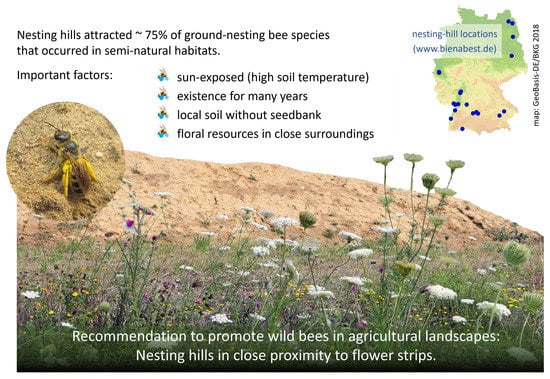
Artificial Nesting Hills Promote Wild Bees in Agricultural Landscapes
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Nesting Hills
2.2. Bee Survey on Nesting Hills
2.3. Abiotic Parameters (Substrate, Soil Temperature, Aspect)
2.4. Comparison of Nesting Hill and Local Bee Community
2.5. Landscape Composition (Cover of Semi-Natural Landscape Elements)
2.6. Pollen Survey
2.7. Statistical Analysis
2.7.1. Comparison of the Nesting Hill and the Local Bee Community
2.7.2. Abiotic Parameters (Substrate, Soil Temperature, Aspect), Colonization Time, and Landscape Composition (Cover of Semi-Natural Landscape Elements)
3. Results
3.1. Comparison of the Nesting to the Local Bee Community
3.2. Abiotic Parameters (Substrate, Soil Temperature, Aspect)
3.3. Landscape Composition (Cover of Semi-Natural Landscape Elements)
3.4. Pollen Foraging Behaviour
4. Discussion
4.1. Successive Colonization
4.2. Nesting Substrate
4.3. Temperature of Nesting Site
4.4. Local bee Communities and Foraging Habitats
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Westrich, P. Die Wildbienen Deutschlands; Eugen Ulmer: Stuttgart, Germany, 2018. [Google Scholar]
- Potts, S.G.; Biesmeijer, J.C.; Kremen, C.; Neumann, P.; Schweiger, O.; Kunin, W.E. Global pollinator declines: Trends, impacts and drivers. Trends Ecol. Evol. 2010, 25, 345–353. [Google Scholar] [CrossRef]
- Zattara, E.E.; Aizen, M.A. Worldwide occurrence records suggest a global decline in bee species richness. One Earth 2021, 4, 114–123. [Google Scholar] [CrossRef]
- Wojcik, V.A. Ecosystem Services: Pollinators and Pollination. In Terrestrial Ecosystems and Biodiversity; Wang, Y., Ed.; CRC Press: Boca Raton, FL, USA, 2020; pp. 157–163. [Google Scholar]
- Buhk, C.; Oppermann, R.; Schanowski, A.; Bleil, R.; Lüdemann, J.; Maus, C. Flower strip networks offer promising long term effects on pollinator species richness in intensively cultivated agricultural areas. BMC Ecol. 2018, 18, 55. [Google Scholar] [CrossRef]
- Neumüller, U.; Burger, H.; Krausch, S.; Blüthgen, N.; Ayasse, M. Interactions of local habitat type, landscape composition and flower availability moderate wild bee communities. Landsc. Ecol. 2020, 35, 2209–2224. [Google Scholar] [CrossRef]
- Schmidt, A.; Kirmer, A.; Hellwig, N.; Kiehl, K.; Tischew, S. Evaluating CAP wildflower strips: High quality seed mixtures significantly improve plant diversity and related pollen and nectar resources. J. Appl. Ecol. 2022, 59, 860–971. [Google Scholar] [CrossRef]
- Schmied, H.; Getrost, L.; Diestelhorst, O.; Maaßen, G.; Gerhard, L. Between perfect habitat and ecological trap: Even wildflower strips mulched annually increase pollinating insect numbers in intensively used agricultural landscapes. J. Insect Conserv. 2022, 26, 425–434. [Google Scholar] [CrossRef]
- Knop, E.; Kleijn, D.; Herzog, F.; Schmid, B. Effectiveness of the Swiss agri-environment scheme in promoting biodiversity. J. Appl. Ecol. 2006, 43, 120–127. [Google Scholar] [CrossRef]
- Bogusch, P.; Bláhová, E.; Horák, J. Pollen specialists are more endangered than non-specialised bees even though they collect pollen on flowers of non-endangered plants. Arthropod-Plant Interact. 2020, 14, 759–769. [Google Scholar] [CrossRef]
- Westerfelt, P.; Weslien, J.; Widenfalk, O. Population patterns in relation to food and nesting resource for two cavity-nesting bee species in young boreal forest stands. For. Ecol. Manag. 2018, 430, 629–638. [Google Scholar] [CrossRef]
- Wenzel, A.; Grass, I.; Belavadi, V.V.; Tscharntke, T. How urbanization is driving pollinator diversity and pollination–A systematic review. Biol. Conserv. 2020, 241, 108321. [Google Scholar] [CrossRef]
- Harmon-Threatt, A. Influence of nesting characteristics on health of wild bee communities. Annu. Rev. Entomol. 2020, 65, 39–56. [Google Scholar] [CrossRef]
- Requier, F.; Leonhardt, S. Beyond flowers: Including non-floral resources in bee conservation schemes. J. Insect Conserv. 2020, 24, 5–16. [Google Scholar] [CrossRef]
- Fortel, L.; Henry, M.; Guilbaud, L.; Mouret, H.; Vaissiere, B.E. Use of human-made nesting structures by wild bees in an urban environment. J. Insect Conserv. 2016, 20, 239–253. [Google Scholar] [CrossRef]
- Zurbuchen, A.; Müller, A. Wildbienenschutz-von der Wissenschaft zur Praxis; Haupt Verlag: Bern, Switzerland, 2012. [Google Scholar]
- Cane, J.H. Soils of ground-nesting bees (Hymenoptera: Apoidea): Texture, moisture, cell depth and climate. J. Kans. Entomol. Soc. 1991, 64, 406–413. [Google Scholar]
- Gregory, S.; Wright, I. Creation of patches of bare ground to enhance the habitat of ground-nesting bees and wasps at Shotover Hill, Oxfordshire, England. Conserv. Evid. 2005, 2, 139–141. [Google Scholar]
- Antoine, C.M.; Forrest, J.R. Nesting habitat of ground-nesting bees: A review. Ecol. Entomol. 2021, 46, 143–159. [Google Scholar] [CrossRef]
- Potts, S.; Willmer, P. Abiotic and biotic factors influencing nest-site selection by Halictus rubicundus, a ground-nesting halictine bee. Ecol. Entomol. 1997, 22, 319–328. [Google Scholar] [CrossRef]
- Zettel, H.; Zimmermann, D.; Wiesbauer, H. Ergänzungen zur Bienenfauna (Hymenoptera: Apidae) von Wien, Österreich. Beiträge Entomofaunist. 2016, 17, 85–107. [Google Scholar]
- Cane, J.H.; Neff, J.L. Predicted fates of ground-nesting bees in soil heated by wildfire: Thermal tolerances of life stages and a survey of nesting depths. Biol. Conserv. 2011, 144, 2631–2636. [Google Scholar] [CrossRef]
- Weissel, N.; Mitesser, O.; Liebig, J.; Poethke, H.-J.; Strohm, E. The influence of soil temperature on the nesting cycle of the halictid bee Lasioglossum malachurum. Insectes Soc. 2006, 53, 390–398. [Google Scholar] [CrossRef]
- Corbet, S.A.; Fussell, M.; Ake, R.; Fraser, A.; Gunson, C.; Savage, A.; Smith, K. Temperature and the pollinating activity of social bees. Ecol. Entomol. 1993, 18, 17–30. [Google Scholar] [CrossRef]
- Stone, G. Activity patterns of females of the solitary bee Anthophora plumipes in relation to temperature, nectar supplies and body size. Ecol. Entomol. 1994, 19, 177–189. [Google Scholar] [CrossRef]
- Biesmeijer, J.; Born, M.; Lukács, S.; Sommeijer, M.J. The response of the stingless bee Melipona beecheii to experimental pollen stress, worker loss and different levels of information input. J. Apic. Res. 1999, 38, 33–41. [Google Scholar] [CrossRef]
- Ganser, D.; Albrecht, M.; Knop, E. Wildflower strips enhance wild bee reproductive success. J. Appl. Ecol. 2021, 58, 486–495. [Google Scholar] [CrossRef]
- Neumüller, U.; Burger, H.; Schwenninger, H.R.; Hopfenmüller, S.; Krausch, S.; Weiß, K.; Ayasse, M. Prolonged blooming season of flower plantings increases wild bee abundance and richness in agricultural landscapes. Biodivers. Conserv. 2021, 30, 3003–3021. [Google Scholar] [CrossRef]
- Verein Deutscher Ingenieure. Biodiversität-Standardisierte Bestandsschonende Erfassung von Wildbienen für ein Langzeitmonitoring (Entwurf); VDI-Gesellschaft Technologies of Life Sciences: Düsseldorf, Germany, 2021. [Google Scholar]
- Dafni, A.; Kevan, P.G.; Husband, B.C. Practical Pollination Biology; Enviroquest Ltd.: Cambridge, UK, 2005. [Google Scholar]
- R CoreTeam. R: A Language and Environment for Statistical Computing; Version 4.0.0; R Foundation for Statistical Computing: Vienna, Austria, 2021. [Google Scholar]
- Hsieh, T.; Ma, K.; Chao, A. iNEXT: An R package for rarefaction and extrapolation of species diversity (Hill numbers). Methods Ecol. Evol. 2016, 7, 1451–1456. [Google Scholar] [CrossRef]
- Arbizu, M. PairwiseAdonis: Pairwise Multilevel Comparison Using Adonis. R Package Version 0.4. Available online: https://github.com/pmartinezarbizu/pairwiseAdonis (accessed on 15 June 2020).
- Legendre, P.; Gallagher, E.D. Ecologically meaningful transformations for ordination of species data. Oecologia 2001, 129, 271–280. [Google Scholar] [CrossRef]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.; O’Hara, R.; Simpson, G.; Solymos, P.; et al. Vegan: Community Ecology Package. R Package Version 2.5-6. Available online: https://cran.microsoft.com/snapshot/2020-04-03/web/packages/vegan/index.html (accessed on 18 November 2020).
- Wood, S. Mixed GAM Computation Vehicle with Automatic Smoothness Estimation. Available online: https://cran.r-project.org/web/packages/mgcv/ (accessed on 18 November 2020).
- Dormann, C.F.; Elith, J.; Bacher, S.; Buchmann, C.; Carl, G.; Carré, G.; Marquéz, J.R.G.; Gruber, B.; Lafourcade, B.; Leitão, P.J. Collinearity: A review of methods to deal with it and a simulation study evaluating their performance. Ecography 2013, 36, 27–46. [Google Scholar] [CrossRef]
- Westrich, P.; Frommer, U.; Mandery, K.; Riemann, H.; Ruhnke, H.; Saure, C.; Voith, J. Rote Liste und Gesamtartenliste der Bienen (Hymenoptera, Apidae) Deutschlands. In Rote Liste Gefährdeter Tiere, Pflanzen und Pilze Deutschlands. Band 3: Wirbellose Tiere (Teil 1); Bundesamt für Naturschutz, Ed.; Naturschutz und Biologische Vielfalt: Bonn-Bad Godesberg, Germany, 2011; Volume 70, pp. 373–416. [Google Scholar]
- Michener, C.D.; Lange, R.B.; Bigarella, J.J.; Salamuni, R. Factors influencing the distribution of bees’ nests in earth banks. Ecology 1958, 39, 207–217. [Google Scholar] [CrossRef]
- Wuellner, C.T. Nest site preference and success in a gregarious, ground-nesting bee Dieunomia triangulifera. Ecol. Entomol. 1999, 24, 471–479. [Google Scholar] [CrossRef]
- López-Uribe, M.M.; Morreale, S.J.; Santiago, C.K.; Danforth, B.N. Nest suitability, fine-scale population structure and male-mediated dispersal of a solitary ground nesting bee in an urban landscape. PLoS ONE 2015, 10, e0125719. [Google Scholar] [CrossRef] [PubMed]
- Yanega, D. Philopatry and nest founding in a primitively social bee, Halictus rubicundus. Behav. Ecol. Sociobiol. 1990, 27, 37–42. [Google Scholar] [CrossRef]
- Pereira, F.W.; Carneiro, L.; Gonçalves, R.B. More losses than gains in ground-nesting bees over 60 years of urbanization. Urban Ecosyst. 2021, 24, 233–242. [Google Scholar] [CrossRef]
- Ayers, A.C.; Rehan, S.M. Supporting bees in cities: How bees are influenced by local and landscape features. Insects 2021, 12, 128. [Google Scholar] [CrossRef] [PubMed]
- Heneberg, P.; Bogusch, P.; Řezáč, M. Numerous drift sand “specialists” among bees and wasps (Hymenoptera: Aculeata) nest in wetlands that spontaneously form de novo in arable fields. Ecol. Eng. 2018, 117, 133–139. [Google Scholar] [CrossRef]
- Lewis, M.; Cheney, C.; O Dochartaigh, B. Guide to Permeability Indices; Natural Environment Research Council: Swindon, UK, 2006; Available online: https://nora.nerc.ac.uk/id/eprint/7457/1/CR06160N.pdf (accessed on 11 July 2022).
- Singh, V.K.; Kumar, D.; Kashyap, P.; Singh, P.K.; Kumar, A.; Singh, S.K. Modelling of soil permeability using different data driven algorithms based on physical properties of soil. J. Hydrol. 2020, 580, 124223. [Google Scholar] [CrossRef]
- Eickwort, G.C. Aspects of the nesting biology of five Nearctic species of Agapostemon (Hymenoptera: Halictidae). J. Kans. Entomol. Soc. 1981, 54, 337–351. [Google Scholar]
- Corbet, S.A. Bee visits and the nectar of Echium vulgare L. and Sinapis alba L. Ecol. Entomol. 1978, 3, 25–37. [Google Scholar] [CrossRef]
- Willmer, P. Pollination and Floral Ecology; Princeton University Press: Princeton, NJ, USA, 2011; p. 778. [Google Scholar]
- Rozen, J.G. Comparative nesting biology of the bee tribe Exomalopsini (Apoidea, Anthophoridae). Am. Mus. Novit. 1984, 2798, 1–37. [Google Scholar]
- Guaraglia, D.O.; Pousa, J.L.; Pilan, L. Predicting temperature and heat flow in a sandy soil by electrical modeling. Soil Sci. Soc. Am. J. 2001, 65, 1074–1080. [Google Scholar] [CrossRef]
- Holmes, T.; Owe, M.; De Jeu, R.; Kooi, H. Estimating the soil temperature profile from a single depth observation: A simple empirical heatflow solution. Water Resour. Res. 2008, 44, 1–11. [Google Scholar] [CrossRef]
- Mallinger, R.E.; Gibbs, J.; Gratton, C. Diverse landscapes have a higher abundance and species richness of spring wild bees by providing complementary floral resources over bees’ foraging periods. Landsc. Ecol. 2016, 31, 1523–1535. [Google Scholar] [CrossRef]
- Hausmann, S.L.; Petermann, J.S.; Rolff, J. Wild bees as pollinators of city trees. Insect Conserv. Divers. 2016, 9, 97–107. [Google Scholar] [CrossRef]






| Pairwise PERMANOVA | df | F | R2 | p |
|---|---|---|---|---|
| Residual habitat vs. hill | 1 | 3.717 | 0.023 | <0.001 |
| Semi-natural grassland vs. hill | 1 | 3.767 | 0.023 | <0.001 |
| Flower planting vs. hill | 1 | 4.549 | 0.027 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neumüller, U.; Burger, H.; Mayr, A.V.; Hopfenmüller, S.; Krausch, S.; Herwig, N.; Burger, R.; Diestelhorst, O.; Emmerich, K.; Haider, M.; et al. Artificial Nesting Hills Promote Wild Bees in Agricultural Landscapes. Insects 2022, 13, 726. https://doi.org/10.3390/insects13080726
Neumüller U, Burger H, Mayr AV, Hopfenmüller S, Krausch S, Herwig N, Burger R, Diestelhorst O, Emmerich K, Haider M, et al. Artificial Nesting Hills Promote Wild Bees in Agricultural Landscapes. Insects. 2022; 13(8):726. https://doi.org/10.3390/insects13080726
Chicago/Turabian StyleNeumüller, Ulrich, Hannah Burger, Antonia V. Mayr, Sebastian Hopfenmüller, Sabrina Krausch, Nadine Herwig, Ronald Burger, Olaf Diestelhorst, Katrin Emmerich, Mare Haider, and et al. 2022. "Artificial Nesting Hills Promote Wild Bees in Agricultural Landscapes" Insects 13, no. 8: 726. https://doi.org/10.3390/insects13080726