| 1,3-dimethyl-2-imidazolinone–ZnCl2 | DMI−ZnCl2 | Zn | [27] |
| Nd | [26] | ||
| 1-alkyl-3-methylimidazolium bromide | EMIBr | Au | [28] |
| Ag | [29] | ||
| 1-allyl-3-methylimidazolium bromide | AMIBr | Zn | [30] |
| Pt | [31] | ||
| 1-butyl-1-methyl-pyrrolidinium bis(tri-fluoromethylsulfonyl) imide | [Bmim]TFSA or [BMPTFSA] or BMPyrrFSI | Cu | [32] |
| Co | [33] | ||
| Zn | [34] | ||
| Al–Co | [35] | ||
| Al–Cu | [35] | ||
| Pr | [36] | ||
| Bi | [36] | ||
| Ti | [37] | ||
| Ta | [38] | ||
| 1-butyl-1-methylpyrrolidinium dicyanamide | [BMP][DCA] | Sm | [39] |
| Ga | [40] | ||
| Au | [41] | ||
| Eu | [42] | ||
| Cr | [43] | ||
| Ag | [41,44,45] | ||
| 1-butyl-1-methylpyrrolidinium triflate | BMPyOTf | Dy | [46] |
| 1-butyl-1-methylpyrrolidinium trifluoromethylsulfonate | [BMIM][OTf] or [Py1,4]TfO | Fe–Cu | [41] |
| Fe–Al | [47] | ||
| 1-butyl-3-(1-methylimidazolium-3-hexyl) imidazolium bromide | Ag | [29] | |
| 1-butyl-3-benzimidazolium bromate | [HBBIm]Br | Pd | [48] |
| Pt | [48] | ||
| 1-butyl-3-butyillimidazolium bromide | DBIz–Br | Ag | [22,29] |
| 1-butyl-3-methylimidazolium–hydrogen sulfate | [BMIM]HSO4 | Ni–Fe | [49] |
| 1-butyl-3-methylimidazolium acetate | [Bmim][Ac] | Cd–Te | [50] |
| 1-butyl-1-methylpyrrolidinium dicyanamide | [BMP][DCA] | Ag | [41,44] |
| Eu | [42] | ||
| Sm | [39] | ||
| Cr | [43] | ||
| Ga–Sb | [40] | ||
| 1-butyl-1-methylpyrrolidinium triflate | BMPyNTf | Dy | [46,51] |
| 1-butyl-3-methylpyrrolidinium dicyanamide | [BMIm][DCA] | Ag | [41] |
| 1-butyl-3-methylimidazolium bis(triflyl)imide | [BMIM][TFI] or C4mimTFSA | Ni–Fe | [52] |
| Cu | [32] | ||
| 1-butyl-3-methylimidazolium bromide | [Bmim][Br] | Ag | [29,53] |
| 1-butyl-3-methylimidazolium chloride | [Bmim][Cl] or [BMIC] or [C4mimCl] | Al | [54,55,56,57,58,59,60,61] |
| Al–Ti | [62] | ||
| Ti–Al | [63] | ||
| Ag–Pd | [64] | ||
| Cu–Sn | [65,66] | ||
| Cu–Ag | [67] | ||
| Pd | [68] | ||
| 1-butyl-3-methylimidazolium hexafluorophosphate | [Bmim]PF6 | Ru | [69] |
| Ir | [70,71] | ||
| 1-butyl-3-methylimidazolium tetrafluoroborate | [BMIm][BF4] | Co | [72] |
| Ir | [73] | ||
| Ce | [74] | ||
| 1-butyl-3-methylimidazolium trifluoromethanesulfonate | [Bmim][TfO] | Zn | [75] |
| Sn | |||
| 1-butyl-3-methylimidazolium dicyanamide | [BMIm][DCA] | Ag | [41] |
| Au | [41] | ||
| Cu–Sn | [76] | ||
| 1-butylimidazolium bromide | [HBIm][B] | Ag | [53] |
| 1-decyl-3-(1-methylimidazolium-3-hexyl) imidazolium bromide | Ag | [29] | |
| 1-dodecyl-3-methylimidazolium chloride | [C12mim][Cl] | Ni–Fe–Mo | [77] |
| Ni–Fe–W | |||
| 1-ethyl-3-methyl imidazolium bromide | [EMIM] [Br] | Ni–Mo | [78,79] |
| 1-ethyl-3-methylimidazolium chloride | [EMIM][Cl] or [EMIC] or [C2mimCl] | Zn | [80] |
| Nd–Fe | [81] | ||
| Al | [11,54,82,83,84,85,86,87,88,89,90] | ||
| Al–Li | [91] | ||
| Al–Ga | [92] | ||
| Al–Mn–Zr | [93] | ||
| Al–Ti | [94] | ||
| Al–W | [95,96,97] | ||
| Al–Mn | [98] | ||
| Co–Zn | [99] | ||
| Cu | [100] | ||
| Cu–Sn | [101] | ||
| Ga | [102] | ||
| Pd | [103,104] | ||
| 1-ethyl-3-methylimidazolium chloride-ethylene glycol | EMIC–EG | Ni–La | [105] |
| 1-ethyl-3-methylimidazolium chloride–urea | EMIC–UA | Cu | [100] |
| 1-ethyl-3-methylimidazolium dicyanamide | [EMIM][DCA] | Cu–Sn | [76] |
| Nd | [81,106] | ||
| Fe | [81,106] | ||
| Nd–Fe | [81] | ||
| 1-ethyl-3-methylimidazolium trifluoroacetate | [Emim]TA | Pd | [107] |
| 1-ethyl-3-methylimidazolium trifluoromethylsulfonate | [EMIm][TfO] | Cu–Zn | [108] |
| Cu | [109] | ||
| Ag | [109] | ||
| 1-ethyl-3-methylimidazolium bis(trifluoromethylsulfonyl)imide | [C4mim][NTf2] | Ga | [102] |
| Pd | [110] | ||
| 1-ethyl-3-methylimidazolium bis(fluorosulfonyl)amide | [C2C1im][FSA] | Na | [111] |
| 1-ethyl-3-methylimidazolium fluoride | [EMIM]F | Cu | [112] |
| 1-heptyl-3-(1-methylimidazolium-3-hexyl) imidazolium bromide | Ag | [29] | |
| 1-hexyl-3-methyl-imidazolium bromide | [HMI][Br] | Ag | [29] |
| 1-hexyl-3-methyl-imidazolium chloride | [HMI][Cl] | Al | [54] |
| 1-ethyl-1-methylpyrrolidinium bis(trifluoromethylsulfonyl) imide | [EMIm][TFSA] | Cu | [113] |
| Cu–Sn | [114] | ||
| Zn | [34,115,116] | ||
| 1-hexyl-3-methylimidazolium hydrogen sulfate | [HMIM][HSO4] | Ag | [117] |
| 1-Methylpiperidinium trifluoromethane sulphonate | [HmPip][OTf] or [MIMTfO] | Cu | [118] |
| Zn | [119] | ||
| 1-octyl-3-methylimidazolium bromide | Ag | [29] | |
| 1-propyl-1-methylpyrrolidinium bis(trifluoromethanesulfonyl)amide | [C3mpyr][TFSA] | Co | [120] |
| 1-tetradecyl-2-aminoethyl imidazolium bromide | [C14PImNH2]Br | Pt | [121] |
| 2,2-diheptyl-1,1,3,3-tetramethylguanidinium bromide | Pt | [122] | |
| 3- butyl-1- ethylimidazolium | Al | [61,123] | |
| Aluminium chloride–4-ethylpyridine | AlCl3–4-EP | Al | [124] |
| Aluminium chloride–triethylamine hydrochloride | AlCl3–TMHC | Al–Zr–Cu | [125] |
| Co–Al | [126] | ||
| Betainium bis((trifluoromethyl)sulfonyl)amide | [Hbet][Tf2N] or [Hbet][TFSA] | Cu | [127] |
| Cu–Pb | [127,128] | ||
| Zn | [129] | ||
| Pb | [129,130] | ||
| Pd | [131] | ||
| Butylpyridinium dicyanamide | Pyri4–DCA | Ag | [45] |
| Choline chloride–ethylene glycol | ChCl–EG | Fe–Cr | [132] |
| Ni–Fe | [133] | ||
| Ni–Sn | [134] | ||
| Ni | [12,18,135,136] | ||
| Ni–Sn–P | [137] | ||
| Co | [138,139] | ||
| Ni–Co | [15] | ||
| Cu | [118,136,140] | ||
| Sn | [136] | ||
| Zn | [17,141,142,143] | ||
| Au | [144] | ||
| Ag | [136,144] | ||
| Mn | [14] | ||
| Choline chloride–Urea | ChCl–UA | Ni | [12,18,145] |
| Co | [139] | ||
| Ni–Co | [13] | ||
| Zn | [17] | ||
| Mn | [14] | ||
| Pr–Mg–Co | [146] | ||
| Pr–Mg–Ni | [147] | ||
| Sn–Co–Ni | [148] | ||
| Sn–Co–Zn | |||
| Choline chloride–malonic acid | ChCl–MA | Co | [139] |
| Co–Cr | [149] | ||
| Choline chloride–oxalic acid | ChCl–OC | Co | [139] |
| Dibutylpyrrolidinium bromide | DBP1–Br | Ag | [22] |
| Ethylene carbonate–Aluminum chloride | EC–AlCl3 | Al–Li | [150] |
| Li | [150] | ||
| Lithium–bis(trifluoromethylsulfonyl)amide | Li–TFSI | Li | [151,152,153] |
| Lithium–bis(fluorosulfonyl)imide | Li–FSI | Li | [153] |
| Perfluoro-3-oxa-4,5 dichloro-pentan-sulphonate | Al | [154] | |
| poly(1-allyl-3-methylimidazolium) | PAMI | Pt | [31] |
| Tetramethyl guanidinium-perfluoro-3-oxa-4,5 dichloro-pentan-sulphonate | [C5H14N3+][CF2ClCFClOCF2CF2SO3−] | Al | [154,155] |
| Tributylhexylphosphonium bis(trifluoromethyl sulfonyl)imide | [P4446][NTf2] | Li | [156] |
| Triethylammonium acetate | [TEAA] | Ag–Cu | [157] |
| Triethyl-n-pentyl phosphonium bis(trifluoromethyl-sulfonyl)amide | [P2225][TFSA] | Ru | [158] |
| Triethyl-n-hexyl phosphonium bis(trifluoromethyl-sulfonyl)amide | [P2226][TFSA] | Pt | [159] |
| Trihexyltetradecylphosphonium bis(2,4,4-trimethylpentyl)phosphinate | (Cyphos IL 104®) | Rh | [160] |
| Trihexyltetradecylphosphonium bis(trifluoromethylsulfonyl)amide | [P6,6,6,14][TFSI] | Nd | [161] |
| Trihexyl(tetradecyl)phosphonium chloride | (Cyphos IL 101) | Ir | [162] |
| N-butyl-N-methylpyrrolidinium bistriflimide | BMPTFSI | La | [163] |
| Sm | |||
| Nd | |||
| Dy | |||
| N-butyl-N-methyl pyrrolidinium dicyanamide | BMP–DCA or [C4mpyr][DCA] | Ni–La | [164] |
| Nd | [165] | ||
| N-methyl-N-butyl-pyrrolidinium bis(trifluoromethanesulfonyl)imide | [C4mpyr][Tf2N] | Li | [166] |
| N-methyl-N-propylpiperidinium bis(trifluoromethanesulfonyl)imide | [MPPip][TFSI] | Zn | [167] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Costa, J.G.d.R.d.; Costa, J.M.; Almeida Neto, A.F.d. Progress on Electrodeposition of Metals and Alloys Using Ionic Liquids as Electrolytes. Metals 2022, 12, 2095. https://doi.org/10.3390/met12122095
Costa JGdRd, Costa JM, Almeida Neto AFd. Progress on Electrodeposition of Metals and Alloys Using Ionic Liquids as Electrolytes. Metals. 2022; 12(12):2095. https://doi.org/10.3390/met12122095
Chicago/Turabian StyleCosta, Javan Grisente dos Reis da, Josiel Martins Costa, and Ambrósio Florêncio de Almeida Neto. 2022. "Progress on Electrodeposition of Metals and Alloys Using Ionic Liquids as Electrolytes" Metals 12, no. 12: 2095. https://doi.org/10.3390/met12122095