A Review of Biomaterials Based on High-Entropy Alloys
Abstract
:1. Introduction
2. Microstructure and Mechanical Performance
2.1. BioHEAs with Single-Phase BCC
2.2. BioHEAs with Dual Phase BCC
2.3. BioHEAs with Single-Phase FCC or Dual FCC
2.4. BioHEAs with Amorphous Phase
2.5. Other Phases Obtained with bioHEAs
3. Biological and Chemical Properties
3.1. Anticorrosive Performance
3.2. Cell Viability
3.3. Antimicrobial Activity
3.4. Magnetic Susceptibility
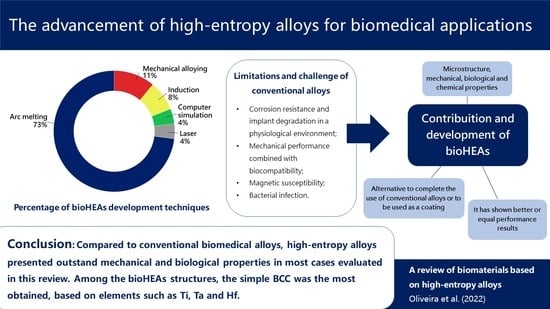
4. Conclusions
- Among the bioHEAs structures, the simple BCC was the most obtained, based on elements such as Ti, Ta, and Hf;
- Although composition is relevant to material hardness, the processing and heat treatment of bioHEAs proved to be more influential for this property. Amorphous HEAs that were used as a coating on conventional alloy substrates showed high hardness [58]. On the other hand, the most suitable Young’s modulus for biomedical applications was found in BCC structures [19,48];
- It is noteworthy that Ti, Nb, and Ta hinder the corrosion dissolution [66,69], allowing greater resistance. In conjunction with this factor, the formation of a protective oxide layer helped in the performance against corrosion [12,45,62,64]. For antibacterial characteristics, Ag, Cu, and Zn are promising elements that can bring good results [9,67];
- Compared to conventional biomedical alloys, high-entropy alloys presented interesting mechanical, chemical, and biological properties in most cases evaluated in this review;
- In the biocompatibility analyses, the predominance of corrosive tests of the alloy was verified. Antibacterial performance, viability, cell density and adhesion, and magnetic susceptibility are other assays performed for bioHEAs;
- Because the study of high-entropy alloys for biomedical applications is a relatively new and current topic, there is a need to evaluate the biological influence of these alloys in long-term applications.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| HEA | High-entropy alloy |
| bioHEA | bio-high-entropy alloy |
| SLM | Selective laser melting |
| BCC | Body-centered cubic |
| HPT | High pressure torsion |
| HT | Heat treatment |
| VAM | Vacuum arc melting |
| VAR | Vacuum arc remelting |
| SPS | Spark plasma sintering |
| DFT | Density functional theory |
| SEM | Scanning electron microscopy |
| SFP | Stationary friction processing |
| FCC | Face centered cubic |
| LPS | Liquid phase separation |
| HCP | Hexagonal close-packed |
| CP | Primitive cubic |
| PBS | Phosphate buffer solution |
| FBS | Fetal bovine serum |
| SBF | Simulated body fluid |
| AS | Artificial saliva |
References
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A.J. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Yeh, J.W. Alloy design strategies and future trends in high-entropy alloys. Jom 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Gao, M.C.; Yeh, J.W.; Liaw, P.K.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Mishra, R.K.; Kumari, P.; Gupta, A.K.; Shahi, R.R. Design and development of Co35Cr5Fe20- xNi20+ xTi20 High Entropy Alloy with excellent magnetic softness. J. Alloys Compd. 2021, 889, 161773. [Google Scholar] [CrossRef]
- Senkov, O.N.; Miracle, D.B.; Chaput, K.J.; Couzinie, J.P. Development and exploration of refractory high entropy alloys—A review. J. Mater. Res. 2018, 33, 3092–3128. [Google Scholar] [CrossRef] [Green Version]
- Murty, B.S.; Yeh, J.W.; Ranganathan, S.; Bhattacharjee, P.P. High-Entropy Alloys, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Gao, J.; Jin, Y.; Fan, Y.; Xu, D.; Meng, L.; Wang, C.; Yu, Y.; Zhang, D.; Wang, F. Fabricating antibacterial CoCrCuFeNi high-entropy alloy via selective laser melting and in-situ alloying. J. Mater. Sci. Technol. 2022, 102, 159–165. [Google Scholar] [CrossRef]
- Hori, T.; Nagase, T.; Todai, M.; Matsugaki, A.; Nakano, T. Development of non-equiatomic TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 2019, 172, 83–87. [Google Scholar] [CrossRef]
- Nagase, T.; Mizuuchi, K.; Nakano, T. Solidification microstructures of the ingots obtained by arc melting and cold crucible levitation melting in TiNbTaZr medium-entropy alloy and TiNbTaZrX (X = V, Mo, W) high-entropy alloys. Entropy 2019, 21, 483. [Google Scholar] [CrossRef] [Green Version]
- Motallebzadeh, A.; Peighambardoust, N.S.; Sheikh, S.; Murakami, H.; Guo, S.; Canadinc, D. Microstructural, mechanical and electrochemical characterization of TiZrTaHfNb and Ti1.5ZrTa0.5Hf0.5Nb0.5 refractory high-entropy alloys for biomedical applications. Intermetallics 2019, 113, 106572. [Google Scholar] [CrossRef]
- Shittu, J.; Pole, M.; Cockerill, I.; Sadeghilaridjani, M.; Reddy, L.V.K.; Manivasagam, G.; Singh, H.; Grewal, H.S.; Arora, H.S.; Mukherjee, S. Biocompatible high entropy alloys with excellent degradation resistance in a simulated physiological environment. ACS Appl. Bio Mater. 2020, 3, 8890–8900. [Google Scholar] [CrossRef] [PubMed]
- Tüten, N.; Canadinc, D.; Motallebzadeh, A.; Bal, B. Microstructure and tribological properties of TiTaHfNbZr high entropy alloy coatings deposited on Ti6Al4V substrates. Intermetallics 2019, 105, 99–106. [Google Scholar] [CrossRef]
- Ishimoto, T.; Ozasa, R.; Nakano, K.; Weinmann, M.; Schnitter, C.; Stenzel, M.; Matsugaki, A.; Nagase, T.; Matsuzaka, T.; Todai, M.; et al. Development of TiNbTaZrMo bio-high entropy alloy (BioHEA) super-solid solution by selective laser melting, and its improved mechanical property and biocompatibility. Scr. Mater. 2021, 194, 113658. [Google Scholar] [CrossRef]
- Alagarsamy, K.; Fortier, A.; Komarasamy, M.; Kumar, N.; Mohammad, A.; Banerjee, S.; Han, H.C.; Mishra, R.S. Mechanical properties of high entropy alloy Al0.1CoCrFeNi for peripheral vascular stent application. Cardiovasc. Eng. Technol. 2016, 7, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Narushima, T. New-generation metallic biomaterials. In Metals for Biomedical Devices; Elsevier: Amsterdam, The Netherlands, 2019; pp. 495–521. [Google Scholar]
- Akmal, M.; Park, H.K.; Ryu, H.J. Plasma spheroidized MoNbTaTiZr high entropy alloy showing improved plasticity. Mater. Chem. Phys. 2021, 273, 125060. [Google Scholar] [CrossRef]
- Berger, J.E.; Jorge, A.M., Jr.; Asato, G.H.; Roche, V. Formation of self-ordered oxide nanotubes layer on the equiatomic TiNbZrHfTa high entropy alloy and bioactivation procedure. J. Alloys Compd. 2021, 865, 158837. [Google Scholar] [CrossRef]
- Hua, N.; Wang, W.; Wang, Q.; Ye, Y.; Lin, S.; Zhang, L.; Guo, Q.; Brechtl, J.; Liaw, P.K. Mechanical, corrosion, and wear properties of biomedical TiZrNbTaMo high entropy alloys. J. Alloys Compd. 2021, 861, 157997. [Google Scholar] [CrossRef]
- Aksoy, C.B.; Canadinc, D.; Yagci, M.B. Assessment of Ni ion release from TiTaHfNbZr high entropy alloy coated NiTi shape memory substrates in artificial saliva and gastric fluid. Mater. Chem. Phys. 2019, 236, 121802. [Google Scholar] [CrossRef]
- Perumal, G.; Grewal, H.S.; Pole, M.; Reddy, L.V.K.; Mukherjee, S.; Singh, H.; Manivasagam, G.; Arora, H.S. Enhanced biocorrosion resistance and cellular response of a dual-phase high entropy alloy through reduced elemental heterogeneity. ACS Appl. Bio Mater. 2020, 3, 1233–1244. [Google Scholar] [CrossRef]
- Yuan, Y.; Wu, Y.; Yang, Z.; Liang, X.; Lei, Z.; Huang, H.; Wang, H.; Liu, X.; An, K.; Wu, W.; et al. Formation, structure and properties of biocompatible TiZrHfNbTa high-entropy alloys. Mater. Res. Lett. 2019, 7, 225–231. [Google Scholar] [CrossRef] [Green Version]
- Calin, M.; Vishnu, J.; Thirathipviwat, P.; Popa, M.M.; Krautz, M.; Manivasagam, G.; Gebert, A. Tailoring biocompatible TiZrNbHfSi metallic glasses based on high-entropy alloys design approach. Mater. Sci. Eng. C 2021, 121, 111733. [Google Scholar] [CrossRef] [PubMed]
- Gurel, S.; Nazarahari, A.; Canadinc, D.; Cabuk, H.; Bal, B. Assessment of biocompatibility of novel TiTaHf-based high entropy alloys for utility in orthopedic implants. Mater. Chem. Phys. 2021, 266, 124573. [Google Scholar] [CrossRef]
- Kokubo, T. Bioceramics and Their Clinical Applications; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Saini, M.; Singh, Y.; Arora, P.; Arora, V.; Jain, K. Implant biomaterials: A comprehensive review. World J. Clin. Cases WJCC 2015, 3, 52. [Google Scholar] [CrossRef] [PubMed]
- Murphy, W.; Black, J.; Hastings, G.W. Handbook of Biomaterial Properties, 2nd ed.; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Chen, Q.; Thouas, G. Biomaterials: A Basic Introduction; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Wagner, W.R.; Sakiyama-Elbert, S.E.; Zhang, G.; Yaszemski, M.J. Biomaterials Science: An Introduction to Materials in Medicine, 4th ed.; Academic Press: Cambridge, MA, USA, 2020. [Google Scholar]
- Leyens, C.; Peters, M. Titanium and Titanium Alloys: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2003. [Google Scholar]
- Ratner, B.D.; Hoffman, A.S.; Schoen, F.J.; Lemons, J.E. Biomaterials Science: An Introduction to Materials in Medicine; Elsevier Academic Press: San Diego, CA, USA, 2004. [Google Scholar]
- Davis, R.; Singh, A.; Jackson, M.J.; Coelho, R.T.; Prakash, D.; Charalambous, C.P.; Ahmed, W.; da Silva, L.R.R.; Lawrence, A.A. A comprehensive review on metallic implant biomaterials and their subtractive manufacturing. Int. J. Adv. Manuf. Technol. 2022, 1–58. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer IARC Monographs on the Identification of Carcinogenic Hazards to Humans. Available online: https://monographs.iarc.who.int/list-of-classifications/ (accessed on 20 September 2022).
- Castro, D.; Jaeger, P.; Baptista, A.; Oliveira, J. An overview of high-entropy alloys as biomaterials. Metals 2021, 11, 648. [Google Scholar] [CrossRef]
- Ahmady, A.R.; Ekhlasi, A.; Nouri, A.; Nazarpak, M.H.; Gong, P.; Solouk, A. High entropy alloy coatings for biomedical applications: A review. Smart Mater. Manuf. 2022, 1, 100009. [Google Scholar] [CrossRef]
- Gurel, S.; Yagci, M.; Canadinc, D.; Gerstein, G.; Bal, B.; Maier, H. Fracture behavior of novel biomedical Ti-based high entropy alloys under impact loading. Mater. Sci. Eng. A 2021, 803, 140456. [Google Scholar] [CrossRef]
- Yang, W.; Pang, S.; Liu, Y.; Wang, Q.; Liaw, P.K.; Zhang, T. Design and properties of novel Ti-Zr-Hf-Nb-Ta high-entropy alloys for biomedical applications. Intermetallics 2022, 141, 107421. [Google Scholar] [CrossRef]
- González-Masís, J.; Cubero-Sesin, J.M.; Campos-Quirós, A.; Edalati, K. Synthesis of biocompatible high-entropy alloy TiNbZrTaHf by high-pressure torsion. Mater. Sci. Eng. A 2021, 825, 141869. [Google Scholar] [CrossRef]
- Bhandari, U.; Ghadimi, H.; Zhang, C.; Gao, F.; Yang, S.; Guo, S. Computational exploration of biomedical HfNbTaTiZr and Hf0.5Nb0.5Ta0.5Ti1.5Zr refractory high-entropy alloys. Mater. Res. Express 2021, 8, 096534. [Google Scholar] [CrossRef]
- Akmal, M.; Hussain, A.; Afzal, M.; Lee, Y.I.; Ryu, H.J. Systematic study of (MoTa)xNbTiZr medium and high-entropy alloys for biomedical implants in vivo biocompatibility examination. J. Mater. Sci. Technol. 2021, 78, 183–191. [Google Scholar] [CrossRef]
- Nagase, T.; Iijima, Y.; Matsugaki, A.; Ameyama, K.; Nakano, T. Design and fabrication of TiZrHfCrMo and TiZrHfCoCrMo high-entropy alloys as metallic biomaterials. Mater. Sci. Eng. C 2020, 107, 110322. [Google Scholar] [CrossRef] [PubMed]
- Málek, J.; Zỳka, J.; Lukáč, F.; Vilémová, M.; Vlasák, T.; Čížek, J.; Melikhova, O.; Macháčková, A.; Kim, H.S. The effect of processing route on properties of HfNbTaTiZr high entropy alloy. Materials 2019, 12, 4022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savin, A.; Craus, M.L.; Bruma, A.; Novy, F.; Malo, S.; Chlada, M.; Steigmann, R.; Vizureanu, P.; Harnois, C.; Turchenko, V.; et al. Microstructural analysis and mechanical properties of TiMo20Zr7Ta15Six alloys as biomaterials. Materials 2020, 13, 4808. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Liu, Y.; Pang, S.; Liaw, P.K.; Zhang, T. Bio-corrosion behavior and in vitro biocompatibility of equimolar TiZrHfNbTa high-entropy alloy. Intermetallics 2020, 124, 106845. [Google Scholar] [CrossRef]
- Gurel, S.; Yagci, M.; Bal, B.; Canadinc, D. Corrosion behavior of novel Titanium-based high entropy alloys designed for medical implants. Mater. Chem. Phys. 2020, 254, 123377. [Google Scholar] [CrossRef]
- Li, C.; Ma, Y.; Yang, X.; Hou, M. New TiTaNbZrMo high-entropy alloys for metallic biomaterials. Mater. Res. Express 2021, 8, 105403. [Google Scholar] [CrossRef]
- Normand, J.; Moriche, R.; García-Garrido, C.; Sepúlveda Ferrer, R.E.; Chicardi, E. Development of a TiNbTaMoZr-based high entropy alloy with low Young’s modulus by mechanical alloying route. Metals 2020, 10, 1463. [Google Scholar] [CrossRef]
- Koval, N.E.; Juaristi, J.I.; Muiño, R.D.; Alducin, M. Elastic properties of the TiZrNbTaMo multi-principal element alloy studied from first principles. Intermetallics 2019, 106, 130–140. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.P.; Xu, J. TiZrNbTaMo high-entropy alloy designed for orthopedic implants: As-cast microstructure and mechanical properties. Mater. Sci. Eng. C 2017, 73, 80–89. [Google Scholar] [CrossRef]
- Nagase, T.; Todai, M.; Hori, T.; Nakano, T. Microstructure of equiatomic and non-equiatomic TiNbTaZrMo high-entropy alloys for metallic biomaterials. J. Alloys Compd. 2018, 753, 412–421. [Google Scholar] [CrossRef]
- Navi, A.S.; Haghighi, S.E.; Haghpanahi, M.; Momeni, A. Investigation of microstructure and corrosion of TiNbTaZrMo high-entropy alloy in the simulated body fluid. J. Bionic Eng. 2021, 18, 118–127. [Google Scholar] [CrossRef]
- Todai, M.; Nagase, T.; Hori, T.; Matsugaki, A.; Sekita, A.; Nakano, T. Novel TiNbTaZrMo high-entropy alloys for metallic biomaterials. Scr. Mater. 2017, 129, 65–68. [Google Scholar] [CrossRef] [Green Version]
- Song, H.; Lee, S.; Lee, K. Thermodynamic parameters, microstructure, and electrochemical properties of equiatomic TiMoVWCr and TiMoVNbZr high-entropy alloys prepared by vacuum arc remelting. Int. J. Refract. Met. Hard Mater. 2021, 99, 105595. [Google Scholar] [CrossRef]
- Chang, S.H.; Wu, S.K.; Liao, B.S.; Su, C.H. Selective leaching and surface properties of CoNiCr-based medium-high-entropy alloys. Appl. Surf. Sci. 2020, 515, 146044. [Google Scholar] [CrossRef]
- Zhou, E.; Qiao, D.; Yang, Y.; Xu, D.; Lu, Y.; Wang, J.; Smith, J.A.; Li, H.; Zhao, H.; Liaw, P.K.; et al. A novel Cu-bearing high-entropy alloy with significant antibacterial behavior against corrosive marine biofilms. J. Mater. Sci. Technol. 2020, 46, 201–210. [Google Scholar] [CrossRef]
- Nagase, T.; Todai, M.; Nakano, T. Liquid phase separation in AgCoCrFeMnNi, CoCrCuFeMnNi and CoCrCuFeMnNiB high entropy alloys for biomedical application. Crystals 2020, 10, 527. [Google Scholar] [CrossRef]
- Alamdari, A.A.; Unal, U.; Motallebzadeh, A. Investigation of microstructure, mechanical properties, and biocorrosion behavior of Ti1.5ZrTa0.5Nb0.5W0.5 refractory high-entropy alloy film doped with Ag nanoparticles. Surfaces Interfaces 2022, 28, 101617. [Google Scholar] [CrossRef]
- Peighambardoust, N.S.; Alamdari, A.A.; Unal, U.; Motallebzadeh, A. In vitro biocompatibility evaluation of Ti1.5ZrTa0.5Nb0.5Hf0.5 refractory high-entropy alloy film for orthopedic implants: Microstructural, mechanical properties and corrosion behavior. J. Alloys Compd. 2021, 883, 160786. [Google Scholar] [CrossRef]
- Motallebzadeh, A.; Yagci, M.; Bedir, E.; Aksoy, C.; Canadinc, D. Mechanical properties of TiTaHfNbZr high-entropy alloy coatings deposited on NiTi shape memory alloy substrates. Metall. Mater. Trans. A 2018, 49, 1992–1997. [Google Scholar] [CrossRef]
- Liu, D.; Ma, Z.; Zhao, H.; Ren, L.; Zhang, W. Nano-indentation of biomimetic artificial bone material based on porous Ti6Al4V substrate with Fe22Co22Ni22Ti22Al12 high entropy alloy coating. Mater. Today Commun. 2021, 28, 102659. [Google Scholar] [CrossRef]
- Socorro-Perdomo, P.; Florido-Suárez, N.; Voiculescu, I.; Mirza-Rosca, J. Comparative EIS study of AlxCoCrFeNi alloys in Ringer’s solution for medical instruments. Metals 2021, 11, 928. [Google Scholar] [CrossRef]
- Ríos, M.L.; Perdomo, P.S.; Voiculescu, I.; Geanta, V.; Crăciun, V.; Boerasu, I.; Rosca, J.M. Effects of nickel content on the microstructure, microhardness and corrosion behavior of high-entropy AlCoCrFeNix alloys. Sci. Rep. 2020, 10, 21119. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, X.; Liu, Q. A novel biomedical high-entropy alloy and its laser-clad coating designed by a cluster-plus-glue-atom model. Mater. Des. 2020, 196, 109085. [Google Scholar] [CrossRef]
- Edalati, P.; Floriano, R.; Tang, Y.; Mohammadi, A.; Pereira, K.D.; Luchessi, A.D.; Edalati, K. Ultrahigh hardness and biocompatibility of high-entropy alloy TiAlFeCoNi processed by high-pressure torsion. Mater. Sci. Eng. C 2020, 112, 110908. [Google Scholar] [CrossRef]
- Razazzadeh, A.; Atapour, M.; Enayati, M.H. Corrosion characteristics of TiNbMoMnFe high entropy thin film deposited on AISI316L for biomedical applications. Met. Mater. Int. 2021, 27, 2341–2352. [Google Scholar] [CrossRef]
- Rodrigues, J.F.Q.; Padilha, G.S.; Bortolozo, A.D.; Osorio, W.R. Effect of sintering time on corrosion behavior of an AgAlNbTiZn alloy system. J. Alloys Compd. 2020, 834, 155039. [Google Scholar] [CrossRef]
- Eliaz, N. Corrosion of metallic biomaterials: A review. Materials 2019, 12, 407. [Google Scholar] [CrossRef]
- Song, Q.; Xu, Y.; Xu, J. Dry-Sliding wear behavior of (TiZrNbTa)90Mo10 high-entropy alloy against Al2O3. Acta Metall. Sin. 2020, 56, 1507–1520. [Google Scholar]
- Iijima, Y.; Nagase, T.; Matsugaki, A.; Wang, P.; Ameyama, K.; Nakano, T. Design and development of TiZrHfNbTaMo high-entropy alloys for metallic biomaterials. Mater. Des. 2021, 202, 109548. [Google Scholar] [CrossRef]
- Zhang, E.; Zhao, X.; Hu, J.; Wang, R.; Fu, S.; Qin, G. Antibacterial metals and alloys for potential biomedical implants. Bioact. Mater. 2021, 6, 2569–2612. [Google Scholar] [CrossRef] [PubMed]










| Young’s Modulus (GPa) [Ref.] | Hardness (HV) [Ref.] | Yield Strength (MPa) [Ref.] | Tensile Strength (MPa) [Ref.] | |
|---|---|---|---|---|
| Cortical bone | 10–30 [17,29] | - | 100–200 [29] | - |
| cp-Ti | 90–110 [17] | 120–200 [30] | 170–310 [31] | >240 [31] |
| Ti6Al4V | 100–110 [28] | 310 [30] | 850–900 [32] | 860 [32] |
| 316L | 200 [29] | 130-160 [33] | 200–700 [29] | 480–1000 [30] |
| CoCrMo alloy | 240 [29] | 298 [33] | 450–1500 [29] | 655–1192 [32] |
| Elements | Ti | Zr | Nb | Ta | Mo | Hf | Fe | Cr | Co |
|---|---|---|---|---|---|---|---|---|---|
| Recurrence | 57 | 49 | 48 | 44 | 28 | 27 | 12 | 12 | 12 |
| Alloy | Route/Method | Post-Processing | Hardness (HV) | Young’s Modulus (GPa) | Reference |
|---|---|---|---|---|---|
| (TiZrHf)(NbTa) | Arc melting | HT 1200 °C-24 h | 287–293 | 56–68 | [38] |
| TiNbZrHfTa | Arc melting | HT 790 °C-1 h | - | 66 | [19] |
| TiTaHfNb | Arc melting | - | - | 112 | [37] |
| TiTaHfNbZr | - | 132 | |||
| TiTaHfMoZr | - | 159 | |||
| TiNbZrTaHf | Mechanical alloying | - | 564 | 79 | [39] |
| HfNbTaTiZr | Computational method | - | 297 | 97 | [40] |
| HfNbTaTiZr | 253 | 86 | |||
| (MoTa)NbTiZr | VAM | HT 1400 °C-4 h | 380–430 | 113–125 | [41] |
| TiNbTaZrMo | SLM | - | - | 140 | [15] |
| TiZrHfCrMo | Arc melting | - | 531 | - | [42] |
| TiZrHfCoCrMo | 532 | - | |||
| HfNbTaTiZr | Arc melting | - | 320 | 112 | [12] |
| HfNbTaTiZr | 307 | 98 | |||
| TaNbHfZrTi | Arc melting | - | - | 73–103 | [23] |
| HfNbTaTiZr | Powder techniques | HPT (2.5 GPa) | 410 | - | [43] |
| TiMoZrTaSi | VAR | - | 337 | 89 | [44] |
| TiMoZrTaSi | 355 | 69 | |||
| TiMoZrTaSi | 356 | 79 | |||
| TiZrHfNbTa | Arc melting | - | - | - | [45] |
| Alloy | Route/Method | Hardness (HV) | Young’s Modulus (GPa) | Reference |
|---|---|---|---|---|
| (MoTa)NbTiZr | VAM | 480 | 140 | [41] |
| MoTaNbTiZr | 510 | 160 | ||
| TiZrNbTaMo | Arc melting | 430–490 | - | [20] |
| TiZrNbTaMo | Induction | 619 | - | [47] |
| Ti(NbTaZr)Mo | 487 | - | ||
| MoNbTaTiZr | VAM | 657 | 164 | [13] |
| TiNbTaMoZr | Mechanical alloying | 591 | 62 | [48] |
| TiZrNbTaMo | Computational method | - | 122–144 | [49] |
| TiZrNbTaMo | Arc melting | 500 | 153 | [50] |
| Alloy | Route/Method | Phases | Reference |
|---|---|---|---|
| CoCrFeCuNi | SLM | FCC | [9] |
| FeCoNiCrPd | VAR | FCC | [55] |
| AlCoCrFeNi | Arc melting | FCC | [16] |
| AlCoCrCuFeNi | Induction | Double FCC | [56] |
| AgCoCrFeMnNi | Arc melting | Double FCC | [57] |
| CuCoCrFeMnNi | |||
| CoCrCuFeMnNi | |||
| CoCrCuFeMnNi | |||
| CoCrCuFeMnNiB | |||
| CoCrCuFeMnNiB | |||
| CoCrCuFeMnNiB |
| Film | Route | Substrate | Hardness (HV) | Young’s Modulus (GPa) | Reference |
|---|---|---|---|---|---|
| TiZrTaNbHf | Arc melting | 316L | 1165 | 180 | [59] |
| CoCrMo | 1172 | 185 | |||
| Ti6Al4V | 1168 | 183 | |||
| TiTaHfNbZr | VAM | Ti6Al4V | 1276 | 181 | [14] |
| TiTaHfNbZr | Arc melting | NiTi | 1285 | 183 | [60] |
| NiTi | 1132 | 173 | |||
| TiTaHfNbZr | VAM | NiTi | 1285 | 183 | [21] |
| TiZrTaNbW | Arc melting | Ti6Al4V | 1835 | 210 | [58] |
| HEA-9Ag NPs | Ti6Al4V | 1631 | 200 |
| Alloy | Route | Structure/Phase | Hardness (HV) | Young’s Modulus (GPa) | Reference |
|---|---|---|---|---|---|
| FeCoNiTiAl (0.5 h ) | - | BCC and FCC | 867 | 100 | [61] |
| 1 h | 2294 | 132 | |||
| 2 h | 1754 | 120 | |||
| 3 h | 1479 | 112 | |||
| AlCoCrFeNi | VAR | BCC and FCC | 245 | - | [62] |
| AlCoCrFeNi | 427 | - | |||
| AlCoCrFeNi | 562 | - | |||
| AlCrFeCoNi | VAR | cP | 562 | - | [63] |
| AlCoCrFeNi | FCC and cP | 455 | - | ||
| AlCoCrFeNi | FCC and cP | 316 | - | ||
| (TiZrNb)SnMo | VAM | BCC and HCP | 551 | 110 | [64] |
| HEA coating | 584 | 89 | |||
| TiAlFeCoNi | Arc melting | BCC and L2 | 635 | 250 | [65] |
| HEA-HPT | 880 | 126 | |||
| TaNbHfZrTi | Arc melting | BCC and HCP | - | 71 | [23] |
| TiNbMoMnFe | Powder metallurgy | BCC and amorphous | - | - | [66] |
| AgAlNbTiZn | Powder metallurgy | BCC and FCC | - | - | [67] |
| Alloy | Solution | Ecorr (mV) | Rp (kcm²) | Icorr (µA/cm²) | Reference |
|---|---|---|---|---|---|
| Ti6Al4V | PBS | −526 | 261 | 0.18 | [12] |
| 316L | −216 | 52 | 1.32 | ||
| CoCrMo | −331 | 163 | 0.28 | ||
| TiZrTaHfNb | −391 | 554 | 0.07 | ||
| TiZrTaHfNb | −396 | 780 | 0.06 | ||
| Ti6Al4V | PBS | −571 | - | - | [50] |
| 316L | −234 | - | - | ||
| CoCrMo | −320 | - | - | ||
| TiZrNbTaMo | −607 | - | - | ||
| TiZrTaNbHf-Ti6Al4V | PBS | −168 | 830 | 0.04 | [59] |
| HEA-316L | −100 | 782 | 0.04 | ||
| HEA-CoCrMo | −88 | 818 | 0.04 | ||
| cp-Ti | PBS | −420 | - | - | [41] |
| 316L | −260 | - | - | ||
| (MoTa)NbTiZr | −530 | - | - | ||
| Ti6Al4V | PBS | −462 | 237 | 0.16 | [58] |
| TiZrTaNbW | −160 | 306 | 0.09 | ||
| HEA-Ag | −190 | 492 | 0.07 | ||
| IM-CoCrFeCuNi | NaCl | - | 38 | - | [9] |
| SLM-CoCrFeCuNi | - | 15 | - | ||
| 316L Substrate | Ringer’s solution | −79 | 600 | 0.03 | [66] |
| TiNbMoMnFe Film | −59 | 900 | 0.02 | ||
| CoNiCr | Ringer’s solution | −430 | - | 0.06 | [55] |
| FeCoNiCr | −150 | - | 0.04 | ||
| FeCoNiCrPd | 60 | - | 0.02 | ||
| cp-Ti | Ringer’s solution | −250 | - | 0.26 | [24] |
| TiZrNbHfSi | −330 | - | 0.08 | ||
| TiZrNbSiGaB | −250 | - | 0.15 | ||
| TiZrNbHfSiGaB | −320 | - | 0.13 | ||
| CoCrMo | Ringer’s solution | −447 | - | 7.34 | [54] |
| TiMoVWCr | −500 | - | 4.51 | ||
| TiMoVNbZr | −481 | - | 2.13 | ||
| AlCrFeCoNi | Ringer’s solution | - | 1300–2200 | [62] | |
| AlCrFeCoNi | - | 1900–6100 | - | ||
| AlCrFeCoNi | - | 3200–3200 | - | ||
| (TiZrNbTa)Mo | Ringer’s solution | - | 850–4600 | 0.01–0.07 | [69] |
| Ti6Al4V | SBF | −1150 | - | 3.89 | [64] |
| (TiZrNb)SnMo | −850 | - | 2.01 | ||
| HEA coating | −1000 | - | 1.10 | ||
| Ti6Al4V | SBF | −140 | 27 | 4.64 | [52] |
| TiNbTaZrMo | −420 | 226 | 0.34 | ||
| SS304 | SBF | −123 | - | 1.70 | [13] |
| MoNbTaTiZr | −118 | - | 0.30 | ||
| Ti6Al4V | Hank’s solution | −325 | 677 | - | [45] |
| TiZrHfNbTa | −395 | 642 | - | ||
| MoNbTaTiZr | Ringer’s solution and SBF | −314 | 29 | 0.22 | [22] |
| MoNbTaTiZr (FSP) | −175 | 450 | 0.11 | ||
| MoNbTaTiZr (SFP) | −142 | 2207 | 0.04 | ||
| AlCoCrFeNi | - | −256 | - | 0.43 | [63] |
| AlCoCrFeNi | −200 | - | 0.75 | ||
| AlCoCrFeNi | −207 | - | 0.37 |
| Alloy | Antibacterial Rates against E. coli | |
|---|---|---|
| In Sessile Cells | In Planktonic Cells | |
| IM-HEA | 94% | 92% |
| SLM-HEA | 98% | 98% |
| Alloy | Magnetic Susceptibility Volume (Xv) [ppm] |
|---|---|
| cp-Ti | 182 |
| 316L | 3520–6700 |
| TiZrNbHfSi | 50 |
| TiZrNbSiGaB | 46 |
| TiZrNbHfSiGaB | 191 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira, T.G.; Fagundes, D.V.; Capellato, P.; Sachs, D.; da Silva, A.A.A.P. A Review of Biomaterials Based on High-Entropy Alloys. Metals 2022, 12, 1940. https://doi.org/10.3390/met12111940
de Oliveira TG, Fagundes DV, Capellato P, Sachs D, da Silva AAAP. A Review of Biomaterials Based on High-Entropy Alloys. Metals. 2022; 12(11):1940. https://doi.org/10.3390/met12111940
Chicago/Turabian Stylede Oliveira, Thiago Gonçalves, Danilo Valim Fagundes, Patrícia Capellato, Daniela Sachs, and Antonio Augusto Araújo Pinto da Silva. 2022. "A Review of Biomaterials Based on High-Entropy Alloys" Metals 12, no. 11: 1940. https://doi.org/10.3390/met12111940