The Study of Graphene Oxide on the Regulations and Controls of the Sol-Gel Film Structure and Its Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Pretreatment of Aluminum Alloy
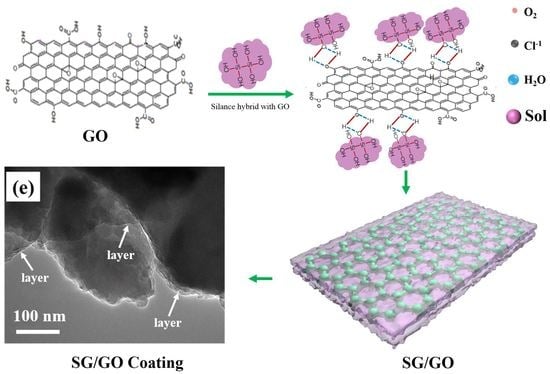
2.2. Synthesis of Graphene and Sol-Gel Film
2.3. Characterization Techniques
3. Results and Discussion
3.1. Chemical Characterization of GO, SG Film and SG/GO Film
3.2. Microstructure of SG Film and SG/GO Film
3.3. Corrosion Protection Performance of Bare Al with SG Film and SG/GO Film
3.4. Coating Formation and Corrosion Protection Mechanism of Samples
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mo, Q.; Qin, G.; Ling, K.; Lv, X.; Wang, N.; Li, W. Layer-by-layer self-assembled polyurea layers onto MAO surface for enhancing corrosion protection to aluminum alloy 6063. Surf. Coat. Technol. 2021, 405, 126653. [Google Scholar] [CrossRef]
- Li, T.; Li, L.; Qi, J.; Chen, F. Corrosion protection of Ti6Al4V by a composite coating with a plasma electrolytic oxidation layer and sol-gel layer filled with graphene oxide. Prog. Org. Coat. 2020, 144, 105632. [Google Scholar] [CrossRef]
- Ji, L. Study on Corrosion Resistance of Anodized 6463 Aluminum Alloy as Construction Material in 3.5% Sodium Chloride Solution. Int. J. Electrochem. Sci. 2021, 16, 2. [Google Scholar] [CrossRef]
- Li, J.; Cao, Y.; Wang, Q.; Shang, W.; Peng, N.; Jiang, J.; Liang, L.; Wen, Y. Construction of anodizing/silane/graphene oxide composite film and its corrosion resistance mechanism on aluminum alloy surface. Mater. Today Commun. 2021, 29, 102999. [Google Scholar] [CrossRef]
- Razavi, S.M.R.; Masoomi, M.; Bagheri, R. Facile strategy toward developing a scalable, environmental friendly and self-cleaning superhydrophobic surface. Colloids Surf. A Physicochem. Eng. Asp. 2018, 541, 108–116. [Google Scholar] [CrossRef]
- del Olmo, R.; Tiringer, U.; Milošev, I.; Visser, P.; Arrabal, R.; Matykina, E.; Mol, J.M.C. Hybrid sol-gel coatings applied on anodized AA2024-T3 for active corrosion protection. Surf. Coat. Technol. 2021, 419, 127251. [Google Scholar] [CrossRef]
- Catauro, M.; Tranquillo, E.; Poggetto, G.D.; Naviglio, S.; Barrino, F. Antibacterial Properties of Sol–Gel Biomaterials with Different Percentages of PEG or PCL. Macromol Symp. 2020, 389, 1900056. [Google Scholar] [CrossRef]
- Catauro, M.; Barrino, F.; Blanco, I.; Piccolella, S.; Pacifico, S. Use of the Sol–Gel Method for the Preparation of Coatings of Titanium Substrates with Hydroxyapatite for Biomedical Application. Coatings 2020, 10, 203. [Google Scholar] [CrossRef] [Green Version]
- Kaliyannan, G.V.; Palanisamy, S.V.; Priyanka, E.B.; Thangavel, S.; Sivaraj, S.; Rathanasamy, R. Investigation on sol-gel based coatings application in energy sector–A review. Mater. Today Proc. 2020, 45, 1138–1143. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Sampath, S.; Aruna, S.T. Silica-alumina based sol-gel coating containing cerium oxide nanofibers as a potent alternative to conversion coating for AA2024 alloy. Surf. Coat. Technol. 2021, 411, 127007. [Google Scholar] [CrossRef]
- Yu, F.; Akid, R. Corrosion protection of AA2024-T3 alloy by modified hybrid titania-containing sol-gel coatings. Prog. Org. Coat. 2017, 102, 120–129. [Google Scholar] [CrossRef]
- Ono, S.; Tsuge, H.; Nishi, Y.; Hirano, S.I. Improvement of Corrosion Resistance of Metals by an Environmentally Friendly Silica Coating Method. J. Sol-Gel Sci. Techn. 2004, 29, 147–153. [Google Scholar] [CrossRef]
- Yu, M.; Liu, Y.; Liu, J.; Li, S.; Xue, B.; Zhang, Y.; Yin, X. Effects of cerium salts on corrosion behaviors of Si–Zr hybrid sol–gel coatings. Chin. J. Aeronaut. 2015, 28, 600–608. [Google Scholar] [CrossRef] [Green Version]
- Amiri, S.; Rahimi, A. Hybrid nanocomposite coating by sol–gel method: A review. Iran. Polym. J. 2016, 25, 559–577. [Google Scholar] [CrossRef]
- Xue, B.; Yu, M.; Liu, J.; Liu, J.; Li, S.; Xiong, L. Corrosion protection of AA2024-T3 by sol-gel film modified with graphene oxide. J. Alloys Compd. 2017, 725, 84–95. [Google Scholar] [CrossRef]
- Zhang, R.; Yu, X.; Yang, Q.; Cui, G.; Li, Z. The role of graphene in anti-corrosion coatings: A review. Constr. Build. Mater. 2021, 294, 123613. [Google Scholar] [CrossRef]
- Yang, X.; Li, L.; Shang, S.; Tao, X.-m. Synthesis and characterization of layer-aligned poly(vinyl alcohol)/graphene nanocomposites. Polymer 2010, 51, 3431–3435. [Google Scholar] [CrossRef]
- Yoshinaga, K.; Yokoyama, T.; Sugawa, Y.; Karakawa, H.; Enomoto, N.; Nishida, H.; Komatsu, M. Preparation of monodispersed polymer-modified silica particles by radical polymerization using silica colloid and introduction of functional groups on the composite surface. Polym. Bull. 1992, 28, 663–668. [Google Scholar] [CrossRef]
- Kang, D.; Kwon, J.Y.; Cho, H.; Sim, J.H.; Hwang, H.S. Oxidation resistance of iron and copper foils coated with reduced graphene oxide multilayers. ACS Nano 2012, 6, 7763–7769. [Google Scholar] [CrossRef]
- Wang, Q.; Li, X.; Shi, B.; Wu, Y. Experimental and Numerical Studies on Preparation of Thin AZ31B/AA5052 Composite Plates Using Improved Explosive Welding Technique. Metals 2020, 10, 1023. [Google Scholar] [CrossRef]
- Hummer, W.S.; Offeman, R.E. Functionalized graphene and graphene oxide: Materials synthesis and electronic applications. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar]
- Tang, L.-C.; Wan, Y.-J.; Yan, D.; Pei, Y.-B.; Zhao, L.; Li, Y.-B.; Wu, L.-B.; Jiang, J.-X.; Lai, G.-Q. The effect of graphene dispersion on the mechanical properties of graphene/epoxy composites. Carbon 2013, 60, 16–27. [Google Scholar] [CrossRef]
- Wan, Y.-J.; Gong, L.-X.; Tang, L.-C.; Wu, L.-B.; Jiang, J.-X. Mechanical properties of epoxy composites filled with silane-functionalized graphene oxide. Compos. Part A Appl. Sci. Manuf. 2014, 64, 79–89. [Google Scholar] [CrossRef]
- Cano, E.; Lafuente, D.; Bastidas, D.M. Use of EIS for the evaluation of the protective properties of coatings for metallic cultural heritage: A review. J. Solid State Electrochem. 2009, 14, 381–391. [Google Scholar] [CrossRef] [Green Version]
- Xue, B.; Yu, M.; Liu, J.; Li, S.; Xiong, L.; Kong, X. Corrosion Protective Properties of Silane Functionalized Graphene Oxide Film on AA2024-T3 Aluminum Alloy. J. Electrochem. Soc. 2016, 163, C798–C806. [Google Scholar] [CrossRef]
- Mora, L.V.; Taylor, A.; Paul, S.; Dawson, R.; Wang, C.; Taleb, W.; Owen, J.; Neville, A.; Barker, R. Impact of silica nanoparticles on the morphology and mechanical properties of sol-gel derived coatings Surf. Coat. Technol. 2018, 342, 48–56. [Google Scholar] [CrossRef]
- Saxena, S.; Tyson, T.A.; Shukla, S.; Negusse, E.; Chen, H.; Bai, J. Investigation of structural and electronic properties of graphene oxide. Appl. Phys. Lett. 2011, 99, 013104. [Google Scholar] [CrossRef] [Green Version]
- Krishnamoorthy, K.; Jeyasubramanian, K.; Premanathan, M.; Subbiah, G.; Shin, H.S.; Kim, S.J. Graphene oxide nanopaint. Carbon 2014, 72, 328–337. [Google Scholar] [CrossRef]








| Elements | Si | Cu | Mg | Zn | Mn | Cr | Fe | Al |
|---|---|---|---|---|---|---|---|---|
| Content | ≦0.25 | ≦0.10 | 2.20~2.80 | ≦0.10 | ≦0.10 | 0.15~0.35 | ≦0.40 | Bal. |
| Simple | Ecorr (V/SCE) | jcorr (10−8 A cm−2) | ba (V dec−1) | −bc (V dec−1) |
|---|---|---|---|---|
| Bare | −0.731 | 82.4 | 0.0157 | 0.128 |
| SG film | −0.749 | 14.4 | 0.0511 | 0.0623 |
| SG/GO film | −0.762 | 0.463 | 0.0613 | 0.0464 |
| Sample | Time (h) | Qcoat | Rcoat (Ω cm2) | Qinter | Rinter (Ω cm2) | Cdl (10−6 Ω−1 cm−2 sn) | Rct (Ω cm2) | χ 2 (10−3) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Y0 (μS sn cm−2) | n | Y0 (μS sn cm−2) | n | |||||||
| Bare Al-alloy | 1 | / | / | / | 7.22 | 0.89 | 10.8 | 2.11 | 2560 | 5.12 |
| 72 | / | / | / | 70.1 | 0.88 | 7.13 | 68.1 | 2500 | 1.12 | |
| 480 | / | / | / | 890.2 | 0.83 | 23.2 | 84.6 | 3215 | 0.71 | |
| 720 | / | / | / | 1356.6 | 0.80 | 38.4 | 89.5 | 3456 | 1.56 | |
| SG film | 1 | 1.67 | 0.80 | 337 | 5.77 | 0.83 | 570,000 | 5.02 | 4640 | 0.42 |
| 72 | / | / | / | 7.12 | 0.83 | 7420. | 6.77 | 5640 | 0.67 | |
| 480 | / | / | / | 12.5 | 0.86 | 84.3 | 9.52 | 3841 | 0.48 | |
| 720 | / | / | / | 16.1 | 0.83 | 54.2 | 12.7 | 4784 | 1.23 | |
| SG/GO film | 1 | 0.11 | 0.82 | 12100 | 5.33 | 0.79 | 1,140,000 | 4.68 | 5740 | 0.24 |
| 72 | 0.54 | 0.76 | 6210 | 5.19 | 0.81 | 714,000 | 5.93 | 4210 | 0.15 | |
| 480 | 0.79 | 0.73 | 2149 | 8.12 | 0.80 | 432,000 | 8.12 | 3854 | 0.98 | |
| 720 | / | / | / | 7.06 | 0.726 | 1308 | 3.32 | 7975 | 4.30 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Y.; Fan, Y.; Zhang, J.; Liu, X.; Wang, N.; Yang, S. The Study of Graphene Oxide on the Regulations and Controls of the Sol-Gel Film Structure and Its Performance. Metals 2022, 12, 20. https://doi.org/10.3390/met12010020
Gao Y, Fan Y, Zhang J, Liu X, Wang N, Yang S. The Study of Graphene Oxide on the Regulations and Controls of the Sol-Gel Film Structure and Its Performance. Metals. 2022; 12(1):20. https://doi.org/10.3390/met12010020
Chicago/Turabian StyleGao, Yan, Yadong Fan, Junxi Zhang, Xuanxuan Liu, Ning Wang, and Shengjie Yang. 2022. "The Study of Graphene Oxide on the Regulations and Controls of the Sol-Gel Film Structure and Its Performance" Metals 12, no. 1: 20. https://doi.org/10.3390/met12010020