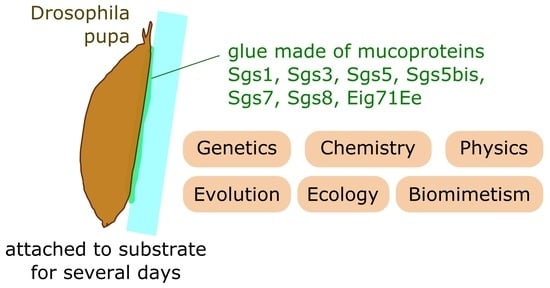
Drosophila Glue: A Promising Model for Bioadhesion
Abstract
:Simple Summary
Abstract
1. Introduction
2. Research Interest in the Adhesive Properties of Drosophila Glue Is New
3. Aspect and Ultrastructure of Drosophila Glue
4. Adhesive Properties of the Glue
5. Production and Expectoration of the Glue
6. Identification of the Glue Genes in D. melanogaster
7. Characteristics and Functions of the Glue Proteins in D. melanogaster
8. Glue Genes and Proteins in other Drosophila Species
9. Glue Gene Evolution
10. Role of Drosophila Glue in Natural Environments
11. Perspectives for Future Applications
12. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A2 | Abdominal segment 2 |
| DOPA | 3,4 dihydroxy phenylalanine |
| Sgs gene | Salivary gland secretion gene |
| T2 | Thoracic segment 2 |
References
- Bianco-Peled, H.; Davidovich-Pinhas, M. Bioadhesion and Biomimetics: From Nature to Applications; CRC Press: Boca Raton, FL, USA, 2015; ISBN 978-981-4463-99-7. [Google Scholar]
- Pandey, N.; Soto-Garcia, L.F.; Liao, J.; Zimmern, P.; Nguyen, K.T.; Hong, Y. Mussel-Inspired Bioadhesives in Healthcare: Design Parameters, Current Trends, and Future Perspectives. Biomater. Sci. 2020, 8, 1240–1255. [Google Scholar] [CrossRef] [PubMed]
- Kord Forooshani, P.; Lee, B.P. Recent Approaches in Designing Bioadhesive Materials Inspired by Mussel Adhesive Protein. J. Polym. Sci. Part Polym. Chem. 2017, 55, 9–33. [Google Scholar] [CrossRef]
- Fraenkel, G.; Brookes, V.J. The Process by which the Puparia of Many Species of Flies Become Fixed to a Substrate. Biol. Bull. 1953, 105, 442–449. [Google Scholar] [CrossRef]
- Ashburner, M. Drosophila: A Laboratory Handbook, 2nd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2004. [Google Scholar]
- O’Grady, P.M.; DeSalle, R. Phylogeny of the Genus Drosophila. Genetics 2018, 209, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Markow, T.; O’Grady, P.M. Evolutionary Genetics of Reproductive Behavior in Drosophila: Connecting the Dots. Annu. Rev. Genet. 2005, 39, 263–291. [Google Scholar] [CrossRef]
- Godoy-Herrera, R.; Cifuentes, L.; Díaz de Arcaya, M.F.; Fernández, M.; Fuentes, M.; Reyes, I.; Valderrama, C. The Behaviour of Drosophila Melanogaster Larvae during Pupation. Anim. Behav. 1989, 37, 820–829. [Google Scholar] [CrossRef]
- Godoy-Herrera, R.; Silva-Cuadra, J.L. The Behavior of Sympatric Chilean Populations of Drosophila Larvae during Pupation. Genet. Mol. Biol. 1998, 21, 31–39. [Google Scholar] [CrossRef]
- Vandal, N.B.; Siddalingamurthy, G.S.; Shivanna, N. Larval Pupation Site Preference on Fruit in Different Species of Drosophila. Entomol. Res. 2008, 38, 188–194. [Google Scholar] [CrossRef]
- Carson, H.L. The Association between Drosophila Carcinophila Wheeler and Its Host, the Land Crab Gecarcinus ruricola (L.). Am. Midl. Nat. 1967, 78, 324–343. [Google Scholar] [CrossRef]
- Carson, H.L.; Wheeler, M.R. Drosophila Endobranchia, a New Drosophilid1 Associated with Land Crabs in the West Indies. Ann. Entomol. Soc. Am. 1968, 61, 675–678. [Google Scholar] [CrossRef]
- Sokolowski, M.B. Genetics and Ecology of Drosophila Melanogaster Larval Foraging and Pupation Behaviour. J. Insect Physiol. 1985, 31, 857–864. [Google Scholar] [CrossRef]
- Beltramí, M.; Medina-Muñoz, M.C.; Arce, D.; Godoy-Herrera, R. Drosophila Pupation Behavior in the Wild. Evol. Ecol. 2010, 24, 347–358. [Google Scholar] [CrossRef]
- Beltramí, M.; Medina-Muñoz, M.C.; Pino, F.D.; Ferveur, J.-F.; Godoy-Herrera, R. Chemical Cues Influence Pupation Behavior of Drosophila Simulans and Drosophila Buzzatii in Nature and in the Laboratory. PLoS ONE 2012, 7, e39393. [Google Scholar] [CrossRef] [PubMed]
- Grossfield, J. Non-Sexual Behavior of Drosophila; Academic Press: London, UK, 1978; Volume 2, pp. 1–126. [Google Scholar]
- Woltz, J.M.; Lee, J.C. Pupation Behavior and Larval and Pupal Biocontrol of Drosophila Suzukii in the Field. Biol. Control 2017, 110, 62–69. [Google Scholar] [CrossRef]
- Crosby, M.A.; Meyerowitz, E.M. Drosophila Glue Gene Sgs-3: Sequences Required for Puffing and Transcriptional Regulation. Dev. Biol. 1986, 118, 593–607. [Google Scholar] [CrossRef]
- Farkaš, R. The Complex Secretions of the Salivary Glands of Drosophila Melanogaster, A Model System. In Extracellular Composite Matrices in Arthropods; Cohen, E., Moussian, B., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 557–600. ISBN 978-3-319-40738-8. [Google Scholar]
- Meyerowitz, E.M.; Crosby, M.A.; Garfinkel, M.D.; Martin, C.H.; Mathers, P.H.; Vijay Raghavan, K. The 68C Glue Puff of Drosophila. Cold Spring Harb. Symp. Quant. Biol. 1985, 50, 347–353. [Google Scholar] [CrossRef]
- Borne, F.; Kovalev, A.; Gorb, S.; Courtier-Orgogozo, V. The Glue Produced by Drosophila melanogaster for Pupa Adhesion Is Universal. J. Exp. Biol. 2020, 223, jeb220608. [Google Scholar] [CrossRef]
- Beňová-Liszeková, D.; Beňo, M.; Farkaš, R. A Protocol for Processing the Delicate Larval and Prepupal Salivary Glands of Drosophila for Scanning Electron Microscopy. Microsc. Res. Tech. 2019, 82, 1145–1156. [Google Scholar] [CrossRef]
- Del Pino, F.; Jara, C.; Pino, L.; Godoy-Herrera, R. The Neuro-Ecology of Drosophila Pupation Behavior. PLoS ONE 2014, 9, e102159. [Google Scholar] [CrossRef]
- Medina-Muñoz, M.C.; Godoy-Herrera, R. Dispersal and Prepupation Behavior of Chilean Sympatric Drosophila Species That Breed in the Same Site in Nature. Behav. Ecol. 2005, 16, 316–322. [Google Scholar] [CrossRef]
- Ringo, J.; Dowse, H. Pupation Site Selection in Four Drosophilid Species: Aggregation and Contact. J. Insect Behav. 2012, 25, 578–589. [Google Scholar] [CrossRef]
- Borne, F.; Prigent, S.R.; Molet, M.; Courtier-Orgogozo, V. Drosophila Glue Protects from Predation. Proc. R. Soc. B Biol. Sci. 2021, 288, 20210088. [Google Scholar] [CrossRef] [PubMed]
- Borne, F.; Kulathinal, R.J.; Courtier-Orgogozo, V. Glue Genes Are Subjected to Diverse Selective Forces during Drosophila Development. Genome Biol. Evol. 2021, 13, evab248. [Google Scholar] [CrossRef] [PubMed]
- Du, D.; Chen, X.; Shi, C.; Zhang, Z.; Shi, D.; Kaneko, D.; Kaneko, T.; Hua, Z. Mussel-Inspired Epoxy Bioadhesive with Enhanced Interfacial Interactions for Wound Repair. Acta Biomater. 2021, 136, 223–232. [Google Scholar] [CrossRef]
- Ebnesajjad, S.; Landrock, A.H. Characteristics of Adhesive Materials. In Adhesives Technology Handbook; Elsevier: Amsterdam, The Netherlands, 2015; pp. 84–159. ISBN 978-0-323-35595-7. [Google Scholar]
- Kress, H. Temporal Relationships between Leaving Food, Ecdysone Release, Mucoprotein Extrusion and Puparium Formation in Drosophila Virilis. J. Insect Physiol. 1974, 20, 1041–1055. [Google Scholar] [CrossRef]
- Reynolds, H.M.; Zhang, L.; Tran, D.T.; Ten Hagen, K.G. Tango1 Coordinates the Formation of Endoplasmic Reticulum/Golgi Docking Sites to Mediate Secretory Granule Formation. J. Biol. Chem. 2019, 294, 19498–19510. [Google Scholar] [CrossRef]
- Ji, S.; Samara, N.L.; Revoredo, L.; Zhang, L.; Tran, D.T.; Muirhead, K.; Tabak, L.A.; Ten Hagen, K.G. A Molecular Switch Orchestrates Enzyme Specificity and Secretory Granule Morphology. Nat. Commun. 2018, 9, 3508. [Google Scholar] [CrossRef]
- Syed, Z.A.; Zhang, L.; Tran, D.T.; Bleck, C.K.E.; Hagen, K.G.T. Orchestrated Restructuring Events During Secretory Granule Maturation Mediate Intragranular Cargo Segregation. bioRxiv 2022. bioRxiv:2021.08.16.456250. [Google Scholar]
- Rousso, T.; Schejter, E.D.; Shilo, B.-Z. Orchestrated Content Release from Drosophila Glue-Protein Vesicles by a Contractile Actomyosin Network. Nat. Cell Biol. 2016, 18, 181–190. [Google Scholar] [CrossRef]
- Duan, J.; Zhao, Y.; Li, H.; Habernig, L.; Gordon, M.D.; Miao, X.; Engström, Y.; Büttner, S. Bab2 Functions as an Ecdysone-Responsive Transcriptional Repressor during Drosophila Development. Cell Rep. 2020, 32, 107972. [Google Scholar] [CrossRef]
- Tran, D.T.; Masedunskas, A.; Weigert, R.; Ten Hagen, K.G. Arp2/3-Mediated F-Actin Formation Controls Regulated Exocytosis in Vivo. Nat. Commun. 2015, 6, 10098. [Google Scholar] [CrossRef] [PubMed]
- von Gaudecker, B. Der Strukturwandel der larvalen Speicheldrüse vonDrosophila melanogaster. Z. Für Zellforsch. Mikrosk. Anat. 1972, 127, 50–86. [Google Scholar] [CrossRef]
- Farkaš, R.; Šuťáková, G. Ultrastructural Changes of Drosophila Larval and Prepupal Salivary Glands Cultured in Vitro with Ecdysone. Vitro Cell. Dev. Biol.—Anim. 1998, 34, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Syed, Z.A.; Zhang, L.; Ten Hagen, K.G. In Vivo Models of Mucin Biosynthesis and Function. Adv. Drug Deliv. Rev. 2022, 184, 114182. [Google Scholar] [CrossRef]
- Lane, N.J.; Carter, Y.R.; Ashburner, M. Puffs and Salivary Gland Function: The Fine Structure of the Larval and Prepupal Salivary Glands OfDrosophila Melanogaster. Wilhelm Roux Arch. Für Entwickl. Org. 1972, 169, 216–238. [Google Scholar] [CrossRef]
- Heredia, F.; Volonté, Y.; Pereirinha, J.; Fernandez-Acosta, M.; Casimiro, A.P.; Belém, C.G.; Viegas, F.; Tanaka, K.; Menezes, J.; Arana, M.; et al. The Steroid-Hormone Ecdysone Coordinates Parallel Pupariation Neuromotor and Morphogenetic Subprograms Via Epider-Mis-To-Neuron Dilp8-Lgr3 Signal Induction. Nat. Commun. 2021, 12, 1–20. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Suleski, M.; Hedges, S.B. TimeTree: A Resource for Timelines, Timetrees, and Divergence Times. Mol. Biol. Evol. 2017, 34, 1812–1819. [Google Scholar] [CrossRef]
- Kodani, M. The Protein of the Salivary Gland Secretion in Drosophila. Proc. Natl. Acad. Sci. USA 1948, 34, 131–135. [Google Scholar] [CrossRef]
- Korge, G. Chromosome Puff Activity and Protein Synthesis in Larval Salivary Glands of Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA 1975, 72, 4550–4554. [Google Scholar] [CrossRef]
- Russell, S.; Ashburner, M. Ecdysone-Regulated Chromosome Puffing in Drosophila Melanogaster. In Metamorphosis; Elsevier: Amsterdam, The Netherlands, 1996; pp. 109–144. [Google Scholar]
- Muskavitch, M.A.; Hogness, D.S. Molecular Analysis of a Gene in a Developmentally Regulated Puff of Drosophila Melanogaster. Proc. Natl. Acad. Sci. USA 1980, 77, 7362–7366. [Google Scholar] [CrossRef]
- Meyerowitz, E.M.; Bond, M.W.; Crowley, T.E. The Structural Genes for Three Drosophila Glue Proteins Reside at a Single Polytene Chromosome Puff Locus. Mol. Cell Biol. 1983, 3, 12. [Google Scholar]
- Velissariou, V.; Ashburner, M. Cytogenetic and Genetic Mapping of a Salivary Gland Secretion Protein in Drosophila Melanogaster. Chromosoma 1981, 84, 173–185. [Google Scholar] [CrossRef] [PubMed]
- Akam, M.E.; Roberts, D.B.; Richards, G.P.; Ashburner, M. Drosophila: The Genetics of Two Major Larval Proteins. Cell 1978, 13, 215–225. [Google Scholar] [CrossRef]
- Da Lage, J.-L.; Thomas, G.W.C.; Bonneau, M.; Courtier-Orgogozo, V. Evolution of Salivary Glue Genes in Drosophila Species. BMC Evol. Biol. 2019, 19, 36. [Google Scholar] [CrossRef] [PubMed]
- Restifo, L.L.; Guild, G.M. An Ecdysterone-Responsive Puff Site in Drosophila Contains a Cluster of Seven Differentially Regulated Genes. J. Mol. Biol. 1986, 188, 517–528. [Google Scholar] [CrossRef]
- Wright, L.G.; Chen, T.; Thummel, C.S.; Guild, G.M. Molecular Characterization of the 71E Late Puff in Drosophila Melanogaster Reveals a Family of Novel Genes. J. Mol. Biol. 1996, 255, 387–400. [Google Scholar] [CrossRef]
- Korayem, A.M.; Fabbri, M.; Takahashi, K.; Scherfer, C.; Lindgren, M.; Schmidt, O.; Ueda, R.; Dushay, M.S.; Theopold, U. A Drosophila Salivary Gland Mucin Is Also Expressed in Immune Tissues: Evidence for a Function in Coagulation and the Entrapment of Bacteria. Insect Biochem. Mol. Biol. 2004, 34, 1297–1304. [Google Scholar] [CrossRef]
- Roth, G.E.; Wattler, S.; Bornschein, H.; Lehmann, M.; Korge, G. Structure and Regulation of the Salivary Gland Secretion Protein Gene Sgs-1 of Drosophila Melanogaster. Genetics 1999, 153, 753–762. [Google Scholar] [CrossRef]
- Garfinkel, M.D.; Pruitt, R.E.; Meyerowitz, E.M. DNA Sequences, Gene Regulation and Modular Protein Evolution in the Drosophila 68C Glue Gene Cluster. J. Mol. Biol. 1983, 168, 765–789. [Google Scholar] [CrossRef]
- Shore, E.M.; Guild, G.M. Larval Salivary Gland Secretion Proteins in Drosophila Structural Analysis of the Sgs-5 Gene. J. Mol. Biol. 1986, 190, 149–158. [Google Scholar] [CrossRef]
- Marin, F.; Luquet, G.; Marie, B.; Medakovic, D. Molluscan Shell Proteins: Primary Structure, Origin, and Evolution. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2007; Volume 80, pp. 209–276. [Google Scholar]
- Lanio, W.; Swida, U.; Kress, H. Molecular Cloning of the Drosophila Virilis Larval Glue Protein Gene Lgp-3 and Its Comparative Analysis with Other Drosophila Glue Protein Genes. Biochim. Biophys. Acta BBA—Gene Struct. Expr. 1994, 1219, 576–580. [Google Scholar] [CrossRef]
- Mateos, B.; Conrad-Billroth, C.; Schiavina, M.; Beier, A.; Kontaxis, G.; Konrat, R.; Felli, I.C.; Pierattelli, R. The Ambivalent Role of Proline Residues in an Intrinsically Disordered Protein: From Disorder Promoters to Compaction Facilitators. J. Mol. Biol. 2020, 432, 3093–3111. [Google Scholar] [CrossRef]
- Mettling, C.; Bourouis, M.; Richards, G. Allelic Variation at the Nueleotide Level in Drosophilaglue Genes. Mol. Gen. Genet. MGG 1985, 201, 265–268. [Google Scholar] [CrossRef]
- Andres, A.J.; Fletcher, J.C.; Karim, F.D.; Thummel, C.S. Molecular Analysis of the Initiation of Insect Metamorphosis: A Comparative Study of Drosophila Ecdysteroid-Regulated Transcription. Dev. Biol. 1993, 160, 388–404. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-R.; White, K.P. Tissue-Specific Gene Expression and Ecdysone-Regulated Genomic Networks in Drosophila. Dev. Cell 2003, 5, 59–72. [Google Scholar] [CrossRef]
- Barnett, S.W.; Flynn, K.; Webster, M.K.; Beckendorf, S.K. Noncoordinate Expression of Drosophila Glue Genes: Sgs-4 Is Expressed at Many Stages and in Two Different Tissues. Dev. Biol. 1990, 140, 362–373. [Google Scholar] [CrossRef]
- Swida, U.; Lucka, L.; Kress, H. Glue Protein Genes in Drosophila Virilis: Their Organization, Developmental Control of Transcription and Specific MRNA Degradation. Development 1990, 108, 269–280. [Google Scholar] [CrossRef]
- Kress, H. Ecdysone-Induced Puffing InDrosophila: A Model. Naturwissenschaften 1981, 68, 28–33. [Google Scholar] [CrossRef]
- Kress, H. Biochemical and Ontogenetic Aspects of Glycoprotein Synthesis in Drosophila Virilis Salivary Glands. Dev. Biol. 1982, 93, 231–239. [Google Scholar] [CrossRef]
- Ramesh, S.R.; Kalisch, W.-E. Glue Proteins InDrosophila Nasuta. Biochem. Genet. 1988, 26, 527–541. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, S.R.; Kalisch, W.-E. Comparative Analysis of Glue Proteins in TheDrosophila Nasuta Subgroup. Biochem. Genet. 1989, 27, 507–520. [Google Scholar] [CrossRef]
- Ramesh, S.R.; Shivanna, N. SDS-PAGE Pattern Polymorphism of X-Chromosomal Glue Proteins in Natural Populations of Two Drosophila Nasuta Subgroup Species. Biochem. Genet. 1999, 37, 1–21. [Google Scholar] [CrossRef]
- Perkowska, E. Some Characteristics of the Salivary Gland Secretion of Drosophila Virilis. Exp. Cell Res. 1963, 32, 259–271. [Google Scholar] [CrossRef]
- Thomopoulos, G.N.; Kastritsis, C.D. A Comparative Ultrastructural Study of ‘Glue’ Production and Secretion of the Salivary Glands in Different Species of TheDrosophila Melanogaster Group. Wilhelm Rouxs Arch. Dev. Biol. 1979, 187, 329–354. [Google Scholar] [CrossRef]
- Paris, M.; Boyer, R.; Jaenichen, R.; Wolf, J.; Karageorgi, M.; Green, J.; Cagnon, M.; Parinello, H.; Estoup, A.; Gautier, M.; et al. Near-chromosome level genome assembly of the fruit pest Drosophila suzukii using long-read sequencing. Sci. Rep. 2020, 10, 1–14. [Google Scholar] [CrossRef]
- Shirk, P.D.; Roberts, P.A.; Harn, C.H. Synthesis and Secretion of Salivary Gland Proteins in Drosophila Gibberosa during Larval and Prepupal Development. Rouxs Arch. Dev. Biol. 1988, 197, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.S. The Golgi Material and Mitochondria in the Salivary Glands of the Larva of Drosophila Melanogaster. J. Cell Sci. 1948, s3-89, 401–414. [Google Scholar] [CrossRef]
- Gregg, T.G.; McCrate, A.; Reveal, G.; Hall, S.; Rypstra, A.L. Insectivory and Social Digestion InDrosophila. Biochem. Genet. 1990, 28, 197–207. [Google Scholar] [CrossRef]
- Ashburner, M. Function and Structure of Polytene Chromosomes During Insect Development. In Advances in Insect Physiology; Elsevier: Amsterdam, The Netherlands, 1970; Volume 7, pp. 1–95. ISBN 978-0-12-024207-8. [Google Scholar]
- Jones, N.A.; Kuo, Y.M.; Sun, Y.H.; Beckendorf, S.K. The Drosophila Pax Gene Eye Gone Is Required for Embryonic Salivary Duct Development. Development 1998, 125, 4163–4174. [Google Scholar] [CrossRef]
- Beňová-Liszeková, D.; Mentelová, L.; Babišová, K.; Beňo, M.; Pechan, T.; Chase, B.A.; Farkaš, R. An Apocrine Mechanism Delivers a Fully Immunocompetent Exocrine Secretion. Sci. Rep. 2021, 11, 15915. [Google Scholar] [CrossRef]
- Seyahooei, M.A.; Kraaijeveld-Smit, F.J.L.; Kraaijeveld, K.; Crooijmans, J.B.M.; Van Dooren, T.J.M.; Van Alphen, J.J.M. Closely Related Parasitoids Induce Different Pupation and Foraging Responses in Drosophila Larvae. Oikos 2009, 118, 1148–1157. [Google Scholar] [CrossRef]
- Rossi, G.; Manfrin, A.; Lutolf, M.P. Progress and Potential in Organoid Research. Nat. Rev. Genet. 2018, 19, 671–687. [Google Scholar] [CrossRef] [PubMed]
- Low, L.A.; Mummery, C.; Berridge, B.R.; Austin, C.P.; Tagle, D.A. Organs-on-Chips: Into the next Decade. Nat. Rev. Drug Discov. 2021, 20, 345–361. [Google Scholar] [CrossRef] [PubMed]


| Sgs Gene Name | Band | Chromosome | Cytogenetic Map | Other Gene Names | Number of Amino Acids | Amino Acid Composition and Glycosylation State | Reference |
|---|---|---|---|---|---|---|---|
| Sgs1 | 1 | 2L | 25B4 | CG3047 | 1286 | Presence of repeats PTTTTPR/STTTTSTSR. Rich in cysteines, prolines, serines and threonines. Glycosylated. | [54] |
| Sgs3 | 3 | 3L | 68C11 | CG11720 | 307 | Presence of repeats KPTTT. Rich in cysteines, prolines, serines and threonines. Glycosylated. | [55] |
| Sgs4 | 4 | X | 3C11-12 | CG12181 | 297 | Presence of repeats. Rich in cysteines, prolines, serines and threonines. Glycosylated. | [46] |
| Sgs5 | 5 | 3R | 90B3-8 | CG7596 | 163 | No repeat. Rich in cysteines, prolines and serines. | [56] |
| Sgs5bis | - | 3R | 90B5 | CG7587 | 142 | No repeat. Rich in cysteines and prolines. | [50] |
| Sgs7 | - | 3L | 68C11 | CG18087 | 74 | No repeat. Rich in cysteines. | [47] |
| Sgs8 | - | 3L | 68C11 | CG6132 | 74 | No repeat. Rich in cysteines. | [47] |
| Eig71Ee | - | 3L | 71E5 | CG7604 VII I71–7 gp150 | 393 | Presence of repeats CTCTESTT/(R/K)TNPT. Rich in cysteines, prolines, serines and threonines. Glycosylated. | [52] |
| Species | Number of Bands | Glue Gene Sequences Identified | Reference |
|---|---|---|---|
| D. simulans D. sechellia | nd | Sgs1; Sgs3; Sgs4; Sgs5; Sgs7; Sgs8; Eig71Ee | [50] |
| D. mauritiana | nd | Sgs1; Sgs3; Sgs4; Sgs5; Sgs7; Eig71Ee | [50] |
| D. santomea D. yakuba | nd | Sgs1; Sgs3; Sgs4; Sgs5; Sgs5bis; Sgs7; Sgs7bis Sgs8; Eig71Ee | [50] |
| D. erecta | nd | Sgs3; Sgs4; Sgs5bis; Sgs7; Sgs8; Eig71Ee | [50] |
| D. eugracilis | nd | Sgs1; Sgs3; Sgs3bis; Sgs5; Sgs5bis; Sgs7; Sgs8; Eig71Ee | [50] |
| D.takahashii | nd | Sgs1; Sgs3; Sgs5; Sgs5bis; Sgs7; Sgs8; Eig71Ee | [50] |
| D. suzukii * | nd | Sgs1; Sgs4; Sgs5; Sgs5bis; 4 copies of Sgs7; Sgs8; Eig71Ee | [50] |
| D. biarmipes | nd | Sgs1; Sgs3; Sgs3bis; Sgs5; Sgs5bis; Sgs7; Sgs8; Eig71Ee | [50] |
| D.elegans | nd | Sgs1; Sgs3a; Sgs3b; Sgs3c; Sgs5 | [50] |
| D. rhopaloa | nd | Sgs1; Sgs3a; Sgs3b; Sgs3c; Sgs3d; Sgs5 | [50] |
| D. ficusphila | nd | Sgs1; Sgs3a; Sgs3b; Sgs3c; Sgs5; Sgs5bis; Eig71Ee | [50] |
| D. kikkawai D. ananassae | nd | Sgs3a; Sgs3b; Sgs5; Sgs5bis | [50] |
| D. bipectinata | nd | Sgs3a; Sgs3b; Sgs7; Sgs8; Sgs5; Sgs5bis; Eig71Ee | [50] |
| D. pseudoobscura | nd | Sgs3a; Sgs3b; Sgs3c; Sgs5bis | [50] |
| D. willistoni | nd | Sgs3a; Sgs3b; Sgs7a; Sgs7b | [50] |
| D. virilis | 3 | Sgs3a (or Lgp1); Sgs3b (or Lgp3) Sgs5bis (or Lgp2) | [50] |
| D. mojavensis | nd | Sgs4; Sgs5; Sgs7 | [19] |
| D. persimilis | nd | Sgs5; Sgs7; Sgs8 | [19] |
| D. n. nasuta | 9 | nd | [68] |
| D. n. albomicans | 10 | nd | [68] |
| D. n. kepulauana | 12 | nd | [68] |
| D. kohkoa | 10 | nd | [68] |
| D. s. albostrigata | 12 | nd | [68] |
| D. s. bilimbata | 14 | nd | [68] |
| D. s. sulfurigaster | 13 | nd | [68] |
| D. gibberosa | 17 | nd | [73] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monier, M.; Courtier-Orgogozo, V. Drosophila Glue: A Promising Model for Bioadhesion. Insects 2022, 13, 734. https://doi.org/10.3390/insects13080734
Monier M, Courtier-Orgogozo V. Drosophila Glue: A Promising Model for Bioadhesion. Insects. 2022; 13(8):734. https://doi.org/10.3390/insects13080734
Chicago/Turabian StyleMonier, Manon, and Virginie Courtier-Orgogozo. 2022. "Drosophila Glue: A Promising Model for Bioadhesion" Insects 13, no. 8: 734. https://doi.org/10.3390/insects13080734