Exploring Honeybee Abdominal Anatomy through Micro-CT and Novel Multi-Staining Approaches
Abstract
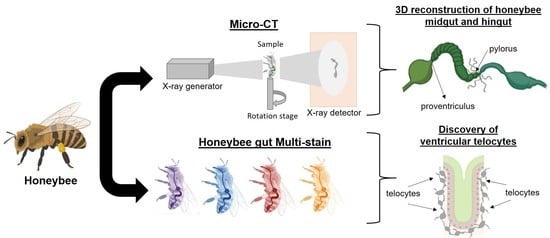
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Honeybee Samples
2.2. Micro-CT Analysis
2.3. Tissue Staining for Optical Microscopy
3. Results
3.1. Micro-CT Abdominal Reconstruction
3.2. Optical Multicolor Microscopy of Honeybee Hindgut
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wallberg, A.; Han, F.; Wellhagen, G.; Dahle, B.; Kawata, M.; Haddad, N.; Simões, Z.L.P.; Allsopp, M.H.; Kandemir, I.; De la Rúa, P.; et al. A Worldwide Survey of Genome Sequence Variation Provides Insight into the Evolutionary History of the Honeybee Apis Mellifera. Nat. Genet. 2014, 46, 1081–1088. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.M.; Loh, E.H.; Rostal, M.K.; Zambrana-Torrelio, C.M.; Mendiola, L.; Daszak, P. Pathogens, Pests, and Economics: Drivers of Honey Bee Colony Declines and Losses. EcoHealth 2013, 10, 434–445. [Google Scholar] [CrossRef]
- Gonçalves Santos, C.; Serrão, J.E. Histology of the Ileum in Bees (Hymenoptera, Apoidea). Braz. J. Morphol. Sci. 2006, 23, 405–413. [Google Scholar]
- Chapman, R.F. The Insects: Structure and Function; Cambridge University Press: Cambridge, UK, 1998; ISBN 978-0-511-81820-2. [Google Scholar]
- Terra, W.R. Physiology and Biochemistry of Insect Digestion: An Evolutionary Perspective. Braz. J. Med. Biol. Res. 1988, 21, 675–734. [Google Scholar]
- Stell, I.M. Understanding Bee Anatomy: A Full Colour Guide; The Catford Press: London, UK, 2012; ISBN 978-0-9574228-0-3. [Google Scholar]
- Lehane, M.J. Peritrophic Matrix Structure and Function. Annu. Rev. Entomol. 1997, 42, 525–550. [Google Scholar] [CrossRef]
- Oliveira, A.H.; Fernandes, K.M.; Gonçalves, W.G.; Zanuncio, J.C.; Serrão, J.E. A Peritrophin Mediates the Peritrophic Matrix Permeability in the Workers of the Bees Melipona Quadrifasciata and Apis Mellifera. Arthropod Struct. Dev. 2019, 53, 100885. [Google Scholar] [CrossRef]
- Serrão, J.E.; Da Cruz-Landim, C. The Ultrastructure of the Pyloric Valve Region in Bees, with Considerations of Fluid Flux in the Digestive Tract. Cytobios 1996, 351, 237–250. [Google Scholar]
- Snodgrass, R.E. Anatomy of the Honeybee; U.S Department of Agriculture, Bureau of Entomology, Technical Series; Cornell University Press: Ithaca, NY, USA, 1956; pp. 154–157. [Google Scholar]
- Boerckel, J.D.; Mason, D.E.; McDermott, A.M.; Alsberg, E. Microcomputed Tomography: Approaches and Applications in Bioengineering. Stem Cell Res. Ther. 2014, 5, 144. [Google Scholar] [CrossRef] [Green Version]
- Alba-Tercedor, J.; Alba-Alejandre, I. Comparing Micro-CT Results of Insects with Classical Anatomical Studies: Te European Honey Bee (Apis Mellifera Linnaeus, 1758) as a Benchmark (Insecta: Hymenoptera, Apidae). Microsc. Anal. 2019, 3, 12–15. [Google Scholar]
- Das, R.; Yadav, R.N.; Sihota, P.; Uniyal, P.; Kumar, N.; Bhushan, B. Biomechanical Evaluation of Wasp and Honeybee Stingers. Sci. Rep. 2018, 8, 14945. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.B.; Bernhardt, G.; Raine, N.E.; Abel, R.L.; Sykes, D.; Ahmed, F.; Pedroso, I.; Gill, R.J. Exploring Miniature Insect Brains Using Micro-CT Scanning Techniques. Sci. Rep. 2016, 6, 21768. [Google Scholar] [CrossRef]
- Rother, L.; Kraft, N.; Smith, D.B.; el Jundi, B.; Gill, R.J.; Pfeiffer, K. A Micro-CT-Based Standard Brain Atlas of the Bumblebee. Cell Tissue Res. 2021, 386, 29–45. [Google Scholar] [CrossRef]
- Hart, A.G.; Bowtell, R.W.; Köckenberger, W.; Wenseleers, T.; Ratnieks, F.L.W. Magnetic Resonance Imaging in Entomology: A Critical Review. J. Insect Sci. 2003, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Tomanek, B.; Jasiński, A.; Sułek, Z.; Muszyńska, J.; Kulinowski, P.; Kwieciński, S.; Krzyzak, A.; Skórka, T.; Kibiński, J. Magnetic Resonance Microscopy of Internal Structure of Drone and Queen Honey Bees. J. Apic. Res. 1996, 35, 3–9. [Google Scholar] [CrossRef]
- Sovran, B.; Hugenholtz, F.; Elderman, M.; Van Beek, A.A.; Graversen, K.; Huijskes, M.; Boekschoten, M.V.; Savelkoul, H.F.J.; De Vos, P.; Dekker, J.; et al. Age-Associated Impairment of the Mucus Barrier Function Is Associated with Profound Changes in Microbiota and Immunity. Sci. Rep. 2019, 9, 1437. [Google Scholar] [CrossRef]
- Cohen, M.; Varki, N.M.; Jankowski, M.D.; Gagneux, P. Using Unfixed, Frozen Tissues to Study Natural Mucin Distribution. JoVE 2012, 67, e3928. [Google Scholar] [CrossRef] [Green Version]
- Lo Cascio, P.; Calabrò, C.; Bertuccio, C.; Iaria, C.; Marino, F.; Denaro, M.G. Immunohistochemical Characterization of PepT1 and Ghrelin in Gastrointestinal Tract of Zebrafish: Effects of Spirulina Vegetarian Diet on the Neuroendocrine System Cells After Alimentary Stress. Front. Physiol. 2018, 9, 614. [Google Scholar] [CrossRef]
- Kali, A. Comparison of Microscopic Morphology of Fungi Using Lactophenol Cotton Blue (LPCB), Iodine Glycerol and Congo Red Formaldehyde Staining. JCDR 2014, 8, DL01. [Google Scholar] [CrossRef]
- Garvican, E.R.; Cree, S.; Bull, L.; Smith, R.K.; Dudhia, J. Viability of Equine Mesenchymal Stem Cells during Transport and Implantation. Stem Cell Res. Ther. 2014, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.S.; Jin, L.H.; Park, S.-H.; Kim, M.S.; Kim, Y.J.; Choi, B.H.; Lee, C.T.; Park, S.R.; Min, B.-H. Extracellular Matrix (ECM) Multilayer Membrane as a Sustained Releasing Growth Factor Delivery System for RhTGF-Β3 in Articular Cartilage Repair. PLoS ONE 2016, 11, e0156292. [Google Scholar] [CrossRef] [Green Version]
- Doello, K. A New Pentachrome Method for the Simultaneous Staining of Collagen and Sulfated Mucopolysaccharides. Yale J. Biol. Med. 2014, 87, 341–347. [Google Scholar]
- Holford, P. X-ray Computerised Microtomography (MicroCT): A New Technique for Assessing External and Internal Morphology of Bees. J. Apic. Res. 2008, 286–291. [Google Scholar] [CrossRef] [Green Version]
- Serrao, J. Proventricular Structure in Solitary Bees (Hymenoptera: Apoidea). Org. Divers. Evol. 2005, 5, 125–133. [Google Scholar] [CrossRef] [Green Version]
- Almehmadi, R.M.; Al-Ghamdi, A.A.; Aljedani, D.M. The Histological Structure of the Pyloric Valve in the Yemeni Honey Bees Queen and Worker (Indigenous) Apis Mellifera Jemenatica (Hymenoptera: Apidae). Cytobios 2015, 12, 145–153. [Google Scholar] [CrossRef]
- Kerkut, G.A. Comprehensive Insect Physiology, Volume 4 Regulation: Digestion, Nutrition, Excretion; Elsevier Science: Kent, UK, 2014; ISBN 978-1-4832-8620-4. [Google Scholar]
- Carreck, N.L.; Andree, M.; Brent, C.S.; Cox-Foster, D.; Dade, H.A.; Ellis, J.D.; Hatjina, F.; van Englesdorp, D. Standard Methods for Apis Mellifera Anatomy and Dissection. J. Apic. Res. 2013, 52, 1–40. [Google Scholar] [CrossRef] [Green Version]
- Popescu, L.M.; Manole, C.G.; Gherghiceanu, M.; Ardelean, A.; Nicolescu, M.I.; Hinescu, M.E.; Kostin, S. Telocytes in Human Epicardium. J. Cell. Mol. Med. 2010, 14, 2085–2093. [Google Scholar] [CrossRef] [Green Version]
- Suciu, L.; Popescu, L.M.; Gherghiceanu, M.; Regalia, T.; Nicolescu, M.I.; Hinescu, M.E.; Faussone-Pellegrini, M.-S. Telocytes in Human Term Placenta: Morphology and Phenotype. Cells Tissues Organs 2010, 192, 325–339. [Google Scholar] [CrossRef]
- Gherghiceanu, M.; Manole, C.G.; Popescu, L.M. TELOCYTES IN ENDOCARDIUM: Electron Microscope Evidence. J. Cell. Mol. Med. 2010, 14, 2330–2334. [Google Scholar] [CrossRef] [Green Version]
- Kaestner, K.H. The Intestinal Stem Cell Niche: A Central Role for Foxl1-Expressing Subepithelial Telocytes. Cell. Mol. Gastroenterol. Hepatol. 2019, 8, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Rosa, I.; Marini, M.; Manetti, M. Telocytes: An Emerging Component of Stem Cell Niche Microenvironment. J. Histochem. Cytochem. 2021, 69, 795–818. [Google Scholar] [CrossRef]
- Varga, I.; Kyselovič, J.; Danišovič, Ľ.; Gálfiová, P.; Kachlík, D.; Polák, Š.; Klein, M. Recently Discovered Interstitial Cells Termed Telocytes: Distinguishing Cell-Biological and Histological Facts from Fictions. Biologia 2019, 74, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Abd-Elhafeez, H.H.; Abdo, W.; Kamal, B.M.; Soliman, S.A. Fish Telocytes and Their Relation to Rodlet Cells in Ruby-Red-Fin Shark (Rainbow Shark) Epalzeorhynchos Frenatum (Teleostei: Cyprinidae). Sci. Rep. 2020, 10, 18907. [Google Scholar] [CrossRef] [PubMed]
- Yemor, T.; Phiancharoen, M.; Eric Benbow, M.; Suwannapong, G. Effects of Stingless Bee Propolis on Nosema Ceranae Infected Asian Honey Bees, Apis cerana. J. Apic. Res. 2015, 54, 468–473. [Google Scholar] [CrossRef]
- Plischuk, S.; Lance, C.E. Detección de Malpighamoeba Mellificae (Protista: Amoebozoa) En Apis Mellifera (Hymenoptera: Apidae) de Argentina. Rev. Soc. Entomol. Argent. 2010, 69, 299–303. [Google Scholar]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Paula, J.C.; Doello, K.; Mesas, C.; Kapravelou, G.; Cornet-Gómez, A.; Orantes, F.J.; Martínez, R.; Linares, F.; Prados, J.C.; Porres, J.M.; et al. Exploring Honeybee Abdominal Anatomy through Micro-CT and Novel Multi-Staining Approaches. Insects 2022, 13, 556. https://doi.org/10.3390/insects13060556
De Paula JC, Doello K, Mesas C, Kapravelou G, Cornet-Gómez A, Orantes FJ, Martínez R, Linares F, Prados JC, Porres JM, et al. Exploring Honeybee Abdominal Anatomy through Micro-CT and Novel Multi-Staining Approaches. Insects. 2022; 13(6):556. https://doi.org/10.3390/insects13060556
Chicago/Turabian StyleDe Paula, Jessica Carreira, Kevin Doello, Cristina Mesas, Garyfalia Kapravelou, Alberto Cornet-Gómez, Francisco José Orantes, Rosario Martínez, Fátima Linares, Jose Carlos Prados, Jesus María Porres, and et al. 2022. "Exploring Honeybee Abdominal Anatomy through Micro-CT and Novel Multi-Staining Approaches" Insects 13, no. 6: 556. https://doi.org/10.3390/insects13060556