1. Introduction
Spodoptera exigua (Hübner), which is native to Southeast Asia, is a serious pest to numerous field crops, vegetables, and ornamentals including corn, soybean, potato, green pea, cotton, onion, peanut, and tomato [
1,
2,
3,
4,
5]. It is a cosmopolitan species that feeds on 170 species, including 35 families of plants [
6,
7,
8].
Spodoptera exigua generally has five, sometimes six instars of larvae [
9]; starting from the fourth instar, larvae cause huge damage by consuming 80–90% of total food [
10,
11]. Chemical insecticides are commonly used to manage
S. exigua. However, management of this pest has failed due to its rapidity in developing resistance to conventional chemical insecticides due to its wide host range, higher mobility and higher reproduction capacity [
9,
12], and a reduction in the haphazard use of broad-spectrum insecticides due to the substantial environmental concerns involved [
13,
14,
15,
16,
17,
18].
The host range of
S. exigua is wide, and it can successfully breed on several plant hosts [
1,
6,
11]. It has been reported that the oviposition and development of
S. exigua can be affected by the physical/chemical attributes of the hosts as well as abiotic factors (temperature, humidity, and light) [
6,
11,
19,
20,
21,
22]. The rate of population build-up of
S. exigua is responsible for the severity of the damage to the hosts, and abiotic factors such as temperature, humidity, and the nutritional quality of the hosts can play a role in population growth and damage intensity [
3,
11,
22]. Consequently, there is a need for the development of environmentally sound alternative control measures for the integrated management of
S. exigua.
It is important to construct a precise predictive model for adult emergence, and a prediction strategy that can serve as a critical component of an integrated pest management system wherein it facilitates decision making and enhances control efficacy [
1,
23]. Predicting the accurate seasonal occurrence of agricultural insect pests including
S. exigua is significant for scheduling, sampling, and the selection of control tactics. Climatic factors in general have been shown to play significant roles in insect life; among such climatic factors, temperature has the greatest influence on population dynamics and the timing of biological events of insect species [
24,
25]. The development of insects occurs within narrow temperature ranges that vary between different insect species [
26], and is sensitive to temperature changes [
26,
27,
28,
29,
30,
31,
32,
33]. Even slight alterations in temperature could cause spatial and temporal changes in the phenology of insects. The thermal requirements of the development of insects and biological agents are often used to predict their activity, seasonality, and population build-up [
34,
35,
36]. The accurate and timely prediction of the occurrence of insect pests is one of the crucial aspects of an integrated pest management system, and it can be a foundation for understanding the sources and dynamics of local insect populations driven by temperature.
Temperature-dependent developmental models are essential for forecasting pest emergence [
34], and there are several models based on the simplified analytic method [
37,
38,
39,
40,
41,
42] and biophysical approaches [
43,
44,
45,
46] that are often used to elucidate the relationship between temperature and the development rate. Although there is some information in the existing literature on the temperature-mediated development of
S. exigua on artificial diets in Korea [
19,
21], there have been no studies examining the effects of temperature on the developmental biology and developmental distribution of
S. exigua on different crop hosts in Korea. Although outbreaks of
S. exigua are sporadic, they are very difficult to control with insecticides due to the rapid development of final instar larvae and insecticide resistance; therefore, early forecasting of the spring emergence of
S. exigua is a crucial aspect of an effective intervention. Therefore, in this study, we examined the effects of constant temperatures on the development, adult longevity, and sex ratio relative to plant hosts soybean (
Glycine max [L.] Merr.; cv. Daewon; Fabaceae), maize (
Zea mays L.; cv. Hangkeummaschal; Poaceae), potato (
Solanum tuberosum L.; cv. Sumi; Solanaceae), and green pea (
Pisum sativum L.; cv. Spakal; Fabaceae), in order to develop empirical developmental models to estimate the thermal requirements of the development of
S. exigua.
4. Discussion
Global warming mediated by anthropogenic activities is anticipated to raise the earth’s temperature by approximately 1.5–5.8 °C by the end of the century, which is expected to lead to serious challenges to pest management and food security [
63,
64,
65]. Temperature is the abiotic factor that determines the development rate and population growth of almost all organisms [
66,
67]. Insects are exothermic organisms, and the temperature is an important determinant of the pre-adult development rates of insects [
68,
69]. Therefore, it is crucial to understand the relationship between temperature and rate of development, because temperature influences insect biology, distribution, abundance, and damaging behavior [
70,
71,
72,
73,
74]. Information on the influence of a constant temperature on the thermal requirements of a given insect pest is significant for the formulation of an integrated pest management program [
75]. We investigated the effects of a wide range of temperatures on
S. exigua development, and we estimated important thermal requirement parameters to understand the biological processes by using developmental rate models (linear and nonlinear) on different crop hosts (soybean, maize, potato, and green pea). This study details the prediction of
S. exigua population dynamics in soybean, maize, potato, and green pea fields in Korea. Temperature affects the developmental time of
S. exigua as it does for several insect pests in crop fields, including
Riptortus pedestris (Thunberg) (Hemiptera: Alydidae),
Halyomorpha halys (Stål) (Heteroptera: Pentatomidae), and
Aphis glycines (Matsumura) (Hemiptera: Aphididae) [
76,
77,
78]. The development trend of
S. exigua at seven constant temperatures revealed decreases in the developmental time from eggs to adults with increases in temperature on all crop hosts (
Table 1). The developmental time (1.81, 2.08, 2.93, 3.84, 5.86, and 13.04 days for eggs, 8.92, 10.73, 13.21, 14.76, 29.77, and 70.83 days for larvae, and 5.56, 5.58, 7.06, 8.76, 14.90, and 41.58 days for pupae at 35, 30, 27, 25, 20, and 15 °C, respectively) estimated on the artificial diet in the present study is similar to the estimated time for
S. exigua on other artificial diets [
19,
21]. However, [
19] estimated a slightly higher number of days for the development at 15 and 20 °C than we estimated in the present work. The developmental time estimated in this study is different from that of a study estimated on an artificial formula [
11], which reported values of 0.76, 0.93, 0.93, 1.04, and 3.20 days for eggs, 5.99, 6.91, 7.77, 10.34, and 17.55 days for larvae, and 3.12, 3.99, 5.64, 6.63, and 7.68 days for pupae at 35, 30, 27, 25, and 20 °C, respectively. These discrepancies may be due to the differences in the diet fed to larvae and its composition, along with factors other than temperature such as genetic makeup, geographic origin, relative humidity, and photoperiod. Among the temperatures,
S. exigua could not complete its development at 40 °C on all the plant hosts due to the fact that all eggs died. This type of unsuccessful egg/pupa development has also been reported by [
11,
79]. This failure in the development of
S. exigua at the highest temperature might be interpreted to mean that high heat induces rapid dehydration of eggs, and that physiological disorders are responsible for the abnormal development and death of eggs. Substantial inhibition and unsuccessful development of eggs at high temperature were also reported in other insect species such as Hawaiian flower thrips,
Thrips hawaiiensis (Morgan) (Thysanoptera: Thripidae) [
80]. Prolonged exposure to high temperatures has been shown to produce various metabolic disorders in insects that ultimately lead to death [
81]. High and low temperature exposure can also inactivate enzymes blocking cell cycle development, thereby substantially narrowing the temperature range for embryonic development in insects compared to the range of thermal tolerance in adults [
82], which would explain why extreme temperatures had such adverse effects on the development of
S. exigua.
The results regarding the combined effects of temperature and plant hosts (
Table 6 and
Figure 6) clearly show that, just like temperature, plant hosts also influenced the developmental time of
S. exigua. This result is similar to that reported by [
3,
8,
11], who also reported that plant hosts significantly affected the development of
S. exigua. However, the developmental time of each stage varied according to the plant host. The results show that the insect had the shortest (109.27 days) developmental time when feeding on green pea, followed by when feeding on potato (114.90 days), and the longest (125.95 days) when feeding on maize (
Table 1). However, the results of the present study contradict those reported by [
3] for
S. exigua, who found a longer developmental time (28 days at 27 °C) on pea. This discrepancy in developmental time may be due to variability in either the nutritional quality, or the quantity of host plant species [
83]. These differences may also be related to the availability of different primary and secondary biochemicals on different host plants or different plant parts consumed by the larvae [
84]. A study by [
85] examining the feeding ecology of several species of bugs reported negative aspects of food containing limited nutrient contents that are essential for the normal development of bugs; specifically, insects feeding on such poor nutrient sources tend to store important nutrient contents such as lipids rather than utilize them for normal development, thus resulting in a longer development time. This plant host-based developmental time (which is shorter on green pea and longer on maize) of
S. exigua is well supported by our nutrient content analysis, which clearly showed variations in the nutrient contents among the host plant leaves (
Table 8). The contents of all nutrients (lipid, protein, raffinose, sucrose, glucose, galactose, and fructose) except stachyose were detected in green pea, with a higher lipid content (5.31%) and protein content (35.94%) than maize (lipid: 4.74%; protein: 29.78%). In contrast to our study, [
84] reported lower developmental times of 3 days for eggs, 14.91 and 13.10 days for larvae, 7.02 and 6.66 days for pupae, and 24.93 and 22.74 days for egg-adults at 26 ± 1 °C on maize (
Z. mays) and soybean (
G. max), respectively. The authors of [
8] reported lower total developmental times of 16.91, 27.21, 41.63, and 120.50 days at 30, 25, 20, and 15 °C, respectively, on sugar beet. These differences may be attributed to food (sugar beet), geographic origin, genetic makeup, and the exposure and experience of differential adaption to the environmental regimes. This phenomenon (variations in developmental time based on the host plant and nutrient quality) of
S. exigua has been reported in several previous studies [
3,
6,
9,
84,
86,
87,
88,
89,
90].
Origination and the development-based lower temperature threshold can be used to estimate the population development of organisms [
91]. The estimations of LTDs for different stages of
S. exigua from the linear model estimated by [
19,
21] on the artificial diet as well as [
8], are all in contrast to the findings of our study. The authors of [
19,
21] estimated higher LTDs for eggs, larvae, pupae and total immatures. However, [
8] reported lower LDTs for the egg, larva, and pupa stages on sugar beet than we estimated on soybean and the artificial diet.
The authors of [
11] also reported lower LDTs for eggs (7.50 °C), larvae (7.96 °C), pupae (5.60 °C), and total immatures (5.60 °C) than our estimates for eggs (12.80 °C), larvae (12.43 °C), pupae (13.11 °C), and total immatures (12.18 °C) on the artificial diet. According to the present study, eggs required 39.91 DD to hatch on the artificial diet, which is similar to the thermal constant (39.37 DD) reported by [
9] and lower than the thermal constant (23.62) reported by [
11]. However, Refs. [
79,
92] reported higher thermal constant values (42.55 and 49.15 DD, respectively) on an artificial diet than the present study. In contrast to our study, Ref. [
8] reported a lower thermal constant (40.16 DD) for egg hatching on sugar beet than we estimated on the plant hosts. The thermal constant of the larval period in our study (195.24 DD) was higher on the artificial diet than that estimated by [
21] (155.72 DD), [
19] (155.80 DD), [
9] (128.70 DD), and [
11] (191.49 DD). Similarly, we also estimated a higher thermal constant on plant hosts than that reported by [
8] on sugar beet (174.83 DD) and by [
9] on Pigweed (123.20 DD) and cotton (157.70 DD). This variation may also be due to differences in the nutrient quality of plant hosts i.e., it is possible that a factor other than temperature affects the thermal requirement, as suggested by the significant differences seen among plant hosts, temperatures, and the interaction between plant host and temperature in this study (
Table 6). With similar findings to this study, Ref. [
93] reported that there is a significant interaction of temperature and diet that ultimately alters the developmental time of larvae of
S. exigua, even at an identical temperature on different diets. This discrepancy could also be due to the differences in each study’s experimental methodology. For example, in this study, for pupal development, we used a Petri dish (5 cm dia.
× 1.5 cm height, with topside ventilation) with the leaves of each plant host above a piece of tissue paper to provide a rough surface. Each Petri dish was then closed tightly with para film to protect the larvae from escaping and to maintain moisture. However, we did not provide soil for pupation, whereas Ref. [
8] provided 1 cm height soil for pupation purposes. One should therefore consider the possibility that the materials and methodologies adopted could affect pupal development and its thermal constants.
In existing research studies, several mathematical functions have been developed and employed to describe the insect development rates across thermal regimes. The selection of models is generally based on the choice of authors and is strongly subject to field -associated biases. While certain models consistently perform better within a specific context, there is no consensus on which are generally best across a wide range of applications [
94]. Many investigations related to temperature-dependent development are carried out with a single model or a few models targeting a particular taxonomic group, often without justification. Some of the important qualities of the model, such as the potentially superior predictive power or other beneficial qualities may be overlooked [
95]. To overcome this limitation, we selected several models and analyzed the observed data using different models. One nonlinear mathematical function (SSI) was used to adequately describe the developmental rate versus temperature curve, because the relationship between the developmental rate and temperature is curvilinear near extremes. Both the temperature threshold and the temperature maximum using the SSI nonlinear model were employed to estimate the temperature ranges for insect development, because the model provides clear biophysical meaning and thermodynamic information among model parameters [
82,
96]. Further, Ref. [
97] suggested that the SSI function performed as well as, or relatively better than, other functions. The developmental rate of
S. exigua was well fitted and described using a nonlinear model, which is indicated by the high coefficient (r
2) values (0.98–0.99) and the minimum variance of the estimated parameters on all plant hosts. The intrinsic optimum temperature (
) value for development is the most critical factor that determines the fitness of an optimum life history strategy [
58], which suggests the involvement of the maximal active state of enzymes in the development process [
98,
99]. The
values in this study using the SSI model were 26.6 °C on soybean, 23.6 °C on maize, 22.3 °C on potato, 33.1 °C on green pea, and 26.7 °C on the artificial diet, which differ from those of [
8], who estimated 28.5 °C on sugar beet. The T low values estimated by the SSI model varied between 12.11 °C and 14.91 °C, and the T high values estimated by the SSI model for the total immature stage were 35.6 °C on soybean, 37.1 °C on maize, 37.9 °C on potato, 50.3 °C on green pea, and 37.0 °C on the artificial diet (
Table 4). The estimated T low and T high values in the present study differ from those reported by [
8], who estimated respective values of 13.3 °C and 34.6 °C on sugar beet. The
value (33.1 °C on green pea) estimated by the SSI model for the total immature stage refers to the temperature that can make the population obtain maximum fitness as the true optimal temperature, in which the developmental rate was 0.0544 day-1.
Standard laboratory tests under a standard set of conditions, such as constant temperature and other controlled and replicable conditions with a particular physiological or behavioral response or survival, often produce relatively simple relationships. However, when comparing laboratory conditions with field conditions, field conditions are much more complex and variable. and within this complexity, several types of known or unknown traits might be involved [
100]. The authors of [
101] reported an alteration in the phenotype of
Drosophila melanogaster Meigen due to the effect of developmental and adult temperature acclimation. The authors of [
100] reported adaptive effects of acclimation in both laboratory and field tests, with stronger effects in the field test. Though there is a difference in the relationship between the laboratory and field, the estimated laboratory-based traits might still be significant and highly relevant to field performance, but this critical assumption needs to be verified [
102].
The results of the present study provide substantial evidence indicating that temperature and plant hosts significantly affect the longevity of
S. exigua (
Table 7). The longest longevity for
S. exigua was found at 30 °C on soybean, while the shortest was found at 35 °C on potato. As stated above, longevity differences among plant hosts can also be related to the nutritional quality and quantity of plant host species as well as the primary and secondary biochemical contents available on those plant hosts [
83,
84]. Significant differences were also found between the longevity values of females and males. On all plant hosts, females had longer longevity than males. Several previous studies have also reported differences in longevity between sexes of
S. exigua [
19,
21] and other insect species, such as the azuki bean weevil (
Callosobruchus chinensis, Coleoptera: Bruchidae) [
26] and the potato leafminer fly (
Liriomyza huidobrensis, Diptera: Agromyzidae) [
103]. These differences in adult longevity could be due to (i) complex interactions between the specific local environmental conditions and sex-specific costs of reproduction, (ii) epigenetic control of longevity by imprinting through DNA methylation, and (iii) increased fecundity and protection from aging stemming from the act of mating or components from the male ejaculate [
104].
Linear models are widely used to estimate the lower temperature threshold and thermal constant of insect species [
105], though researchers have highlighted many drawbacks [
106,
107]. Despite these shortcomings, linear models remain widely used because they require minimal data input for formulation with easy calculation. Therefore, their application has generally been found to yield correct values with negligible differences in accuracy [
36]. For these reasons, we adopted and used a linear model to estimate the parameters of temperature-dependent development on different plant hosts and on an artificial diet. By using a linear model with 397.27 DD on soybean, 458.34 DD on maize, 446.23 DD on potato, 439.75 DD on green pea, and 355.82 DD on the artificial diet, we tested the simple application of a degree-day model with the biofix of 1 January [
33,
108] to predict the number of generations of
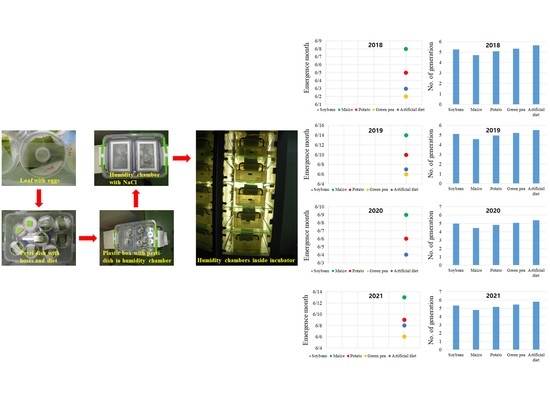
S. exigua, which resulted in 5.2, 4.6, 5.0, 5.3, and 5.6 generations on soybean, maize, potato, green pea, respectively. The resulting spring emergence date of
S. exigua was 2–8 June in 2018, 6–14 June in 2019, 4–9 June in 2020, and 6–13 June in 2021 on plant hosts in Korea (
Table 5) (Maharjan, unpublished). As a result, this study provides important information on the temperature-dependent development of this polyphagous pest in Korea, which is expected to be useful for the prediction modeling of the distribution expansion and population regulation of
S. exigua from a climate change perspective [
107,
109]. The model developed in the present study could contribute to the development of integrated pest management strategies including spray timing [
110,
111], even with limited capacity of extrapolation from the laboratory-based parameter estimation [
112,
113].