Invasion, Distribution, Monitoring and Farmers Perception of Fall Armyworm (Spodoptera frugiperda) and Farm-Level Management Practices in Bangladesh
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigation of Invasion and Distribution of Fall Armyworm in Bangladesh
2.2. Monitoring of Fall Armyworm in Bangladesh
2.3. Farmer Perception and Farm-Level Management Practices of Fall Armyworm
2.4. Economic Benefit of Pesticide Use
2.5. Statistical Analyses
3. Results
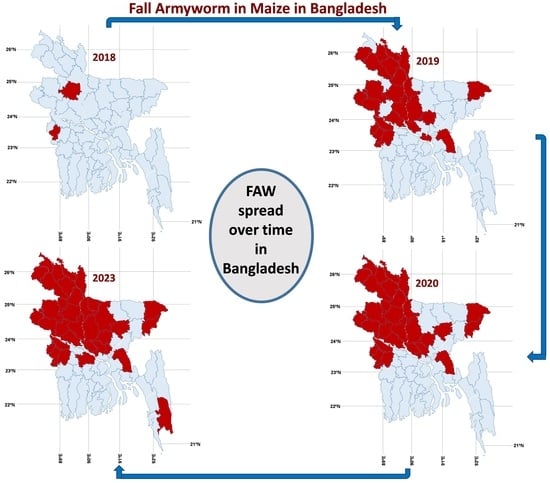
3.1. Invasion and Distribution of FAW in Bangladesh
3.2. Monitoring of FAW in Bangladesh
3.3. Farmer Perception and Farm-Level Management of FAW
3.3.1. Maize Production Systems
3.3.2. FAW Infestation Knowledge
3.3.3. Farmers’ Knowledge, Perceptions, and Practices concerning FAW Management
3.3.4. Pesticides Used by Farmers
3.3.5. Economic Benefit of Pesticide Use
4. Discussion
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sparks, A.N. A review of the biology of the fall armyworm. Fla. Entomol. 1979, 62, 276–283. [Google Scholar] [CrossRef]
- Dumas, P.; Legeai, F.; Lemaitre, C.; Scaon, E.; Orsucci, M.; Labadie, K.; Gimenez, S.; Clamens, A.-L.; Henri, H.; Vavre, F.; et al. Spodoptera frugiperda (Lepidoptera: Noctuidae) host-plant variants: Two host strains or two distinct species? Genetica 2015, 143, 305–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Montezano, D.; Specht, A.; Sosa-G’omez, D.; Roque-Specht, V.; Sousa-Silva, J.; Paula- Moraes, S.; Peterson, J.; Hunt, T. Host plants of Spodoptera frugiperda (Lepidoptera: Noctuidae) in the Americas. Afr. Entomol. 2018, 26, 286–300. [Google Scholar] [CrossRef] [Green Version]
- Groot, A.T.; Marr, M.; Heckel, D.G.; Schofl, G. The roles and interactions of reproductive isolation mechanisms in fall armyworm (Lepidoptera: Noctuidae) host strains. Ecol. Entomol. 2010, 35, 105–118. [Google Scholar] [CrossRef]
- Schofl, G.; Heckel, D.G.; Groot, A.T. Time-shifted reproductive behaviours among fall armyworm (Noctuidae: Spodoptera frugiperda) host strains: Evidence for differing modes of inheritance. J. Evol. Biol. 2009, 22, 1447–1459. [Google Scholar] [CrossRef]
- Nagoshi, R.N.; Adamczyk, J.J.; Meagher, R.L.; Gore, J.; Jackson, R. Using stable isotope analysis to examine fall armyworm (Lepidoptera: Noctuidae) host strains in a cotton habitat. J. Econ. Entomol. 2014, 100, 1569–1576. [Google Scholar] [CrossRef]
- CABI. Invasive Species Compendium Datasheets—Spodoptera frugiperda (Fall Armyworm). Available online: https://www.cabi.org/isc/datasheet/29810 (accessed on 11 August 2021).
- Hruska, A.J.; Gould, F. Fall armyworm (Lepidoptera: Noctuidae) and Diatraea ineolate (Lepidoptera: Pyralidae): Impact of larval population level and temporal occurrence on maize yield in Nicaragua. J. Econ. Entomol. 1997, 90, 611–622. [Google Scholar] [CrossRef]
- Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Day, R.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. In Evidence Note (2); CABI: London, UK, 2017. [Google Scholar]
- Qin, Y.J.; Yamng, D.C.; Kang, D.L.; Zhao, Z.H.; Zhao, Z.H.; Yang, P.Y.; Li, Z.H. Potential economic loss assessment of maize industry caused by fall armyworm (Spodoptera frugiperda) in China. Plant. Prot. 2020, 46, 69–73. (In Chinese) [Google Scholar]
- CIMMYT (The International Maize and Wheat Improvement Center). Fighting Back Against Fall Armyworm (FAW) in Bangladesh. 2021. Semi-Annual Report October 2020–March 2021. Available online: https://csisa.org/wp-content/uploads/sites/2/2021/04/210426-FIGHTING-FAW-2020-21-SEMI-ANNUAL-REPORT.pdf (accessed on 11 January 2023).
- Ullah, M.S.; Hossain, M.A.; Jahan, M.; Sarker, M.A.; Hamim, I. A Field Guide of Major Insect Pests and Diseases of Different Crops in Bangladesh; Bangladesh Agricultural University Extension Centre (BAUEC): Mymensingh, Bangladesh, 2021; pp. 26–27. [Google Scholar]
- Ullah, M.S.; Sharmin, D.; Chaudhary, M. Distribution of Fall armyworm Spodoptera frugiperda in Bangladesh and farmers’ perception on its management. In Proceedings of the Developing Smallholder Oriented IPM Strategies for Fall Armyworm Management, Online Conference, 24–26 August 2021. [Google Scholar]
- Bessin, R. Fall Armyworm in Corn; University of Kentucky College of Agriculture Cooperative Extension Service: Lexington, KY, USA, 2003. [Google Scholar]
- Wan, J.; Huang, C.; Li, C.-Y.; Zhou, H.-X.; Ren, Y.-L.; Li, Z.-Y.; Xing, L.; Zhang, B.; Qiao, X.; Liu, B.; et al. Biology, invasion and management of the agricultural invader: Fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae). J. Integr. Agric. 2021, 20, 646–663. [Google Scholar] [CrossRef]
- Knipling, E.F. Regional management of the fall armyworm- a realistic approach? Fla. Entomol. 1980, 63, 468–480. [Google Scholar] [CrossRef]
- Goergen, G.; Kumar, P.L.; Sankung, S.B.; Togola, A.; Tamo, M. First report of outbreaks of the fall armyworm Spodoptera frugiperda (J E Smith) (Lepidoptera, Noctuidae), a new alien invasive pest in West and Central Africa. PLoS ONE 2016, 11, e0165632. [Google Scholar] [CrossRef] [Green Version]
- FAO. Briefing Note on FAO Actions on Fall Armyworm in Africa, 16 February 2018. Available online: http://www.fao.org/3/a-bt415e.pdf (accessed on 11 August 2021).
- FAO. Briefing Note on FAO Actions on Fall Armyworm, 5 March 2019. Available online: http://www.fao.org/3/BS183E/bs183e.pdf (accessed on 11 August 2021).
- EPPO Global Database. First Report of Spodoptera frugiperda in Thailand. EPPO Reporting Service no. 01–2019 Num. Article: 2019/006. 2019. Available online: https://gd.eppo.int/reporting/article-6436 (accessed on 11 August 2021).
- Wu, Q.L.; He, L.M.; Shen, X.J.; Jiang, Y.Y.; Liu, J.; Hu, G.; Wu, K.M. Estimation of the potential infestation area of newly invaded fall armyworm Spodoptera frugiperda in the Yangtze River Valley of China. Insects 2019, 10, 298. [Google Scholar] [CrossRef] [Green Version]
- Nagoshi, R.N.; Fleischer, S.; Meagher, R.L.; Hay-Roe, M.; Khan, A.; Murua, M.G.; Silvie, P.; Vergara, C.; Westbrook, J. Fall armyworm migration across the Lesser Antilles and the potential for genetic exchanges between North and South American populations. PLoS ONE 2017, 12, e0171743. [Google Scholar] [CrossRef] [Green Version]
- Early, R.; González-Moreno, P.; Murphy, S.T.; Day, R. Forecasting the global extent of invasion of the cereal pest Spodoptera frugiperda, the fall armyworm. NeoBiota 2018, 40, 25–50. [Google Scholar] [CrossRef] [Green Version]
- Gallien, L.; Munkemuller, T.; Albert, C.H.; Boulangeat, I.; Thuiller, W. Predicting potential distributions of invasive species: Where to go from here? Divers. Distrib. 2010, 16, 331–342. [Google Scholar] [CrossRef]
- Matova, P.M.; Kamutando, C.N.; Kutywayo, D.; Magorokosho, C.; Labuschagne, M. Fall armyworm tolerance of maize parental lines, experimental hybrids, and commercial cultivars in southern Africa. Agronomy 2022, 12, 1463. [Google Scholar] [CrossRef]
- Gutiérez-Moreno, R.; Mota-Sanchez, D.; Blanco, C.A.; Whalon, M.E.; Terán-Santofimio, H.; Rodriguez-Maciel, J.C.; DiFonzo, C. Field-evolved resistance of the fall armyworm (Lepidoptera: Noctuidae) to synthetic insecticides in Puerto Rico and Mexico. J. Econ. Entomol. 2019, 112, 792–802. [Google Scholar] [CrossRef]
- Adnan, K.M.M.; Sarker, S.A.; Tama, R.A.Z.; Shan, T.B.; Datta, T.; Monshi, M.H.; Hossain, M.S.; Akhi, K. Catastrophic risk perceptions and the analysis of risk attitudes of Maize farming in Bangladesh. J. Agric. Food Res. 2023, 11, 100471. [Google Scholar] [CrossRef]
- Kansiime, M.K.; Mugambi, I.; Rwomushana, I.; Nunda, W.; Lamontagne-Godwin, J.; Rware, H.; Phiri, N.A.; Chipabika, G.; Ndlovu, M.; Day, R. Farmer perception of fall armyworm (Spodoptera frugiderda J.E. Smith) and farm-level management practices in Zambia. Pest Manag. Sci. 2019, 75, 2840–2850. [Google Scholar] [CrossRef] [Green Version]
- Midega, C.A.; Pittchar, J.O.; Pickett, J.A.; Hailu, G.W.; Khan, Z.R. A climate-adapted push-pull system effectively controls fall armyworm, Spodoptera frugiperda (J.E. Smith), in maize in East Africa. Crop Prot. 2018, 105, 10–15. [Google Scholar] [CrossRef]
- Abro, Z.; Kimathi, E.; De Groote, H.; Tefera, T.; Sevgan, S.; Niassy, S.; Kassie, M. Socioeconomic and health impacts of fall armyworm in Ethiopia. PLoS ONE 2021, 16, e0257736. [Google Scholar] [CrossRef] [PubMed]
- Day, R.; Abrahams, P.; Bateman, M.; Beale, T.; Clottey, V.; Cock, M.; Colmenarez, Y.; Corniani, N.; Early, R.; Godwin, J.; et al. Fall armyworm: Impacts and implications for Africa. Outlooks Pest Manag. 2017, 28, 196–201. [Google Scholar] [CrossRef] [Green Version]
- Kumela, T.; Simiyu, J.; Sisay, B.; Likhayo, P.; Mendesil, E.; Gohole, L.; Tefera, T. Farmers’ knowledge, perceptions, and management practices of the new invasive pest, fall armyworm (Spodoptera frugiperda) in Ethiopia and Kenya. Int. J. Pest Manag. 2018, 65, 1–9. [Google Scholar] [CrossRef]
- Baudron, F.; Zaman-Allah, M.A.; Chaipa, I.; Chari, N.; Chinwada, P. Understanding the factors influencing fall armyworm (Spodoptera frugiperda JE Smith) damage in African smallholder maize fields and quantifying its impact on yield. A case study in Eastern Zimbabwe. Crop Prot. 2019, 120, 141–150. [Google Scholar] [CrossRef]
- Skendžić, S.; Zovko, M.; Živković, I.P.; Lešić, V.; Lemić, D. The impact of climate change on agricultural insect pests. Insects 2021, 12, 440. [Google Scholar] [CrossRef]
- Nayyar, N.; Gracy, R.G.; Ashika, T.R.; Mohan, G.; Swathi, R.S.; Mohan, M.; Chaudhary, M.; Bakthavatsalam, N.; Venkatesan, T. Population structure and genetic diversity of invasive Fall Armyworm after 2 years of introduction in India. Sci. Rep. 2021, 11, 7760. [Google Scholar] [CrossRef]






| Agro-Ecological Zone (AEZ) | Division | District | Upazila | No. of Respondent Farmer |
|---|---|---|---|---|
| Zone I—Old Himalayan Piedmont Plain | Rangpur | Dinajpur | Birol | 20 |
| Zone 3—Tista Meander Floodplain | Rangpur | Rangpur | Badarganj | 31 |
| Rangpur | Lalmonirhat | Aditmari | 11 | |
| Rangpur | Kurigram | Rajarhat | 23 | |
| Rangpur | Gaibandha | Palashbari | 25 | |
| Zone 7—Active Brahmaputra—Jamuna Floodplain | Dhaka | Dhaka | Dhamrai | 16 |
| Zone 9—Old Brahmaputra Floodplain | Mymensingh | Sherpur | Nalitabari | 17 |
| AEZ 10—Active Ganges Floodplain | Khulna | Kushtia | Bheramara | 39 |
| Zone 11—High Ganges River Floodplain | Khulna | Chuadanga | Sadar | 20 |
| Rajshahi | Chapai Nawabganj | Nachole | 21 |
| Farmer Category | Frequency (Number) | Percentage (%) | |
|---|---|---|---|
| Sex | |||
| Male | 220 | 98.7 | |
| Female | 3 | 1.3 | |
| Land owned for maize cultivation (acre) | |||
| <1 | 74 | 33.2 | |
| 1 | 77 | 34.5 | |
| 2 | 42 | 18.8 | |
| 3 | 14 | 6.3 | |
| 4 | 11 | 4.9 | |
| >4 | 5 | 2.3 | |
| Experience in maize farming (years) | |||
| 0–3 | 67 | 30.0 | |
| 4–6 | 74 | 33.2 | |
| >6 | 82 | 36.8 | |
| Maize variety cultivated | |||
| Local | 0 | 0.00 | |
| Hybrid | 223 | 100.00 | |
| Parameters a | Experience (in Years) of Cultivating Maize | ||||
|---|---|---|---|---|---|
| 0–3 | 4–6 | >6 | Total | ||
| FAW infested cropping stage * | |||||
| Early whorl stage | 1 (0.5) | 4 (1.8) | 6 (2.7) | 11 (4.9) | |
| Late whorl stage | 57 (25.6) | 65 (29.2) | 75 (33.6) | 197 (88.3) | |
| Tasseling/Silking | 3 (1.4) | 4 (1.8) | 1 (0.5) | 8 (3.6) | |
| Mature/Cob formation | 6 (2.7) | 1 (0.5) | 0 (0.0) | 7 (3.1) | |
| FAW infested more in the month *** | |||||
| January | 10 (4.5) | 10 (4.5) | 15 (6.7) | 35 (15.7) | |
| Feb | 29 (13.0) | 24 (10.8) | 20 (9.0) | 73 (32.7) | |
| March | 13 (5.8) | 8 (3.6) | 9 (4.0) | 30 (13.5) | |
| April | 5 (2.2) | 23 (10.3) | 9 (4.0) | 37 (16.6) | |
| May | 7 (3.1) | 1 (0.5) | 1 (0.5) | 9 (4.0) | |
| June | 0 | 0 | 0 | 0 | |
| July | 0 | 0 | 0 | 0 | |
| August | 0 | 0 | 0 | 0 | |
| September | 0 | 0 | 0 | 0 | |
| October | 0 | 3 (1.3) | 7 (3.1) | 10 (4.5) | |
| November | 1 (0.5) | 5 (2.2) | 12 (5.4) | 18 (8.1) | |
| December | 2 (0.9) | 0 | 9 (5.4) | 11 (4.9) | |
| Methods used to control FAW | |||||
| Chemical | 2 (0.9) | 4 (1.8) | 1 (0.5) | 7 (3.1) | |
| Mechanical–Chemical | 64 (28.7) | 67 (30.0) | 80 (35.9) | 211 (94.6) | |
| Biological–Chemical | 1 (0.5) | 3 (1.4) | 1 (0.5) | 5 (2.2) | |
| Agricultural practices used in the field against FAW | |||||
| Larvae killed by hand | 56 (25.1) | 51 (22.9) | 62 (27.8) | 169 (75.8) | |
| Egg mass destruction | 20 (9.0) | 32 (14.4) | 38 (1.0) | 90 (40.4) | |
| Tobacco | 0 | 3 (1.4) | 2 (0.9) | 5 (2.2) | |
| Chilli powder | 0 | 0 | 1 (0.5) | 1 (0.5) | |
| Neem | 0 | 0 | 0 | 0 | |
| Ash | 3 (1.4) | 6 (2.7) | 3 (1.4) | 12 (5.4) | |
| Bishkatali | 0 | 0 | 0 | 0 | |
| Mud or sand | 1 (0.5) | 2 (0.9) | 3 (1.4) | 6 (2.7) | |
| Biocontrol agent | 0 | 2 (0.9) | 0 | 2 (0.9) | |
| Used the chemicals in a season *** | |||||
| 1 time | 3 (1.4) | 3 (1.4) | 0 | 6 (2.7) | |
| 2 times | 31 (13.9) | 18 (8.1) | 26 (11.7) | 75 (33.6) | |
| 3 times | 18 (8.1) | 38 (17.0) | 50 (22.4) | 106 (47.5) | |
| 4 times | 5 (2.2) | 5 (2.2) | 3 (1.4) | 13 (5.8) | |
| >4 times | 10 (4.5) | 10 (4.5) | 3 (1.4) | 23 (10.3) | |
| Intervals in use of chemicals *** | |||||
| 3 days | 2 (0.9) | 0 | 0 | 2 (0.9) | |
| 7 days | 33 (14.8) | 51 (22.9) | 36 (16.1) | 120 (53.8) | |
| 10 days | 7 (3.1) | 1 (0.5) | 1 (0.5) | 9 (4.0) | |
| 15 days | 22 (9.9) | 19 (8.5) | 45 (20.2) | 86 (38.6) | |
| Damage (%) in the absence of control measures of FAW | |||||
| <10 | 0 | 2 (0.9) | 1 (0.5) | 3 (1.4) | |
| 10–20 | 11 (4.9) | 9 (4.0) | 9 (4.0) | 29 (13.0) | |
| 21–30 | 6 (2.7) | 12 (5.4) | 20 (9.0) | 38 (17.0) | |
| 31–40 | 21 (9.4) | 14 (6.3) | 24 (10.8) | 59 (26.5) | |
| >40 | 29 (13.0) | 37 (16.6) | 28 (12.6) | 94 (42.2) | |
| Pesticide Name (Trade Name with Formulation) | Active Ingredient | Freq. a | % | WHO Class b | DAE List c | Applied in Stage of Plant |
|---|---|---|---|---|---|---|
| Nitro 505 EC | Chlorpyrifos (50%) + Cypermethrin (5%) | 67 | 17.54 | II | Y | Vegetative |
| Tracer 45 SC | Spinosad | 60 | 15.71 | III | Y | Vegetative |
| Karate 2.5 EC | Lambda Cyhalothrin | 37 | 9.69 | II | Y | Vegetative |
| Proclaim 5 SG | Emamectin Benzoate | 36 | 9.42 | II | Y | Vegetative |
| Success 2.5 SC | Spinosad | 31 | 8.12 | III | Y | Seedling, vegetative |
| Virtako 40 WG | Thiamethoxam (20%) + Chlorantraniliprole (20%) | 31 | 8.12 | Thiamethoxam—II Chlorantraniliprole—U | Y | Vegetative |
| Lumectin 10 WDG | Lufenuron (5%) + Emamectin Benzoate (5%) | 18 | 4.71 | II | Y | Vegetative |
| AC Mix 55 EC | Chlorpyrifos (50%) + Cypermethrin (5%) | 13 | 3.40 | II | Y | Seedling, vegetative |
| Saham 5 SG | Emamectin Benzoate | 11 | 2.88 | II | Y | Vegetative |
| Protect 5 SG | Emamectin Benzoate | 10 | 2.62 | II | Y | Vegetative |
| Setara 55 EC | Chlorpyrifos (50%) + Cypermethrin (5%) | 9 | 2.36 | II | Y | Vegetative |
| Sevin 85 SP | Carbaryl | 9 | 2.36 | II | Y | Vegetative |
| Morter 48 EC | Chlorpyriphos | 8 | 2.09 | II | Y | Vegetative |
| Master Plus 48 EC | Chlorpyriphos | 8 | 2.09 | II | Y | Vegetative |
| Reload 18 SC | Thiamethoxam (12%) + Fipronil (6%) | 7 | 1.83 | II | Y | Seedling, vegetative |
| Shobicron 425 EC | Profenofos (40%) + Cypermethrin (2.5%) | 4 | 1.05 | II | Y | Vegetative, flowering |
| Venom 25 WDG | Acetamiprid (20%) + Emamectin Benzoate (5%) | 4 | 1.05 | II | Y | Vegetative |
| Benten 1.8 EC | Abamectin | 3 | 0.79 | III | Y | Seedling, vegetative |
| Cartap 50 SP | Cartap | 3 | 0.79 | II | Y | Vegetative |
| Canopy 20 SL | Imidacloprid | 3 | 0.79 | II | Y | Flowering |
| Emitaf 20 SL | Imidacloprid | 3 | 0.79 | II | Y | Vegetative, flowering |
| Emithrin Plus 3% WD | Abamectin (1%) + Beta Cypermethrin (2%) | 2 | 0.52 | II | Y | Vegetative |
| Moxie 17.5 WDG | Imidacloprid (15%) + Lamda Cyhalothrin (5%) | 2 | 0.52 | II | Y | Vegetative, flowering |
| Catrapid 95 SP | Acetamiprid (3%) + Cartap (92%) | 1 | 0.26 | II | Y | Vegetative |
| Coragen 18.5 SC | Chlorantraniliprole | 1 | 0.26 | U | Y | Vegetative |
| Diazinon 60 EC | Diazinon | 1 | 0.26 | II | Y | Vegetative |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, M.S.; Sharmin, D.; Tumpa, T.A.; Rashed, M.T.N.N.; Mondal, P.; Akram, M.W.; Chowdhury, S.; Ahmad, M.; Gotoh, T.; Chaudhary, M. Invasion, Distribution, Monitoring and Farmers Perception of Fall Armyworm (Spodoptera frugiperda) and Farm-Level Management Practices in Bangladesh. Insects 2023, 14, 343. https://doi.org/10.3390/insects14040343
Ullah MS, Sharmin D, Tumpa TA, Rashed MTNN, Mondal P, Akram MW, Chowdhury S, Ahmad M, Gotoh T, Chaudhary M. Invasion, Distribution, Monitoring and Farmers Perception of Fall Armyworm (Spodoptera frugiperda) and Farm-Level Management Practices in Bangladesh. Insects. 2023; 14(4):343. https://doi.org/10.3390/insects14040343
Chicago/Turabian StyleUllah, Mohammad Shaef, Dilruba Sharmin, Toufica Ahmed Tumpa, Md Tafsir Nur Nabi Rashed, Powlomee Mondal, Md Wasim Akram, Setu Chowdhury, Masum Ahmad, Tetsuo Gotoh, and Malvika Chaudhary. 2023. "Invasion, Distribution, Monitoring and Farmers Perception of Fall Armyworm (Spodoptera frugiperda) and Farm-Level Management Practices in Bangladesh" Insects 14, no. 4: 343. https://doi.org/10.3390/insects14040343