Can Mixed Intercropping Protect Cereals from Aphid-Borne Viruses? An Experimental Approach
Abstract
:Simple Summary
Abstract
1. Introduction
- The Rhopalosiphum padi total abundance in a multiple plants system is decreased in the presence of clover.
- The BYDV-PAV incidence in barley is reduced in the presence of clover in arena.
- The spatial distributions of aphids and virus in arena are modified in the presence of clover.
- The effects of clover are more important for wingless founder aphids than for alate founder aphids.
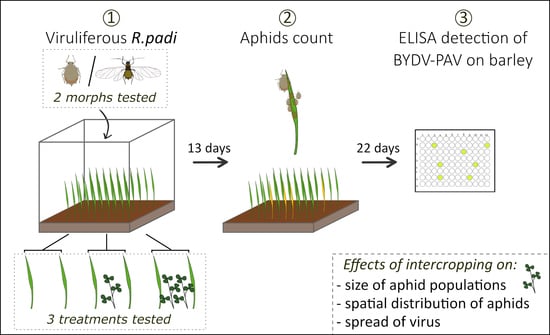
2. Materials and Methods
2.1. Plants, Insects and Virus
2.2. Arena Experiment
2.3. Serological Detection of BYDV-PAV in Plants
2.4. Statistical Analyses
2.4.1. Population Scale Analysis
2.4.2. Tiller Scale Analysis
3. Results
3.1. Morph and Virus Transmission
3.2. Morph and Clover Effect at Population Scale
3.3. Clover and Distance Effect at Tiller Scale
| Variable | Fixed Factors | Wingless | Alate | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| p-Value | R2 | p-Value | R2 | |||||||
| Aphids | occurrence per tiller | Distance | p < 0.001 | R2m: 0.07 R2c: 0.18 | p < 0.001 | 0.16 | ||||
| (a) | Treatment | 0.03 | 0.33 | |||||||
| D × T | 0.96 | p < 0.001 | ||||||||
| per infested tiller | Distance | p < 0.001 | 0.57 | p < 0.001 | 0.51 | |||||
| (b) | Treatment | 0.04 | 0.15 | |||||||
| D × T | 0.21 | 0.26 | ||||||||
| per tiller | Distance | p < 0.001 | 0.55 | p < 0.001 | 0.42 | |||||
| (c) | Treatment | 0.004 | 0.42 | |||||||
| D × T | 0.10 | 0.02 | ||||||||
| Virus | occurrence per tiller | Distance | p < 0.001 | R2m: 0.03 R2c: 0.46 | p < 0.001 | R2m: 0.06 R2c: 0.19 | ||||
| (d) | Treatment | 0.81 | 0.79 | |||||||
| D × T | 0.57 | 0.10 | ||||||||
| occurrence per non-infested tiller | Distance | 0.066 | 0.008 | p < 0.001 | 0.008 | |||||
| (e) | Treatment | 0.57 | 0.61 | |||||||
| D × T | 0.81 | 0.90 | ||||||||
| occurrence per infested tiller | Distance | 0.004 | 0.02 | p < 0.001 | 0.13 | |||||
| (f) | Treatment | 0.76 | 0.76 | |||||||
| D × T | 0.40 | 0.07 | ||||||||
3.3.1. Wingless Founder Morph
 . Areas are the 0.95 margin error for each curve.
. Areas are the 0.95 margin error for each curve.
 . Areas are the 0.95 margin error for each curve.
. Areas are the 0.95 margin error for each curve.
3.3.2. Alate Founder Morph
 . Areas are the 0.95 margin error for each curve.
. Areas are the 0.95 margin error for each curve.
 . Areas are the 0.95 margin error for each curve.
. Areas are the 0.95 margin error for each curve.
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perry, K.L.; Kolb, F.L.; Sammons, B.; Lawson, C.; Cisar, G.; Ohm, H. Yield effects of Barley yellow dwarf virus in soft red winter wheat. Phytopathology 2000, 90, 1043–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nancarrow, N.; Aftab, M.; Hollaway, G.; Rodoni, B.; Trębicki, P. Yield losses caused by Barley Yellow Dwarf Virus-PAV Infection in wheat and barley: A three-year field study in South-Eastern Australia. Microorganisms 2021, 9, 645. [Google Scholar] [CrossRef] [PubMed]
- Gray, S.M.; Gildow, F.E. Luteovirus-aphid interactions. Annu. Rev. Phytopathol. 2003, 41, 539–566. [Google Scholar] [CrossRef] [PubMed]
- Halbert, S.E.; Voegtlin, D. Biology and taxonomy of vectors of barley yellow dwarf viruses. In Barley Yellow Dwarf: 40 Years of Progress; D’Arcy, C.J., Burnett, P.A., Eds.; APS Press: St. Paul, MN, USA, 1995; pp. 217–258. [Google Scholar]
- Lister, R.M.; Ranieri, R. Distribution and economic importance of barley yellow dwarf. In Barley Yellow Dwarf: 40 Years of Progress; D’Arcy, C.J., Burnett, P.A., Eds.; APS Press: St. Paul, MN, USA, 1995; pp. 29–53. [Google Scholar]
- Wiktelius, S. Diurnal flight periodicities and temperature thresholds for flight for different migrant forms of Rhopalosiphum padi L. (Hom., Aphididae). Z. Angew. Entomol. 1981, 92, 449–457. [Google Scholar] [CrossRef]
- Elliott, N.C.; Kieckhefer, R.W. Effects of constant and fluctuating temperatures on immature development and age-specific life tables of Rhopalosiphum padi (L.) (Homoptera: Aphididae). Can. Entomol. 1989, 121, 131–140. [Google Scholar] [CrossRef]
- Carrigan, L.L.; Ohm, H.W.; Foster, J.E.; Patterson, F.L. Response of winter wheat cultivars to barley yellow dwarf virus infection. Crop Sci. 1981, 21, 377–380. [Google Scholar] [CrossRef]
- Leclercq-Le Quillec, F.; Tanguy, S.; Dedryver, C.A. Aerial flow of barley yellow dwarf viruses and of their vectors in western France. Ann. Appl. Biol. 1995, 126, 75–90. [Google Scholar] [CrossRef]
- Brabec, M.; Honěk, A.; Pekár, S.; Martinková, Z. Population dynamics of aphids on cereals: Digging in the time-series data to reveal population regulation caused by temperature. PLoS ONE 2014, 9, e106228. [Google Scholar] [CrossRef]
- Van den Eynde, R.; Van Leeuwen, T.; Haesaert, G. Identifying drivers of spatio-temporal dynamics in barley yellow dwarf virus epidemiology as acritical factor in disease control. Pest Manag. Sci. 2020, 76, 2548–2556. [Google Scholar] [CrossRef]
- Poehling, H.M.; Freier, B.; Klüken, A.M. IPM Case Studies: Grain. In Aphids as Crop Pests; Van Emden, H.F., Harrington, R., Eds.; CABI Press: Wallingford, UK, 2007; pp. 597–611. [Google Scholar]
- Nicholls, C.I.; Altieri, M.A. Agroecological bases of ecological engineering for pest management. In Ecological Engineering for Pest Management: Advances in Habitat Manipulation for Arthropods; Gurr, G.M., Wratten, S.D., Altieri, M.A., Eds.; CSIRO Publishing: Collingwood, Australia, 2004; pp. 33–54. [Google Scholar]
- Smith, J.G. Some effects of crop background on populations of aphids and their natural enemies on brussels sprouts. Ann. Appl. Biol. 1969, 63, 326–329. [Google Scholar] [CrossRef]
- Smith, J.G. Influence of crop background on aphids and other phytophagous insects on Brussels sprouts. Ann. Appl. Biol. 1976, 83, 1–13. [Google Scholar] [CrossRef]
- Horn, D.J. Effect of weedy backgrounds on colonization of collards by green peach aphid, Myzus persicae, and its major predators. Environ. Entomol. 1981, 10, 285–289. [Google Scholar] [CrossRef]
- Costello, M.J.; Altieri, M.A. Abundance, growth rate and parasitism of Brevicoryne brassicae and Myzus persicae (Homoptera: Aphididae) on broccoli grown in living mulches. Agric. Ecosyst. Environ. 1995, 52, 187–196. [Google Scholar] [CrossRef] [Green Version]
- Hooks, C.R.R.; Valenzuela, H.R.; Defrank, J. Incidence of pest and arthropod natural enemies in zucchini grown in living mulches. Agric. Ecosyst. Environ. 1998, 69, 217–231. [Google Scholar] [CrossRef]
- Showler, A.T.; Greenberg, S.M. Effects of weeds on selected arthropod herbivore and natural enemy populations, and on cotton growth and yield. Environ. Entomol. 2003, 32, 39–50. [Google Scholar] [CrossRef]
- Nottingham, S.F.; Hardie, J.; Dawson, G.W.; Hick, A.J.; Pickett, J.A.; Wadhams, L.J.; Woodcock, C.M. Behavioral and electrophysiological responses of aphids to host and nonhost plant volatiles. J. Chem. Ecol. 1991, 17, 1231–1242. [Google Scholar] [CrossRef] [PubMed]
- Nottingham, S.F.; Hardie, J. Flight behaviour of the black bean aphid, Aphis fabae, and the cabbage aphid, Brevicoryne brassicae, in host and non-host plant odour. Physiol. Entomol. 1993, 18, 389–394. [Google Scholar] [CrossRef]
- Perrin, R.M.; Phillips, M.L. Some effects of mixed cropping on the population dynamics of insect pests. Entomol. Exp. Appl. 1978, 24, 585–593. [Google Scholar] [CrossRef]
- Powell, G.; Tosh, C.R.; Hardie, J. Host plant selection by aphids: Behavioral, evolutionary, and applied perspectives. Annu. Rev. Entomol. 2006, 51, 309–330. [Google Scholar] [CrossRef]
- Mansion-Vaquié, A.; Ferrer, A.; Ramon-Portugal, F.; Wezel, A.; Magro, A. Intercropping impacts the host location behaviour and population growth of aphids. Entomol. Exp. Appl. 2020, 168, 41–52. [Google Scholar] [CrossRef] [Green Version]
- Hooks, C.R.R.; Fereres, A. Protecting crops from non-persistently aphid-transmitted viruses: A review on the use of barrier plants as a management tool. Virus Res. 2006, 120, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Bergkvist, G.; Stenberg, M.; Wetterlind, J.; Båth, B.; Elfstrand, S. Clover cover crops under-sown in winter wheat increase yield of subsequent spring barley—Effect of N dose and companion grass. Field Crops Res. 2011, 120, 292–298. [Google Scholar] [CrossRef]
- Amossé, C. Analyse Expérimentale de L’effet de Couverts de Légumineuses Associés en Relais à un Blé D’hiver, Conduit en Agriculture Biologique, sur les Performances des Cultures, la Maîtrise des Adventices et la Dynamique de L’azote. Ph.D. Thesis, AgroParisTech, Paris, France, 2013. [Google Scholar]
- Chain, F.; Riault, G.; Trottet, M.; Jacquot, E. Evaluation of the durability of the Barley yellow dwarf virus-resistant Zhong ZH and TC14 wheat lines. Eur. J. Plant Pathol. 2007, 117, 35–43. [Google Scholar] [CrossRef]
- Henry, M.; Dedryver, C.A. Fluctuations in cereal aphid populations on maize (Zea mays) in Western France in relation to the epidemiology of barley yellow dwarf virus. J. Appl. Entomol. 1989, 107, 401–410. [Google Scholar] [CrossRef]
- Henry, M.; Dedryver, C.A. Occurrence of barley yellow dwarf virus in pastures of western France. Plant Pathol. 1991, 40, 93–99. [Google Scholar] [CrossRef]
- Moericke, V. Über die Lebensgewohnheiten der geflügelten Blattläuse (Aphidina) unter besonderer Berücksichtigung des Verhaltens beim Landen. Z. Angew. Entomol. 1955, 37, 29–91. [Google Scholar] [CrossRef]
- Hodgson, C. Dispersal of apterous aphids (Homoptera: Aphididae) from their host plant and its significance. Bull. Entomol. Res. 1991, 81, 417–427. [Google Scholar] [CrossRef]
- Döring, T.F. How aphids find their host plants, and how they don’t. Ann. Appl. Biol. 2014, 165, 3–26. [Google Scholar] [CrossRef]
- Braendle, C.; Davis, G.K.; Brisson, J.A.; Stern, D.L. Wing dimorphism in aphids. Heredity 2006, 97, 192–199. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Port, G. Performance of clones and morphs of two cereal aphids on wheat plants with high and low nitrogen content. Entomol. Sci. 2008, 11, 159–165. [Google Scholar] [CrossRef]
- Power, A.G. Virus spread and vector dynamics in genetically diverse plant populations. Ecology 1991, 72, 232–241. [Google Scholar] [CrossRef]
- Rochow, W.J. Vectors and variations. In Proceedings of the 3rd International Symposium of Virus Diseases of Ornamental Plants, College Park, MD, USA, 11–15 September 1972; Lawson, R.H., Corbett, M.K., Eds.; International Society for Horticultural Science: The Hague, The Netherlands, 1974; pp. 75–83. [Google Scholar]
- Harrewijn, P. Integrated control of potato aphids. In Aphids: Their Biology, Natural Enemies and Control; Minks, A.K., Harrewijn, P., Eds.; Elsevier: Amsterdam, The Netherlands, 1989; Volume 2, pp. 279–284. [Google Scholar]
- Power, A.G. Leafhopper response to genetically diverse host plant stands. Entomol. Exp. Appl. 1988, 49, 213–219. [Google Scholar] [CrossRef]
- Medina-Ortega, K.J.; Bosque-Pérez, N.A.; Ngumbi, E.; Jiménez-Martínez, E.S.; Eigenbrode, S.D. Rhopalosiphum padi (Hemiptera: Aphididae) responses to volatile cues from Barley yellow dwarf virus-infected wheat. Environ. Entomol. 2009, 38, 836–845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ingwell, L.L.; Eigenbrode, S.D.; Bosque-Pérez, N.A. Plant viruses alter insect behavior to enhance their spread. Sci. Rep. 2012, 2, 578. [Google Scholar] [CrossRef] [Green Version]
- Fingu-Mabola, J.C.; Martin, C.; Bawin, T.; Verheggen, F.J.; Francis, F. Does the infectious status of aphids influence their preference towards healthy, virus-infected and endophytically colonized plants? Insects 2020, 11, 435. [Google Scholar] [CrossRef]
- Jiménez-Martínez, E.S.; Bosque-Pérez, N.A.; Berger, P.H.; Zemetra, R.S.; Ding, H.; Eigenbrode, S.D. Volatile cues influence the response of Rhopalosiphum padi (Homoptera: Aphididae) to barley yellow dwarf virus-infected transgenic and untransformed wheat. Environ. Entomol. 2004, 33, 1207–1216. [Google Scholar] [CrossRef] [Green Version]
- Mauck, K.E.; De Moraes, C.M.; Mescher, M.C. Effects of pathogens on sensory-mediated interactions between plants and insect vectors. Curr. Opin. Plant Biol. 2016, 32, 53–61. [Google Scholar] [CrossRef] [Green Version]


| Founder Aphid Morph * | ||
|---|---|---|
| Treatment | Wingless | Alate |
| b | 13 (6) | 10 (7) |
| bc1 | 12 (11) | 9 (5) |
| bc2 | 15 (12) | 6 (5) |
| Variable | Wingless | Alate | p-Value | R2 | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All Arenas | b | bc1 | bc2 | All Arenas | b | bc1 | bc2 | M | T | M × T | R2m | R2c | |||||||
| (a) | Distance founder tiller–deposit tiller | 1.2 ± 0.1 | 1.1 ± 0.1 | 1.2 ± 0.1 | 1.4 ± 0.1 | 2.3 ± 0.2 | 2.8 ± 0.3 | 2.5 ± 0.4 | 1.1 ± 0.4 | 0.001 | 0.11 | p < 0.001 | 0.19 | 0.19 | |||||
| (b) | Aphids | per founder tiller | 85.4 ± 3.9 | 93.4 ± 6.2 | 79.7 ± 8.3 | 83.0 ± 6.0 | 51.1 ± 4.9 | 42.7 ± 8.0 | 61.4 ± 8.8 | 49.5 ± 6.8 | p < 0.001 | 0.92 | 0.22 | 0.29 | 0.29 | ||||
| (c) | per arena | 342.2 ± 21.6 | 412.9 ± 39.1 | 316.3 ± 32.2 | 301.7 ± 34.6 | 132.7 ± 13.5 | 112.4 ± 18.4 | 137.9 ± 23.4 | 158.7 ± 32.4 | p < 0.001 | 0.44 | 0.009 | 0.53 | 0.63 | |||||
| (d) | occurrence per tiller (%) | 72.4 ± 2.7 | 80.5 ± 3.0 | 70.8 ± 5.7 | 66.7 ± 4.3 | 35.2 ± 3.9 | 28.6 ± 4.3 | 36.1 ± 8.7 | 44.9 ± 6.3 | p < 0.001 | 0.41 | p < 0.001 | 0.66 | 0.85 | |||||
| (e) | Virus | occurrence per tiller (%) | 43.1 ± 5.8 | 50.5 ± 13.2 | 37.1 ± 7.7 | 44.9 ± 10.5 | 29.9 ± 3.9 | 24.6 ± 5.7 | 36.1 ± 7.5 | 31.1 ± 8.2 | p < 0.001 | 0.57 | 0.01 | 0.38 | 0.62 | ||||
| (f) | occurrence per non-infested tillers (%) | 7.2 ± 1.5 | 7.9 ± 2.8 | 7.3 ± 2.5 | 6.7 ± 2.5 | 11.6 ± 1.3 | 11.1 ± 2.5 | 13.9 ± 9.9 | 9.4 ± 3.2 | 0.11 | 0.39 | 0.78 | 0.06 | 0.33 | |||||
| (g) | occurrence per infested tillers (%) | 35.9 ± 4.9 | 42.6 ± 11.6 | 29.8 ± 6.1 | 38.2 ± 8.9 | 18.5 ± 3.3 | 13.5 ± 3.2 | 22.2 ± 6.9 | 21.7 ± 7.8 | p < 0.001 | 0.28 | p < 0.001 | 0.56 | 0.66 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grauby, S.; Ferrer, A.; Tolon, V.; Roume, A.; Wezel, A.; Jacquot, E. Can Mixed Intercropping Protect Cereals from Aphid-Borne Viruses? An Experimental Approach. Insects 2022, 13, 521. https://doi.org/10.3390/insects13060521
Grauby S, Ferrer A, Tolon V, Roume A, Wezel A, Jacquot E. Can Mixed Intercropping Protect Cereals from Aphid-Borne Viruses? An Experimental Approach. Insects. 2022; 13(6):521. https://doi.org/10.3390/insects13060521
Chicago/Turabian StyleGrauby, Sarah, Aurélie Ferrer, Vincent Tolon, Anthony Roume, Alexander Wezel, and Emmanuel Jacquot. 2022. "Can Mixed Intercropping Protect Cereals from Aphid-Borne Viruses? An Experimental Approach" Insects 13, no. 6: 521. https://doi.org/10.3390/insects13060521