Crystallization of Fe-W-B Amorphous Powder Prepared by Gas Atomization
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Powder
2.2. Performance Testing Method
3. Results and Discussion
3.1. Effect of Alloy Composition on the Morphology and Structure of Gas Atomized Fe-W-B Powder
3.1.1. Morphology and Microstructure Analysis
3.1.2. Phase Analysis
3.2. Thermal Characteristics and Crystallization Behavior of the Fe-W-B Amorphous Powder
3.3. Nuclear Shielding Performance
4. Conclusions
- Whether the atomized powder was crystalline or non-crystalline depends on the specific composition of the master alloy. Fe-W-B amorphous powder was formed under the master alloy composition of tungsten 19.9 at.%, boron 13.6 at.% and iron 66.4 at.%.
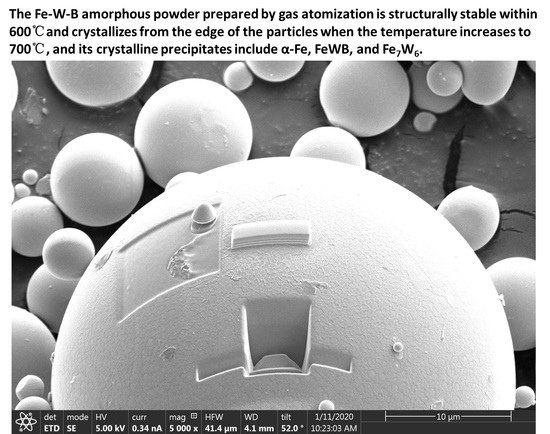
- The structure of Fe-W-B amorphous powder is stable, which remains even as the temperature reaches 600 °C. After annealing at 700 °C, crystallization occurs preferentially at the edge of the particle. The precipitates in crystallization were α-Fe, FeWB and Fe7W6, respectively.
- Compared with iron, Fe-W-B amorphous powder has better shielding performance for γ-rays and neutrons.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Yang, G.P.; Liu, Y.; Qiu, Y.C.; Li, J. A novel route of synthesising Fe–W–B alloy powders and characterisation thereof. Powder Metall. 2017, 60, 241–248. [Google Scholar] [CrossRef]
- Klein, P.; Varga, R.; Kammouni, E.L.R.; Vazquez, M. Magnetic Properties of Glass-Coated FeWB Microwires. Acta Phys. Pol. 2014, 126, 70–71. [Google Scholar] [CrossRef]
- Yang, G.P.; Li, J. Research on Microstructure and Properties of Fe-W-B Coatings Prepared by Plasma Spraying. Guangzhou Chem. Ind. 2016, 44, 143–145. [Google Scholar]
- Li, C.; Li, J.; Liu, Y. Phase evolution of Fe–W–B powders and stability of FeWB ternary boride prepared by reactive synthesis. Mater. Res. Express 2018, 5, 016517. [Google Scholar] [CrossRef]
- Li, J.L.; Li, J.; Li, C.; Liu, Y. Reactive synthesis of FeWB powders and preparation of bulk materials. Int. J. Refract. Met. Hard Mater. 2014, 46, 80–83. [Google Scholar] [CrossRef]
- Li, C.; Li, J.L.; Li, J.; Liu, Y. Study on the synthesis behavior of Fe–W–B powders and the preparation of bulk. Adv. Powder Technol. 2015, 26, 1410–1416. [Google Scholar] [CrossRef]
- Hang, Y.X.; Schwen, D.; Zhang, Y.F.; Bai, X.M. Effects of oversized tungsten on the primary damage behavior in Fe-W alloys. J. Alloys Compd. 2019, 794, 482–490. [Google Scholar]
- Wang, Q.; Du, G.P.; Chen, N.; Jiang, C.S. First-principles study of bubble formation and cohesion properties of hydrogen at Fe/W interfaces. Int. J. Hydrog. Energy 2019, 44, 26469–26476. [Google Scholar] [CrossRef]
- Azakli, Y.; Tarakci, M. Microstructural characterisation of borided binary Fe–W alloys. Surf. Eng. 2018, 34, 226–234. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Z.; Zhong, Z.; Wang, L.; Yang, W.; Wang, Y. Effect of alloying elements on mechanical, electronic and magnetic properties of Fe2B by first-principles investigations. Comput. Mater. Sci. 2018, 147, 322–330. [Google Scholar] [CrossRef]
- Tarek, B.; Rakia, D.; Lusia, E.; Joan, J.S.; Mohamed, K. Amorphization of Al50(Fe2B)30Nb20 mixture by mechanical alloying. Metall. Mater. Trans. A 2013, 44, 4718–4724. [Google Scholar]
- Kirkovska, I.; Homolová, V.; Petryshynets, I.; Csanádi, T. The influence of the third element on nano-mechanical properties of iron borides FeB and Fe2B formed in Fe-B-X (X = C, Cr, Mn, V, W, Mn + V) alloys. Materials 2020, 13, 4155. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M.; Koshimura, M.; Abuzuka, T. Phase Diagram of Amorphous and Crystallized Fe-B Alloy System. Jpn. J. Appl. Phys. 1981, 20, 1821–1832. [Google Scholar] [CrossRef]
- Chang, L.; Zhang, Y.Q.; Dong, Y.Q.; Li, Q.; He, A.N.; Chang, C.T.; Wang, X.M. Enhanced magnetic properties of Fe-based nanocrystalline composites by addition of carbonyl iron powders. SN Appl. Sci. 2019, 1, 902. [Google Scholar] [CrossRef] [Green Version]
- Alvarez, K.L.; Martín, J.M.; Patov, M.; Gonzalez, J. Soft magnetic amorphous alloys (Fe-rich) obtained by gas atomisation technique. J. Alloys Compd. 2018, 735, 2646–2652. [Google Scholar] [CrossRef]
- Gheiratmand, T.; Hosseini, H.M. Finemet nanocrystalline soft magnetic alloy: Investigation of glass forming ability, crystallization mechanism, production techniques, magnetic softness and the effect of replacing the main constituents by other elements. J. Magn. Magn. Mater. 2016, 408, 177–192. [Google Scholar] [CrossRef]
- Ge, Y.; Ying, G.; Bangwei, Z.; Lingling, W.; Yifang, O.; Shuzhi, L. Preparation and thermal properties of amorphous Fe-W-B alloy nano-powders. J. Mater. Process. Technol. 1998, 74, 10–13. [Google Scholar] [CrossRef]
- Zhao, X.M.; Xu, J.; Zhu, X.X.; Zhang, S.M.; Zhao, W.D.; Yuan, G.L. Characterization of 17-4PH stainless steel powders produced by supersonic gas atomization. Int. J. Miner. Metall. Mater. 2012, 19, 83–88. [Google Scholar] [CrossRef]
- Zhao, X.M.; Xu, J.; Zhu, X.X.; Zhang, S.M. Effect of atomization gas pressure variation on gas flow field in supersonic gas atomization. Sci. China 2009, 52, 3046–3053. [Google Scholar] [CrossRef]
- Zhao, X.M.; Xu, J.; Zhu, X.X.; Zhang, S.M. Effect of closed-couple gas atomization pressure on the performances of Al-20Sn-1Cu powders. Rare Met. 2008, 27, 439–443. [Google Scholar] [CrossRef]
- Alvarez, K.L.; Martín, J.M.; Burgos, N.; Ipatov, M.; Domínguez, L.; González, J. Structural and magnetic properties of amorphous and nanocrystalline Fe-Si-B-P-Nb-Cu alloys produced by gas atomization. J. Alloys Compd. 2019, 810, 151754. [Google Scholar] [CrossRef]
- Chang, L.; Xie, L.; Liu, M. Novel Fe-based nanocrystalline powder cores with excellent magnetic properties produced using gas-atomized powder. J. Magn. Magn. Mater. 2018, 452, 442–446. [Google Scholar] [CrossRef]
- Chang, C.; Dong, Y.; Liu, M.; Guo, H.; Xiao, Q.; Zhang, Y. Low core loss combined with high permeability for Fe-based amorphous powder cores produced by gas atomization powders. J. Alloys Compd. 2018, 766, 959–963. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Z.; Liu, M.; Chang, C.; Li, F.; Wang, X. The effects of field annealing on the magnetic properties of FeSiB amorphous powder cores. Mater. Res. Bull. 2017, 96, 160–163. [Google Scholar] [CrossRef]
- Wang, W.H. The nature and properties of amorphous matter. Prog. Phys. 2013, 33, 177–351. [Google Scholar]
- Guo, J.J.; Fu, H.Z. Alloy Melt and Treatment; China Machine Press: Beijing, China, 2005; pp. 35–36. [Google Scholar]
- Lashgari, H.R.; Chu, D.; Xie, S.S.; Sun, H.D.; Ferry, M.; Li, S. Composition dependence of the microstructure and soft magnetic properties of Fe-based amorphous/nanocrystalline alloys: A review study. J. Non-Cryst. Solids 2014, 391, 61–82. [Google Scholar] [CrossRef]
- Lu, Z.P.; Liu, C.T. A new glass-forming ability criterion for bulk metallic glasses. Acta Mater. 2002, 50, 3501–3512. [Google Scholar] [CrossRef]
- Öztürk, P.; Hitit, A. Effects of Tungsten and Boron Contents on Crystallization Temperature and Microhardness of Tungsten Based Metallic Glasses. Acta Metall. Sin. (Engl. Lett.) 2015, 28, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Ouyang, X.M.; Yin, F.C.; Hu, J.X.; Zhao, M.X. Experimental investigation and thermodynamic calculation of the B-Fe-W ternary system. Calphad 2018, 63, 212–219. [Google Scholar] [CrossRef]
- Marshall, J.M.; Walker, D.; Thomas, P.A. HRXRD study of the theoretical densities of novel reactive sintered boride candidate neutron shielding materials. Nucl. Mater. Energy 2020, 22, 100732. [Google Scholar] [CrossRef]
- Dewen, T.; Shuliang, Z.; Liang, Y. Influence of boron contents on microstructure, mechanical properties and shielding effect of Fe–W–C alloy. J. Alloys Compd. 2019, 803, 466–475. [Google Scholar] [CrossRef]
- Zhao, S.; Huo, Z.; Zhong, G.; Zhang, H.; Hu, L. Research Progress of Neutron and Gamma-ray Composite Shielding Materials. J. Funct. Mater. 2021, 52, 03001–03015. [Google Scholar]
- Wang, J.; Zou, S.L. Comparative Study of Tungsten and Lead as Gamma Ray Shielding Material. J. Univ. South China (Sci. Technol.) 2011, 25, 19–22. [Google Scholar]
- Abouhaswa, A.S.; Sayyed, M.I.; Altowyan, A.S.; Al-Hadeethi, Y.; Mahmoud, K.A. Evaluation of optical and gamma ray shielding features for tungsten-based bismuth borate glasses. Opt. Mater. 2020, 106, 109981. [Google Scholar] [CrossRef]










| Serial Number | Sample Number | Compositions of Master Alloys | |||
|---|---|---|---|---|---|
| Percentage | Elements | ||||
| Fe | W | B | |||
| Series 1 | Sample 1 | Mass (wt.%) | 81.3 | 18 | 0.7 |
| Atomic (at.%) | 89.9 | 6.1 | 4 | ||
| Sample 2 | Mass (wt.%) | 71.4 | 25.8 | 2.8 | |
| Atomic (at.%) | 76 | 8.4 | 15.6 | ||
| Sample 3 | Mass (wt.%) | 61.9 | 35.6 | 2.5 | |
| Atomic (at.%) | 72.5 | 12.7 | 14.9 | ||
| Series 2 | Sample 4 | Mass (wt.%) | 53.7 | 44.2 | 2.1 |
| Atomic (at.%) | 68.7 | 17.2 | 14.1 | ||
| Sample 5 | Mass (wt.%) | 54.5 | 44.8 | 0.7 | |
| Atomic (at.%) | 75.9 | 19 | 5.2 | ||
| Sample 6 | Mass (wt.%) | 49.4 | 48.6 | 2 | |
| Atomic (at.%) | 66.4 | 19.9 | 13.6 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, S.; Lv, Z.; Wang, J.; Wang, H.; Yang, J.; Yang, Z.; Li, J.; Xue, Z. Crystallization of Fe-W-B Amorphous Powder Prepared by Gas Atomization. Metals 2022, 12, 1855. https://doi.org/10.3390/met12111855
Ma S, Lv Z, Wang J, Wang H, Yang J, Yang Z, Li J, Xue Z. Crystallization of Fe-W-B Amorphous Powder Prepared by Gas Atomization. Metals. 2022; 12(11):1855. https://doi.org/10.3390/met12111855
Chicago/Turabian StyleMa, Shuwang, Zheng Lv, Jian Wang, Haicheng Wang, Jian Yang, Zhimin Yang, Jingli Li, and Zhiyong Xue. 2022. "Crystallization of Fe-W-B Amorphous Powder Prepared by Gas Atomization" Metals 12, no. 11: 1855. https://doi.org/10.3390/met12111855