The Symbiotic Fungus Leucoagaricus gongylophorus (Möller) Singer (Agaricales, Agaricaceae) as a Target Organism to Control Leaf-Cutting Ants
Abstract
:Simple Summary
Abstract
1. Introduction
1.1. Damage Caused by Leaf-Cutting Ants
1.2. Biological Relationship between Leaf-Cutting Ants and Their Mutualistic Fungus
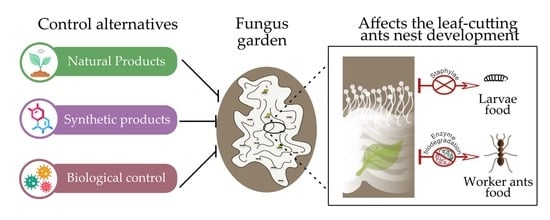
2. Fungal Control
2.1. Chemical Control
2.1.1. Natural Compound
| Species | Extract | Part of Plant | Inhibitory Effect | Reference |
|---|---|---|---|---|
| Achyrocline tomentosa | Ethanol extract | Leaves | 500 µg/spot–5 mm * (B) | [63] |
| Ageratum conyzoides | Hexane extract | Leaves | 25 mg/mL–81% (C) | [64] |
| Allium cepa | - | Bulbs | IC50–1241.55 μg/mL (A)/2000 µg/mL–0.0 to 0.02 g ** (D)/2000 µg/mL–Fungistatic (E) | [19] |
| Allium sativum | - | Seed pods | IC50–358.36 μg/mL (A)/2000 µg/mL–0.0 g ** (D)/2000 µg/mL–Fungicidal (E) | [19] |
| Aristolochia argentina | Ethanol extract | - | 500 µg/spot–15 mm * (B)/MIC–1.95 µg/mL (F) | [63] |
| Baccharis linearifolia | Ethanol extract | - | 500 µg/spot–5 mm * (B) | [63] |
| Baccharis coridifolia | Ethanol extract | - | 500 µg/spot–5 mm * (B) | [63] |
| Baccharis flabellata | Ethanol extract | - | 500 µg/spot–10 mm * (B) | [63] |
| Capsicum baccatum | Ethanol extract | Leaves | 0.1% w/v–40% (A) | [65] |
| Capsicum frutescens | Ethanol extract | Leaves | 0.1% w/v–20% (A) | [65] |
| Cipadessa fruticosa | Dichloromethane extract | Fruits | 1000 mg/mL–80% (A) | [69] |
| Hexane extract | Branches | 1000 mg/mL–80% (A) | ||
| Dichloromethane extract | Branches | 1000 mg/mL–40% (A) | ||
| Hexane extract | Leaves | 1000 mg/mL–20% (A) | ||
| Dichloromethane extract | Leaves | 1000 mg/mL–20% (A) | ||
| Coriandrum sativum | Hexane extract | Leaves | 100 mg/mL–100% (A) | [64] |
| Dalea elegans | Ethanol extract | - | 500 µg/spot–10 mm * (B) | [63] |
| Flourensia oolepis | Ethanol extract | - | 500 µg/spot–5 mm * (B)/MIC–7.8 µg/mL (F) | [63] |
| Grindelia pulchella | Ethanol extract | - | 500 µg/spot–5 mm * (B) | [63] |
| Lycopersicon esculentum | - | Green fruits | IC50−2262.29 μg/mL (A)/2000 µg/mL–0.02 to 0.05 g ** (D)/2000 µg/mL–Fungistatic (E) | [19] |
| Manihot esculenta | - | Leaves | IC50−553.32 μg/mL (A)/2000 µg/mL–0.0 g ** (D)/2000 µg/mL–Fungicidal (E) | [19] |
| Mentha piperita | Hexane extract | Leaves | 25 mg/mL–96% (C) | [64] |
| Myrcia lundiana | Essential oil (Citral chemotype) | Leaves | IC50−104.8 μL/L # (A)/IC50–217.9 µL/L ## (A) | [67] |
| Essential oil (Isopulegol chemotype) | Leaves | IC50−145.1 μL/L # (A)/IC50–238.1 µL/L ## (A) | ||
| Pilocarpus grandiflorus | Dichloromethane extract | Stems | 1000 µg/mL–10% (A) | [72] |
| Piper holtonii | Ethanol extract | Leaves | IC50−102 ppm (A) | [68] |
| Essential oil | Leaves | 1000 ppm–100% (A) | ||
| Pterocaulon alopecuroides | Ethanol extract | - | 500 µg/spot–15 mm * (B)/MIC–7.8 µg/mL (F) | [63] |
| Raulinoa echinata | Methanol extract | Stems | 1000 μg/mL–80% (A) | [17] |
| Methanol extract | Leaves | 1000 μg/mL–80% (A) | ||
| Methanol extract (hexane fraction) | Leaves | 500 μg/mL–80% (A) | ||
| Senna alata | - | Leaves | IC50−251.51 μg/mL (A)/500 µg/mL–0.0 g ** (D)/500 µg/mL–Fungicidal (E) | [19] |
| Sesamum indicum | Chloroform extract | Leaves | 60 mg/mL–60% (A) | [8] |
| Methanol extract | Leaves | 60 mg/mL–60% (A) | ||
| Methanol + Chloroform extract | Leaves | 60 + 60 mg/mL–>80% (A) | ||
| Chloroform extract | Leaves (30 days old) | 60 mg/mL–60% (A) | ||
| Chloroform extract | Leaves (60 days old) | 60 mg/mL–60% (A) | ||
| Chloroform extract | Green leaves (90 days old) | 60 mg/mL–40% (A) | ||
| Chloroform extract | Yellow leaves (90 days old) | 60 mg/mL–60% (A) | ||
| Chloroform extract | Green fruit | 30 mg/mL–40% (A) | ||
| Chloroform extract | Ripe fruit | 30 mg/mL–60% (A) | ||
| Chloroform extract | Green seed | 30 mg/mL–60% (A) | ||
| Chloroform extract | Ripe seed | 30 mg/mL–60% (A) | ||
| Trichocline reptans | Ethanol extract | - | 500 µg/spot–5 mm * (B) | [63] |
| Zanthoxylum coco | Ethanol extract | - | 500 µg/spot–10 mm * (B) | [63] |
| Microorganism | Strain | Extract | Inhibitory Effect (Antifungal Assay) | Reference |
|---|---|---|---|---|
| Escovopsioides nivea | LESF596 + Absence of L. gongylophorus | Crude extract | 3–5 cm2 * (A) | [66] |
| LESF596 + Presence of L. gongylophorus | Crude extract | 3–5 cm2 * (A) | ||
| LESF599 + Absence of L. gongylophorus | Crude extract | 4–5 cm2 * (A) | ||
| LESF599 + Presence of L. gongylophorus | Crude extract | 4–5 cm2 * (A) | ||
| Escovopsis sp. | LESF017 + Absence of L. gongylophorus | Crude extract | 3–5 cm2 * (A) | [66] |
| LESF017 + Presence of L. gongylophorus | Crude extract | 2–3 cm2 * (A) | ||
| LESF019 + Absence of L. gongylophorus | Crude extract | 4–5 cm2 * (A) | ||
| LESF019 + Presence of L. gongylophorus | Crude extract | 3–4 cm2 * (A) | ||
| LESF021 + Absence of L. gongylophorus | Crude extract | 2–4 cm2 * (A) | ||
| LESF021 + Presence of L. gongylophorus | Crude extract | 1–3 cm2 * (A) | ||
| LESF023 + Absence of L. gongylophorus | Crude extract | 4–6 cm2 * (A) | ||
| LESF023 + Presence of L. gongylophorus | Crude extract | 3–5 cm2 * (A) | ||
| LESF033 + Absence of L. gongylophorus | Crude extract | 1–3 cm2 * (A) | ||
| LESF033 + Presence of L. gongylophorus | Crude extract | 1–2 cm2 * (A) | ||
| LESF039 + Absence of L. gongylophorus | Crude extract | 3–5 cm2 * (A) | ||
| LESF039 + Presence of L. gongylophorus | Crude extract | 3–4 cm2 * (A) | ||
| LESF040 + Absence of L. gongylophorus | Crude extract | 4–6 cm2 * (A) | ||
| LESF040 + Presence of L. gongylophorus | Crude extract | 3–5 cm2 * (A) | ||
| LESF041 + Absence of L. gongylophorus | Crude extract | 3–5 cm2 * (A) | ||
| LESF041 + Presence of L. gongylophorus | Crude extract | 5–6 cm2 * (A) | ||
| LESF043 + Absence of L. gongylophorus | Crude extract | 4–5 cm2 * (A) | ||
| LESF043 + Presence of L. gongylophorus | Crude extract | 3–4 cm2 * (A) | ||
| LESF045 + Absence of L. gongylophorus | Crude extract | 1–3 cm2 * (A) | ||
| LESF045 + Presence of L. gongylophorus | Crude extract | 3–5 cm2 * (A) |
| Compound | Class | Species (Part) | Characterization Method | Inhibitory Effect | Reference |
|---|---|---|---|---|---|
| 2-n-Nonyl-4-quinolone (N1) | Alkaloid | Raulinoa echinata (leaves) | 1H NMR, 13C NMR, and EIMS | 100 μg/mL–50% (A) | [17] |
| 3β-Hydroxystigmast-5-en-7-one (N2) | Steroid | Pilocarpus grandiflorus (stems) | - | 60 µg/mL–20% (A) | [72] |
| 7-Hydroxy-3-(1′1′-dimethylallyl)-8-methoxycoumarin (N3) | Coumarin | Pilocarpus riedelianus (stems) | 1H NMR, 13C NMR, MS and IR | 75 µg/mL–80% (A) | [75] |
| Angelicin (N4) | Coumarin | Citrus limonia (roots) | 1H NMR, 13C NMR, MS and IR | 72 µg/mL–40% (A) | [75] |
| Antimycin A1 (N5) | Macrolide | Streptomyces sp. | LC-ESI-MS | 5.8 nmol–+ * (B) | [79] |
| Antimycin A2 (N6) | Macrolide | Streptomyces sp. | LC-ESI-MS | 5.8 nmol–+ * (B) | [79] |
| Antimycin A3 (N7) | Macrolide | Streptomyces sp. | LC-ESI-MS | 5.8 nmol–+ * (B) | [79] |
| Antimycin A4 (N8) | Macrolide | Streptomyces sp. | LC-ESI-MS | 5.8 nmol–+ * (B) | [79] |
| Argentilactone (N9) | Fatty acid lactone derivative | Aristolochia argentina (aerial parts) | 1H NMR, 13C NMR, and GC-MS | MIC–0.90 µg/mL (C) | [63] |
| Caffeine (N10) | Alkaloid | - | - | 0.50% w/v–100% (A) | [71] |
| Caryophyllene epoxide (N11) | Terpene | Hymenaea courbaril (leaves) | GC-MS and 13C NMR | 3 mg/mL–100% (A) | [72] |
| Citral (N12) | Terpene | Myrcia lundiana (leaves) | GC-MS and GC-FID | IC50−31.7 μL/L # (A)/IC50−289.9 µL/L ## (A) | [67] |
| (2R,3R)-2,3-Di-(3′,4′-dimethoxybenzyl)-butyrolactone (N13) | Lignan | Virola sebifera (leaves) | - | 200 μg/mL–20% (A) | [74] |
| (2R,3R)-3-(3″,4″-Dimethoxybenzyl)-2-(3′,4′-methylenedioxybenzyl)-butyrolactone (N14) | Lignan | Virola sebifera (leaves) | - | 210 μg/mL–60% (A) | [74] |
| Dictamine (N15) | Alkaloid | Pilocarpus grandiflorus (stems) | - | 40 µg/mL–40% (A) | [72] |
| Dillapiole (N16) | Phenylpropanoid | Piper holtonii (leaves) | GC-MS, 1H NMR, 13C NMR, and HMBC | IC50−38 ppm (A) | [68] |
| Epigalgavrin (N17) | Lignan | Virola sp. (leaves) | - | 200 μg/mL–>80% (A) | [74] |
| Eudesmin (N18) | Lignan | Virola sebifera (leaves) | - | 160 μg/mL–40% (A) | [74] |
| Flindersiamine (N19) | Alkaloid | Raulinoa echinata (stems) | 1H NMR, 13C NMR, HMBC, and X-ray | 100 μg/mL–50% (A) | [17] |
| Isopimpinellin (N20) | Coumarin | Adiscanthus fusciflorus (roots) | 1H NMR, 13C NMR, MS, and IR | 80 µg/mL–100% (A) | [75] |
| Isopulegol (N21) | Terpene | Myrcia lundiana (leaves) | GC-MS and GC-FID | IC50−150.1 μL/L # (A)/IC50−696.8 µL/L ## (A) | [67] |
| Kokusagine (N22) | Alkaloid | Raulinoa echinata (stems) | 1H NMR and 13C NMR | 100 μg/mL–100% (A) | [17] |
| Maculine (N23) | Alkaloid | Raulinoa echinata (stems) | 1H NMR and 13C NMR | 100 μg/mL–50% (A) | [17] |
| Philygenol (N24) | Lignan | Otoba parvifolia (fruits) | - | 200 μg/mL–20% (A) | [74] |
| Platydesmine (N25) | Alkaloid | Pilocarpus grandiflorus (stems) | - | 50 µg/mL–80% (A) | [72] |
| Quebracho tannin (N26) | Tannin | - | - | 0.25% w/v–-0.02 & (D)/0.25% w/v–0.0 to 0.5 mg && (E) | [78] |
| Sesamin (N27) | Lignan | Virola sebifera (leaves) | - | 70 μg/mL–>80% (A) | [74] |
| Skimmianine (N28) | Alkaloid | Raulinoa echinata (stems) | 1H NMR and 13C NMR | 100 μg/mL–80% (A) | [17] |
| Suberosin (N29) | Coumarin | Citrus limonia (roots) | 1H NMR, 13C NMR, MS, and IR | 64 µg/mL–100% (A) | [75] |
| Syringaldehyde (N30) | Benzaldehyde | Pilocarpus grandiflorus (stems) | - | 50 µg/mL–80% (A) | [72] |
| Tannic acid (N31) | Tannin | - | - | 0.25% w/v–0.08 & (D)/0.025% w/v–0.5 to 1.0 mg && (E) | [77] |
| Umbelliferone (N32) | Coumarin | Picramnia teapensis (bark) | 1H NMR, 13C NMR, MS, and IR | 65 µg/mL–60% (A) | [75] |
| Vanillic acid (N33) | Phenolic acid | Pilocarpus grandiflorus (stems) | - | 50 µg/mL–80% (A) | [75] |
| Xanthoxyletin (N34) | Coumarin | Citrus limonia (roots) | 1H NMR, 13C NMR, MS, and IR | 70 µg/mL–100% (A) | [75] |
| Xanthyletin (N35) | Coumarin | Pilocarpus riedelianus (stems) | 1H NMR, 13C NMR, MS, and IR | 25 µg/mL–100% (A) | [75] |
2.1.2. Synthetic Compounds
| Compound | Class | Characterization Method | Inhibitory Effect (Antifungal Assay) | Reference |
|---|---|---|---|---|
| 1-(3,4-Methylenedioxybenzyloxy)ethane (S1) | Piperonyl-alkane | 1H NMR, 13C NMR, and EIMS | 330 μg/mL–80% (A) | [81] |
| 1-(3,4-Methylenedioxybenzyloxy)butane (S2) | Piperonyl-alkane | 1H NMR, 13C NMR, and EIMS | 170 μg/mL–100% (A) | |
| 1-(3,4-Methylenedioxybenzyloxy)hexane (S3) | Piperonyl-alkane | 1H NMR, 13C NMR, and EIMS | 160 μg/mL–100% (A) | |
| 1-(3,4-Methylenedioxybenzyloxy)octane (S4) | Piperonyl-alkane | 1H NMR, 13C NMR, and EIMS | 15 μg/mL–80% (A) | |
| 1-(3,4-Methylenedioxybenzyloxy)decane (S5) | Piperonyl-alkane | 1H NMR, 13C NMR, and EIMS | 100 μg/mL–20% (A) | |
| N-Piperidine-3-(3,4-methylenedioxyphenyl)-2-(E)-propenamide (S6) | Piperonyl-amide | 1H NMR, 13C NMR, MS, IR, and TLC | 50 µg/mL–100% (A) | [82] |
| N,N-Diethyl-3-(3,4-methylenedioxyphenyl)-2-(E)-propenamide (S7) | Piperonyl-amide | 1H NMR, 13C NMR, MS, IR, and TLC | 50 µg/mL–100% (A) | |
| N-Pyrrolidine-3-(3,4-methylenedioxyphenyl)-2-(E)-propenamide (S8) | Piperonyl-amide | 1H NMR, 13C NMR, MS, IR, and TLC | 100 µg/mL–100% (A) | |
| N-(2-Methylbutyl)-3-(3,4-methylenedioxyphenyl)-2-(E)-propenamide (S9) | Piperonyl-amide | 1H NMR, 13C NMR, MS, IR, and TLC | 100 µg/mL–80% (A) | |
| N-Morpholine-3-(3,4-methylenedioxyphenyl)-2-(E)-propenamide (S10) | Piperonyl-amide | 1H NMR, 13C NMR, MS, IR, and TLC | 100 µg/mL–40% (A) | |
| N-Aniline-3-(3,4-methylenedioxyphenyl)-2-(E)-propenamide (S11) | Piperonyl-amide | 1H NMR, 13C NMR, MS, IR, and TLC | 100 µg/mL–20% (A) | |
| Hexanoic acid (S12) | Saturated fatty acid | - | 100 µg/mL–100% (A) | [62] |
| Heptanoic acid (S13) | Saturated fatty acid | - | 100 µg/mL–100% (A) | |
| Octanoic acid (S14) | Saturated fatty acid | - | 100 µg/mL–100% (A) | |
| Nonanoic acid (S15) | Saturated fatty acid | - | 100 µg/mL–100% (A) | |
| Decanoic acid (S16) | Saturated fatty acids | - | 100 µg/mL–100% (A) | |
| Undecanoic acid (S17) | Saturated fatty acid | - | 100 µg/mL–100% (A) | |
| Lauric acid (S18) | Saturated fatty acid | - | 100 µg/mL–100% (A) |
2.2. Biological Control Using Microorganisms
| Organism | Strain | Inhibitory Effect (Antifungal Assay) | Reference |
|---|---|---|---|
| Acremonium kiliense | C1 | 26% (A) | [84] |
| Escovopsioides nivea | LESF596 | 56% (A) | [67] |
| LESF599 | 45% (A) | ||
| Escovopsis sp. | AP090209–01 | + * (A) | [85] |
| AP090225-01 | + * (A/B/C) | ||
| AP090731-01 | + * (A/C) | ||
| AP100526-01 | + * (A/B/C) | ||
| DE090731-01 | + * (A/C) | ||
| LD100306-01 | + * (B/C) | ||
| RM090730-01 | + * (B) | ||
| RM090730-02 | + * (A/B/C) | ||
| LESF017 | 78% (A) | [67] | |
| LESF043 | 70% (A) | ||
| LESF041 | 68% (A) | ||
| LESF039 | 65% (A) | ||
| LESF019 | 64% (A) | ||
| LESF021 | 62% (A) | ||
| LESF045 | 61% (A) | ||
| LESF033 | 59% (A) | ||
| LESF040 | 58% (A) | ||
| LESF023 | 56% (A) | ||
| Escovopsis weberi | CBS 810.71 | 68% (A) | [85] |
| A088 | 67% (A) | ||
| A086 | 43% (A) | ||
| Gliocladium sp. | G-56 | 9% (A) | [87] |
| G-55 | 10% (A) | ||
| Syncephalastrum sp. | LESF130 | 8.71 ± 1.85 mm² ** (A) | [88] |
| LESF125 | 8.22 ± 1.64 mm² ** (A) | ||
| LESF127 | 8.80 ± 1.60 mm² ** (A) | ||
| Trichoderma koningii | T-83 | 1% (A) | [87] |
| Trichoderma harzianum | T-21 | 22% (A) | [87] |
| T-86 | 30% (A) | ||
| Trichoderma koningiopsis | HEP4 | 67.37% (A) | [86] |
| HEP12 | 69.78% (A) | ||
| HEP20 | 58.03% (A) | ||
| Trichoderma lignorum | T-28 | 11% (A) | [87] |
| T-19 | 32% (A) | ||
| T-26 | 53% (A) | ||
| T-20 | 6% (A) | ||
| T-30 | 28% (A) | ||
| Trichoderma sp. | T-22 | 4% (A) | [87] |
| T-27 | 4% (A) | ||
| T-24 | 9% (A) | ||
| T-29 | 42% (A) | ||
| T-109 | 47% (A) | ||
| T-110 | 9% (A) | ||
| T-71 | 19% (A) | ||
| Trichoderma viridae | T-25 | 21% (A) | [89] |
| T-23 | 33% (A) |
3. Future Perspectives
3.1. Standardization of Antifungal Assay
3.2. Evaluation of the Antifungal Mechanism of Action
3.3. Product Development
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zunino, M.P.; Herrera, J.M.; Pizzolitto, R.P.; Rubinstein, H.R.; Zygadlo, J.A.; Dambolena, J.S. Effect of selected volatiles on two stored pests: The fungus Fusarium verticillioides and the maize weevil Sithophilus zeamais. J. Agric. Food Chem. 2015, 63, 7743–7749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramalho, S.R.; Bezerra, C.S.; Lourenço de Oliveira, D.G.; Souza Lima, L.; Maria Neto, S.; Ramalho de Oliveira, C.F.; Verbisck, N.V.; Rodrigues Macedo, M.L. Novel peptidase kunitz inhibitor from Platypodium elegans seeds is active against Spodoptera frugiperda larvae. J. Agric. Food Chem. 2018, 66, 1349–1358. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.M.; Song, Y.F.; Sun, X.X.; Shen, X.J.; Wu, Q.L.; Zhang, H.W.; Zhang, D.D.; Zhao, S.Y.; Liang, G.M.; Wu, K.M. Population occurrence of the fall armyworm, Spodoptera frugiperda (Lepidoptera: Noctuidae), in the winter season of China. J. Integr. Agric. 2021, 20, 772–782. [Google Scholar] [CrossRef]
- Reddy, G.V.P.; Guerrero, A. Behavioral responses of the diamondback moth, Plutella xylostella, to green leaf volatiles of Brassica oleracea subsp. capitata. J. Agric. Food Chem. 2000, 48, 6025–6029. [Google Scholar] [CrossRef] [PubMed]
- Chapman, J.W.; Reynolds, D.R.; Smith, A.D.; Riley, J.R.; Pedgley, D.E.; Woiwod, I.P. High-altitude migration of the diamondback moth Plutella xylostella to the U.K.: A study using radar, aerial netting, and ground trapping. Ecol. Entomol. 2002, 27, 641–650. [Google Scholar] [CrossRef] [Green Version]
- Ward, P.S.; Brady, S.G.; Fisher, B.L.; Schultz, T.R. The evolution of myrmicine ants: Phylogeny and biogeography of a hyperdiverse ant clade (Hymenoptera: Formicidae). Syst. Entomol. 2014, 40, 61–81. [Google Scholar] [CrossRef] [Green Version]
- Mehdiabadi, N.J.; Schultz, T.R. Natural history and phylogeny of the fungus-farming ants (Hymenoptera: Formicidae: Myrmicinae: Attini). Myrmecol. News 2010, 13, 37–55. [Google Scholar]
- Pagnocca, F.; Da Silva, O.; Hebling-Beraldo, M.; Bueno, O.; Fernandes, J.B.; Vieira, P.C. Toxicity of sesame extracts to the symbiotic fungus of leaf-cutting ants. Bull. Entomol. Res. 1990, 80, 349–352. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, R.; Zanuncio, J.; Santos, J.; Da Silva, W.; Ribeiro, G.; Lemes, P. An overview of integrated management of leaf-cutting ants (Hymenoptera: Formicidae) in Brazilian forest plantations. Forests 2014, 5, 439–454. [Google Scholar] [CrossRef] [Green Version]
- Forim, M.R.; Costa, E.S.; da Silva, M.F.G.F.; Fernandes, J.B.; Mondego, J.M.; Boiça Junior, A.L. Development of a new method to prepare nano-/microparticles loaded with extracts of Azadirachta indica, their characterization and use in controlling Plutella xylostella. J. Agric. Food Chem. 2013, 61, 9131–9139. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Zhu, X.; Li, T.; Liang, C.; Zhang, M.; Yu, S.; Tao, J.; Sun, R. Dehydrozingerone inspired discovery of potential broad-spectrum fungicidal agents as ergosterol biosynthesis inhibitors. J. Agric. Food Chem. 2019, 67, 11354–11363. [Google Scholar] [CrossRef] [PubMed]
- Ramos, V.M.; Ferreira Leite, R.G.; Almeida, V.T.; Camargo, R.S.; Souza Cruz, J.V.; Leão, R.M.; Prado, M.V.; Sousa Pereira, M.C. Bioactivity of Asclepias curassavica, equisetum spp. and Rosmarinus officinalis extracts against leaf-cutting ants. Sociobioly 2019, 66, 536–544. [Google Scholar] [CrossRef]
- Bicalho, K.U.; Terezan, A.P.; Martins, D.C.; Freitas, T.G.; Fernandes, J.B.; Silva, M.F.G.F.; Vieira, P.C.; Pagnocca, F.C.; Bueno, O.C. Evaluation of the toxicity of Virola sebifera crude extracts, fractions and isolated compounds on the nest of leaf-cutting ants. Psyche J. Entom. 2011, 2012, 785424. [Google Scholar]
- Terezan, A.P.; Rossi, R.A.; Almeida, R.N.A.; Freitas, T.G.; Vieira, P.C.; Fernandes, J.B.; Silva, M.F.G.F.; Bueno, O.C.; Pagnocca, F.C.; Pirani, J.R. Activities of extracts and compounds from Spiranthera odoratissima St. Hil. (Rutaceae) in leaf-cutting ants and their symbiotic fungus. J. Braz. Chem. Soc. 2010, 21, 882–886. [Google Scholar] [CrossRef] [Green Version]
- Marsaro, A.L.; Souza, R.C.; Della Lucia, T.M.C.; Vieira, P.C.; Fernandes, J.B.; Silva, M.F.G.F. Behavioral changes in workers of the leaf-cutting ant Atta sexdens rubropilosa induced by chemical components of Eucalyptus maculata leaves. J. Chem. Ecol. 2004, 30, 1771–1780. [Google Scholar] [CrossRef]
- Almeida, R.; Peñaflor, M.; Simote, S.; Bueno, O.; Hebling, M.J.A.; Pagnocca, F.; Fernandes, J.B.; Vieira, P.C.; Silva, M.F.G.F. Toxicity of substances isolated from Helietta puberula RE Fr. (Rutaceae) to the Leaf-cutting Ant Atta sexdens L. (Hymenoptera: Formicidae) and the Symbiotic Fungus Leucoagaricus gongylophorus (Singer) Möller. BioAssay 2009, 2. [Google Scholar] [CrossRef] [Green Version]
- Biavatti, M.W.; Vieira, P.C.; Silva, M.F.G.F.; Fernandes, J.B.; Victor, S.R.; Pagnocca, F.C.; Albuquerque, S.; Caracelli, I.; Zukerman-Schpector, J. Biological activity of quinoline alkaloids from Raulinoa echinata and X-ray structure of flindersiamine. J. Braz. Chem. Soc. 2002, 13, 66–70. [Google Scholar] [CrossRef]
- Boulogne, I.; Petit, P.; Ozier-Lafontaine, H.; Desfontaines, L.; Loranger-Merciris, G. Insecticidal and antifungal chemicals produced by plants: A review. Environ. Chem. Lett. 2012, 10, 325–347. [Google Scholar] [CrossRef] [Green Version]
- Boulogne, I.; Ozier-Lafontaine, H.; Germosén-Robineau, L.; Desfontaines, L.; Loranger-Merciris, G. Acromyrmex octospinosus (Hymenoptera: Formicidae) Management: Effects of TRAMILs fungicidal plant extracts. J. Econ. Entomol. 2012, 105, 1224–1233. [Google Scholar] [CrossRef]
- Vinha, G.L.; Alcántara-de la Cruz, R.; Della Lucia, T.M.C.; Wilcken, C.F.; da Silva, E.D.; Lemes, P.G.; Zanuncio, J.C. Leaf-cutting ants in commercial forest plantations of Brazil: Biological aspects and control methods. South. For. J. For. Sci. 2020, 82, 95–103. [Google Scholar] [CrossRef]
- Cherrett, J.M. Fire Ants and Leaf-Cutting Ants; CRC Press: New York, NY, USA, 2019; pp. 357–368. [Google Scholar]
- Forti, L.C.; Pretto, D.R.; Nagamoto, N.S.; Padovani, C.R.; Camargo, R.S.; Andrade, A.P.P. Dispersal of the delayed action insecticide sulfluramid in nests of the leaf-cutting ant Atta sexdens rubropilosa (Hymenoptera: Formicidae). Sociobiology 2007, 50, 1149–1163. [Google Scholar]
- Della Lucia, T.M.C.; Gandra, L.C.; Guedes, R.N.C. Managing leaf-cutting ants: Peculiarities, trends and challenges. Pest Manag. Sci. 2014, 70, 14–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rust, M.K.; Reierson, D.A.; Klotz, J.H. Delayed toxicity as a critical factor in the efficacy of aqueous baits for controlling Argentine ants (Hymenoptera: Formicidae). J. Econ. Entomol. 2004, 97, 1017–1024. [Google Scholar] [CrossRef] [PubMed]
- Gilljam, J.L.; Leonel, J.; Cousins, I.T.; Benskin, J.P. Additions and correction to is ongoing sulfluramid use in South America a significant soure of perfluorooctanesulfonate (PFOS)? Production Inventories, environmental fate, and local occurance. Environ. Sci. Technol. 2016, 50, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Nascimento, R.A.; Nunoo, D.B.O.; Bizkarguenaga, E.; Schultes, L.; Zabaleta, I.; Benskin, J.P.; Spanó, S.; Leonel, J. Sulfluramid use in Brazilian agriculture: A source of per-and polyfluoroalkyl substances (PFASs) to the environment. Environ. Pollut. 2018, 242, 1436–1443. [Google Scholar] [CrossRef] [PubMed]
- Toledo, M.A.; Ribeiro, P.L.; Carrossoni, P.S.F.; Tomotani, J.V.; Hoffman, A.N.; Klebaner, D.; Watel, H.R.; Iannini, C.A.N.; Helene, A.F. Two castes sizes of leafcutter ants in task partitioning in foraging activity. Cienc. Rural 2016, 46, 1902–1908. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, H.L.; Cherrett, J.M. Leaf-cutting ants and early forest regeneration in central Amazonia: Effects of herbivory on tree seedling establishment. J. Trop. Ecol. 1997, 13, 357–370. [Google Scholar] [CrossRef]
- Zanetti, R.; Zanuncio, J.C.; Mayhé-Nunes, A.J.; Medeiros, A.G.B.; Souza-Silva, A. Combate sistemático de formigas-cortadeiras com iscas granuladas, em eucaliptais com cultivo mínimo. Rev. Arvore 2003, 27, 387–392. [Google Scholar] [CrossRef] [Green Version]
- Zanetti, R.; Zanuncio, J.C.; Souza-Silva, A.; Abreu, L.G. Eficiência de isca formicida aplicada sobre o monte de terra solta de ninhos de Atta sexdens rubropilosa (Hymenoptera: Formicidae). Rev. Arvore 2003, 27, 407–410. [Google Scholar] [CrossRef] [Green Version]
- Matrangolo, C.A.R.; Castro, R.V.O.; Della Lucia, T.M.C.; Della Lucia, R.M.; Mendes, A.F.N.; Costa, J.M.F.N.; Leite, H.G. Crescimento de eucalipto sob efeito de desfolhamento artificial. Pesqui. Agropecu. Bras. 2010, 45, 952–957. [Google Scholar] [CrossRef] [Green Version]
- Cantarelli, E.B.; Costa, E.C.; Pezzutti, R.; Oliveira, L.S. Quantificação das perdas no desenvolvimento de Pinus taeda após o ataque de formigas cortadeiras. Ciência Florestal 2008, 18, 39–45. [Google Scholar] [CrossRef]
- Hernández, J.V.; Jaffé, K. Dano econômico causado por populações de formigas Atta laevigata (F. Smith) em plantações de Pinus caribaea Mor. e elementos para o manejo da praga. An. Soc. Entomol. Bras. 1995, 24, 287–298. [Google Scholar] [CrossRef]
- Souza, A.; Zanetti, R.; Calegario, N. Economic damage level for leaf-cutting ants in function of the productivity index of eucapyptus plantations in an Atlantic Forest region. Neotrop. Entomol. 2011, 40, 483–488. [Google Scholar] [PubMed]
- Zanetti, R.; Zanuncio, J.C.; Vilela, E.F.; Leite, H.G.; Jaffé, K.; Oliveira, A.C. Level of economic damage for leaf-cutting ants (Hymenoptera: Formicidae) in Eucalyptus plantations in Brazil. Sociobioly 2003, 42, 433–444. [Google Scholar]
- Zanuncio, J.C.; Lemes, P.G.; Antunes, L.R.; Maia, J.L.S.; Mendes, J.E.P.; Tanganelli, K.M.; Salvador, J.F.; Serrão, J.E. The impact of the Forest Stewardship Council (FSC) pesticide policy on the management of leaf-cutting ants and termites in certified forests in Brazil. Ann. For. Sci. 2016, 73, 205–214. [Google Scholar] [CrossRef] [Green Version]
- Boulogne, I.; Ozier-Lafontaine, G.; Loranger-Merciris, G. Leaf cutting ants, biology and control. Sustain. Agric. Rev. 2015, 13, 450–455. [Google Scholar]
- Dos Santos, A.; Santos, I.C.L.; Da Silva, N.; Zanetti, R.; Oumar, Z.; Guimarães, L.F.R.; De Camargo, M.B.; Zanuncio, J.C. Mapping defoliation by leaf-cutting ants Atta species in Eucalyptus plantations using the Sentinel-2 sensor. Int. J. Remote Sens. 2020, 41, 1542–1554. [Google Scholar] [CrossRef]
- Zanetti, R.; Zanuncio, J.C.; Vilela, E.F.; Leite, H.G.; Della Lucia, T.M.C.; Couto, L. Efeito da espécie de eucalipto e da vegetação nativa circundante sobre o custo de combate a sauveiros em eucaliptais. Rev. Arvore 1999, 23, 321–325. [Google Scholar]
- Montoya-Lerma, J.; Giraldo-Echeverri, C.; Armbrecht, I.; Farji-Brener, A.; Calle, Z. Leaf-cutting ants revisited: Towards rational management and control. Int. J. Pest Manag. 2012, 58, 225–247. [Google Scholar] [CrossRef]
- Biedermann, P.H.; Rohlfs, M. Evolutionary feedbacks between insect sociality and microbial management. Curr. Opin. Insect Sci. 2017, 22, 92–100. [Google Scholar] [CrossRef] [PubMed]
- De Fine Licht, H.H.; Schiøtt, M.; Rogowska-Wrzesinska, A.; Nygaard, S.; Roepstorff, P.; Boomsma, J.J. Laccase detoxification mediates the nutritional alliance between leaf-cutting ants and fungus-garden symbionts. Proc. Natl. Acad. Sci. USA 2013, 110, 583–587. [Google Scholar] [CrossRef] [Green Version]
- Goes, A.C.; Barcoto, M.O.; Kooij, P.W.; Bueno, O.C.; Rodrigues, A. How Do Leaf-Cutting Ants Recognize Antagonistic Microbes in Their Fungal Crops? Front. Ecol. Evol. 2020, 8, 95. [Google Scholar] [CrossRef]
- Bacci, M., Jr.; Bueno, O.C.; Rodrigues, A.; Pagnocca, F.C.; Somera, A.F.; Silva, A. A metabolic pathway assembled by enzyme selection may support herbivory of leaf-cutter ants on plant starch. J. Insect Physiol. 2013, 59, 525–531. [Google Scholar] [CrossRef]
- Aylward, F.O.; Burnum-Johnson, K.E.; Tringe, S.G.; Teiling, C.; Tremmel, D.M.; Moeller, J.A.; Scott, J.J.; Barry, K.W.; Piehowski, P.D.; Nicora, C.D.; et al. Leucoagaricus gongylophorus produces diverse enzymes for the degradation of recalcitrant plant polymers in leaf-cutter ant fungus gardens. Appl. Environ. Microbiol. 2013, 79, 3770–3778. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, A.; Bacci, M.; Siqueira, C.G.; Bueno, O.C.; Pagnocca, F.C.; Hebling, M.J.A. Survival of Atta sexdens on different food sources. J. Insect Physiol. 2003, 49, 307–313. [Google Scholar] [CrossRef]
- Silva, A.; Bacci, M.; Bueno, O.C.; Pagnocca, F.C.; Hebling, M.J.A. Starch metabolism in Leucoagaricus gongylophorus, the symbiotic fungus of leaf-cutting ants. Microbiol. Res. 2006, 161, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Khadempour, L.; Burnum-Johnson, K.E.; Baker, E.S.; Nicora, C.D.; Webb-Robertson, B.-J.M.; White, R.A.; Monroe, M.E.; Huang, E.L.; Smith, R.D.; Currie, C.R. The fungal cultivar of leaf-cutter ants produces specific enzymes in response to different plant substrates. Mol. Ecol. 2016, 25, 5795–5805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aylward, F.O.; Burnum, K.E.; Scott, J.J.; Suen, G.; Tringe, S.G.; Adams, S.M.; Barry, K.W.; Nicora, C.D.; Piehowski, P.D.; Purvine, S.O.; et al. Metagenomic and metaproteomic insights into bacterial communities in leaf-cutter ant fungus gardens. ISME J. 2012, 6, 1688–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mikheyev, A.S.; Mueller, U.G.; Abbot, P. Comparative Dating of Attine Ant and Lepiotaceous Cultivar Phylogenies Reveals Coevolutionary Synchrony and Discord. Am. Nat. 2010, 175, E126–E133. [Google Scholar] [CrossRef] [Green Version]
- Erthal, M.; Peres Silva, C.; Ian Samuels, R. Digestive enzymes in larvae of the leaf cutting ant, Acromyrmex subterraneus (Hymenoptera: Formicidae: Attini). J. Insect Physiol. 2007, 53, 1101–1111. [Google Scholar] [CrossRef] [PubMed]
- Bass, M.; Cherret, J.M. Fungal hyphae as a source of nutrients for the leaf-cutting ant Atta sexdens. Physiol. Entomol. 1995, 20, 1–6. [Google Scholar] [CrossRef]
- Ford, L.C.; Andrade, A.P.P. Ingestão de líquidos por Atta sexdens (L.) (Hymenoptera, Formicidae) durante a atividade forrageira e na preparação do substrato em condições de laboratório. Naturalia 1999, 24, 61–63. [Google Scholar]
- De Fine Licht, H.H.; Boomsma, J.; Tunlid, A. Symbiotic adaptations in the fungal cultivar of leaf-cutting ants. Nat. Commun. 2014, 5, 5675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bizarria, R., Jr.; Kooij, P.W.; Rodrigues, A. Climate Change Influences Basidiome Emergence of Leaf-Cutting Ant Cultivars. J. Fungi 2021, 7, 912. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Carletti, C.D.; Bueno, O.C.; Pagnocca, F.C. Leaf-cutting ant faecal fluid and mandibular gland secretion: Effects on microfungi spore germination. Braz. J. Microbiol. 2008, 39, 64–67. [Google Scholar] [CrossRef] [Green Version]
- Fernández-Marín, H.; Zimmerman, J.K.; Rehner, S.A.; Wcislo, W.T. Active use of the metapleural glands by ants in controlling fungal infection. Proc. Biol. Sci. 2006, 273, 1689–1695. [Google Scholar] [CrossRef] [Green Version]
- Currie, C.R. A Community of ants, fungi, and bacteria: A multilateral aproach to studying symbiosis. Annu. Rev. Microbiol. 2001, 55, 357–380. [Google Scholar] [CrossRef] [Green Version]
- Lemes, P.G.; Zanuncio, J.C.; Serrão, J.E.; Lawson, S.A. Forest stewardship council (FSC) pesticide policy and integrated pest management in certified tropical plantations. Environ. Sci. Pollut. Res. 2017, 24, 1283–1295. [Google Scholar] [CrossRef] [Green Version]
- Aldholmi, M.; Marchand, P.; Ourliac-Garnier, I.; Le Pape, P.; Ganesan, A. A decade of antifungal leads from natural products: 2010–2019. Pharmaceuticals 2019, 12, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katiyar, C.; Gupta, A.; Kanjilal, S.; Katiyar, S. Drug discovery from plant sources: An integrated approach. Ayu 2012, 33, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Peñaflor, M.F.G.V.; Victor, S.R.; Bueno, O.C.; Hebling, M.J.A.; Pagnocca, F.C.; Leita, A.C.; Fernandes, J.B.; Vieira, P.C.; da Silva, M.F.G.F. Toxicity of straight-chain fatty acids to leaf-cutting ants Atta sexdens rubropilosa (Hymenoptera: Formicidae) and the symbiotic fungus Leucoagaricus gongylophorus. Sociobiology 2006, 47, 843–858. [Google Scholar]
- Napal, G.N.D.; Buffa, L.M.; Nolli, L.C.; Defagó, M.T.; Valladares, G.R.; Carpinella, M.C.; Ruiz, G.; Palacios, S.M. Screening of native plants from central Argentina against the leaf-cutting ant Acromyrmex lundi (Guérin) and its symbiotic fungus. Ind. Crops Prod. 2015, 76, 275–280. [Google Scholar] [CrossRef]
- Morais, W.C.C.; Lima, M.A.P.; Zanuncio, J.C.; Oliveira, M.A.; Bragança, M.A.L.; Serrão, J.E.; Della Lucia, T.M.C. Extracts of Ageratum conyzoides, Coriandrum sativum and Mentha piperita inhibit the growth of the symbiotic fungus of leaf-cutting ants. Ind. Crops Prod. 2015, 65, 463–466. [Google Scholar] [CrossRef]
- Lobo-Echeverri, T.; Salazar, L.C.; Hernández, A.; Ortiz-Reyes, A. Efectos de Capsicum baccatum y C. frutescens sobre Atta cephalotes (Hymenoptera: Formicidae) y el hongo simbionte Leucoagaricus gongylophorus. Rev. Colomb. Entomol. 2016, 42, 137–145. [Google Scholar] [CrossRef] [Green Version]
- Varanda-Haifig, S.S.; Albarici, T.R.; Nunes, P.H.; Haifig, I.; Vieira, P.C.; Rodrigues, A. Nature of the interactions between hypocrealean fungi and the mutualistic fungus of leaf-cutter ants. Antonie Van Leeuwenhoek 2017, 110, 593–605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, C.R.; Oliveira, B.M.S.; Santos, A.C.C.; Silva, E.J.; Ribeiro, G.T.; Blank, A.F.; Araújo, A.P.A.; Bacci, L. Synergistic effect of aromatic plant essential oils on the ant Acromyrmex balzani (Hymenoptera: Formicidae) and antifungal activity on its symbiotic fungus Leucoagaricus gongylophorus (Agaricales: Agaricaceae). Environ. Sci. Pollut. Res. 2020, 27, 17303–17313. [Google Scholar] [CrossRef] [PubMed]
- Salazar, L.C.; Ortiz-Reyes, A.; Rosero, D.M.; Lobo-Echeverri, T.; Ortiz-Reyes, A.; Rosero, D.M.; Lobo-Echeverri, T. Dillapiole in Piper holtonii as an inhibitor of the symbiotic fungus Leucoagaricus gongylophorus of Leaf-Cutting Ants. J. Chem. Ecol. 2020, 46, 668–674. [Google Scholar] [CrossRef] [PubMed]
- Leite, A.C.; Bueno, F.C.; Oliveira, C.G.; Fernandes, J.B.; Vieira, P.C.; Silva, M.F.G.F.; Bueno, O.C.; Pagnocca, F.C.; Hebling, M.J.A.; Bacci, M. Limonoids from Cipadessa fruticosa and Cedrela fissilis and their insecticidal activity. J. Braz. Chem. Soc. 2005, 16, 1391–1395. [Google Scholar] [CrossRef] [Green Version]
- Hubbell, S.P.; Howard, J.J.; Wiemer, D.F. Chemical leaf repellency to an Attine ant: Seasonal distribution among potential host plant species. Ecology 1983, 65, 1067–1076. [Google Scholar] [CrossRef]
- Miyashira, C.H.; Tanigushi, D.G.; Gugliotta, A.M.; Santos, D.Y. Influence of caffeine on the survival of leaf-cutting ants Atta sexdens rubropilosa and in vitro growth of their mutualistic fungus. Pest. Manag. Sci. 2012, 68, 935–940. [Google Scholar] [CrossRef]
- De Souza, R.C.; Fernandes, J.B.; Vieira, P.C.; da Silva, M.F.G.F.; Godoy, M.F.P.; Pagnocca, F.C.; Bueno, O.C.; Hebling, M.J.A.; Pirani, J.R. A new imidazole alkaloid and other constituents from Pilocarpus grandiflorus and their antifungal activity. Z. Nat. 2005, 60, 787–791. [Google Scholar] [CrossRef] [Green Version]
- Bueno, O.C.; Hebling-Beraldo, M.J.A.; Castro, S.L.R.; Silva, O.A.; Pagnocca, F.C. Testes de preferência de Atta sexdens rubropilosa por folhas de Sesamum indicum e Virola sebifera. In Anais IX Encontro de Mirmecologia; Impresa Universitária, Universidade Federal de Viçosa: Viçosa, Brazil, 1989; pp. 24–28. [Google Scholar]
- Pagnocca, F.C.; Ribeiro, S.B.; Torkomian, V.L.; Hebling, M.J.; Bueno, O.C.; Da Silva, O.A.; Fernandes, J.B.; Vieira, P.C.; Da Silva, M.F.; Ferreira, A.G. Toxicity of lignans to symbiotic fungus of leaf-cutting ants. J. Chem. Ecol. 1996, 22, 1325–1330. [Google Scholar] [CrossRef] [PubMed]
- Godoy, M.F.P.; Victor, S.R.; Bellini, A.M.; Guerreiro, G.R.; Waldireny, C.; Bueno, O.C.; Hebling, M.J.A.; Bacci, M., Jr.; Silva, M.F.G.F.; Vieira, P.C.; et al. Inhibition of the symbiotic fungus of leaf-cutting ants by coumarins. J. Braz. Chem. Soc. 2005, 16, 669–672. [Google Scholar] [CrossRef] [Green Version]
- Dini, A.; Ramundo, E.; Saturnino, P.; Scimone, A.; Stagno, I.; Stagno d’Alcontres, I. Isolation, characterization and antimicrobial activity of coumarin derivatives from Cyperus incompletes. Boll. Soc. Ital. Biol. Sper. 1992, 68, 453–461. [Google Scholar]
- Nichols-Orians, C. Condensed tannins, attine ants, and the performance of a symbiotic fungus. J. Chem. Ecol. 1991, 17, 1177–1195. [Google Scholar] [CrossRef] [PubMed]
- Nichols-Orians, C. Differential effects of condensed and hydrolyzable tannin on polyphenol oxidase activity of attine symbiotic fungus. J. Chem. Ecol. 1991, 17, 1811–1819. [Google Scholar] [CrossRef] [PubMed]
- Schoenian, I.; Spiteller, M.; Ghaste, M.; Wirth, R.; Herz, H.; Spiteller, D. Chemical basis of the synergism and antagonism in microbial communities in the nests of leaf-cutting ants. Proc. Natl. Acad. Sci. USA 2011, 108, 1955–1960. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, C.M.; Da Silva, D.; Haranahalli, K.; McCarthy, J.B.; Mallamo, J.; Ojima, I.; Poeta, M. The future of antifungal drug therapy: Novel compounds and targets. Antimicrob. Agents Chemother. 2020, 65, e01719-20. [Google Scholar] [CrossRef]
- Victor, S.R.; Crisóstomo, F.R.; Bueno, F.C.; Pagnocca, F.C.; Fernandes, J.B.; Correa, A.G.; Bueno, O.C.; Hebling, M.J.A.; Bacci, M.; Vieira, P.C.; et al. Toxicity of synthetic piperonyl compounds to leaf-cutting ants and their symbiotic fungus. Pest. Manag. Sci. 2001, 57, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Pagnocca, F.C.; Victor, S.R.; Bueno, F.C.; Crisóstomo, F.R.; Castral, T.C.; Fernandes, J.B.; Corrêa, A.G.; Bueno, O.C.; Bacci, M.; Hebling, M.J.A.; et al. Synthetic amides toxic to the leaf-cutting ant Atta sexdens rubropilosa L. and its symbiotic fungus. Agric. For. Entomol. 2006, 8, 17–23. [Google Scholar] [CrossRef]
- Brink, H.J.M.; Gorcom, R.F.M.; Hondel, C.A.M.J.J.; Punt, P.J. Cytochrome P450 enzyme systems in fungi. Fungal Genet. Biol. 1998, 23, 1–17. [Google Scholar] [CrossRef]
- Silva, A.; Rodrigues, A.; Bacci, M.; Pagnocca, F.C.; Bueno, O.C. Susceptibility of the ant-cultivated fungus Leucoagaricus gongylophorus (Agaricales: Basidiomycota) towards microfungi. Mycopathologia 2006, 162, 115–119. [Google Scholar] [CrossRef]
- Wallace, D.E.E.; Asensio, J.G.V.; Tomás, A.A.P. Correlation between virulence and genetic structure of Escovopsis strains from leaf-cutting ant colonies in Costa Rica. Microbiology 2014, 160, 1727–1736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castrillo, M.L.; Bich, G.A.; Zapata, P.D.; Villalba, L.L. Biocontrol of Leucoagaricus gongylophorus of leaf-cutting ants with the mycoparasitic agent Trichoderma koningiopsis. Mycosphere 2016, 7, 810–819. [Google Scholar] [CrossRef]
- Ortiz, A.; Orduz, S. In vitro evaluation of Trichoderma and Gliocladium antagonism against the symbiotic fungus of the leaf-cutting ant Atta cephalotes. Mycopathologia 2001, 150, 53–60. [Google Scholar] [CrossRef]
- Barcoto, M.O.; Pedrosa, F.; Bueno, O.C.; Rodrigues, A. Pathogenic nature of Syncephalastrum in Atta sexdens rubropilosa fungus gardens. Pest. Manag. Sci. 2017, 73, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Lopez, E.; Orduz, S. Metarhizium anisopliae and Trichoderma viride for control of nests of the fungus-growing ant, Atta cephalotes. Biol. Control. 2003, 27, 194–200. [Google Scholar] [CrossRef]
- NCCLS. Método de Referência para Testes de Diluição em Caldo para Determinação da Sensibilidade a Terapia Antifúngica de Fungos Filamentosos: Norma Aprovada; Documento M38-A do NCCLS; NCCLS: Wayne, PA, USA, 2002; ISBN 1562384708. [Google Scholar]
- Souza, D.P.; Pimentel, R.B.Q.; Santos, A.S.; Albuquerque, P.M.; Fernandes, A.V.; Junior, S.D.; Oliveira, J.T.A.; Ramos, M.V.; Rathinasabapathie, B.; Gonçalves, J.F.C. Fungicidal properties and insights on the mechanisms of the action of volatile oils from Amazonian Aniba trees. Ind. Crops Prod. 2020, 143, 111914. [Google Scholar] [CrossRef]
- Li, D.; Calderone, R. Exploiting mitochondria as targets for the development of new antifungals. Virulence 2017, 8, 159–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avenot, H.F.; Morgan, D.P.; Michailides, T.J. Resistance to pyraclostrobin, boscalid and multiple resistance to Pristine® (pyraclostrobin þ boscalid) fungicide in Alternaria alternata causing alternaria late blight of pistachios in California. Plant Pathol. 2008, 57, 135–140. [Google Scholar] [CrossRef]
- Campia, P.; Venturini, G.; Moreno-Sanz, P.; Casati, P.; Toffolatti, S.L. Genetic structure and fungicide sensitivity of Botrytis cinerea populations isolated from grapevine in northern Italy. Plant Pathol. 2017, 66, 890–899. [Google Scholar] [CrossRef]
- Miyamoto, T.; Ishii, H.; Seko, T.; Kobori, S.; Tomita, Y. Occurrence of Corynespora cassiicola isolates resistant to boscalid on cucumber in Ibaraki prefecture, Japan. Plant Pathol. 2009, 58, 1144–1151. [Google Scholar] [CrossRef]
- Wang, Y.; Duan, Y.B.; Zhou, M.G. Molecular and biochemical characterization of boscalid resistance in laboratory mutants of Sclerotinia sclerotiorum. Plant Pathol. 2015, 64, 101–108. [Google Scholar] [CrossRef]
- Xu, Q.; Zhang, K.; Fu, Y.; Ma, H.; Zhu, F. Toxic action and baseline sensitivity of boscalid against Penicillium digitataum. Crop Prot. 2020, 137, 105272. [Google Scholar] [CrossRef]
- Beattie, S.R.; Krysan, D.J. Antifungal drug screening: Thinking outside the box to identify novel antifungal scaffolds. Curr. Opin. Microbiol. 2020, 57, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Perfect, J.R. The antifungal pipeline: A reality check. Nat. Rev. Drug Discov. 2017, 16, 603–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kah, M.; Hofmann, T. Nanopesticides research: Current trends and future priorities. Environ. Int. 2014, 63, 224–235. [Google Scholar] [CrossRef]
- Kah, M.; Beulke, S.; Tiede, K.; Hofmann, T. Nano-pesticides: State of knowledge, environmental fate and exposure modelling. Crit. Rev. Environ. Sci. Technol. 2013, 43, 1823–1867. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef]
- Marín, F.H.; Nash, D.R.; Higginbotham, S.; Estrada, C.; Zweden, J.S.; d’Ettorre, P.; Wcislo, W.T.; Boomsma, J.J. Functional role of phenylacetic acid from metapleural gland secretions in controlling fungal pathogens in evolutionary derived leaf-cutting ants. Proc. R. Soc. 2015, 282, 201–212. [Google Scholar]
- Silva, P.C.; Rezende, E.M.; Pereira, L.A.S.; Reis, M.V.; Lago, A.M.T.; Carvalho, G.R.; Paiva, R.; Oliveira, J.E.; Marconcini, J.M. Production and efficacy of neem nanoemulsion in the control of Aspergillus flavus and Penicillium citrinum in soybean seeds. Eur. J. Plant Pathol. 2019, 155, 1105–1116. [Google Scholar] [CrossRef]
- Hassanim, M.M.H.; Abd-El-Sayed, M.A.; Abdallah, M.A. Antifungal activity of some essential oil emulsions and nanoemulsions against Fusarium oxysporum pathogen affecting cumin and geranium plants. Sci. J. Flowers Ornam. Plants 2017, 4, 245–258. [Google Scholar] [CrossRef]
- Gündel, S.S.; Reis, T.R.; Copetti, P.M.; Favarin, F.R.; Sagrillo, M.R.; Silva, A.S.; Segat, J.C.; Baretta, D.; Ourique, A.F. Evaluation of cytotoxicity, genotoxicity and ecotoxicity of nanoemulsions containing Mancozeb and Eugenol. Ecotoxicol. Environ. Saf. 2019, 169, 207–215. [Google Scholar] [CrossRef]
- Hernández-Díaz, J.A.; Garza-García, J.J.; Zamudio-Ojeda, A.; León-Morales, J.M.; López-Velázquez, J.C.; García-Morales, S. Plant-mediated synthesis of nanoparticles and their antimicrobial activity against phytopathogens. J. Sci. Food Agric. 2021, 101, 1270–1287. [Google Scholar] [CrossRef] [PubMed]
- Sukhwal, A.; Jain, D.; Joshi, A.; Rawal, P.; Kushwaha, H. Biosynthesised silver nanoparticles using aqueous leaf extract of Tagetes patula L. and evaluation of their antifungal activity against phytopathogenic fungi. IET Nanobiotechnol. 2017, 11, 531–537. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, C.R.; Bilesky-José, N.; Lima, R.; Fraceto, L.F. Encapsulation of Trichoderma harzianum preserves enzymatic activity and enhances the potential for biological control. Front. Bioeng. Biotechnol. 2020, 8, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Araújo, S.; Seibert, J.; Ruani, A.; Alcántara-de la Cruz, R.; Cruz, A.; Pereira, A.; Zandonai, D.; Forim, M.; Silva, M.F.; Bueno, O.; et al. The Symbiotic Fungus Leucoagaricus gongylophorus (Möller) Singer (Agaricales, Agaricaceae) as a Target Organism to Control Leaf-Cutting Ants. Insects 2022, 13, 359. https://doi.org/10.3390/insects13040359
Araújo S, Seibert J, Ruani A, Alcántara-de la Cruz R, Cruz A, Pereira A, Zandonai D, Forim M, Silva MF, Bueno O, et al. The Symbiotic Fungus Leucoagaricus gongylophorus (Möller) Singer (Agaricales, Agaricaceae) as a Target Organism to Control Leaf-Cutting Ants. Insects. 2022; 13(4):359. https://doi.org/10.3390/insects13040359
Chicago/Turabian StyleAraújo, Sean, Janaína Seibert, Ana Ruani, Ricardo Alcántara-de la Cruz, Artur Cruz, Alana Pereira, Doraí Zandonai, Moacir Forim, Maria Fátima Silva, Odair Bueno, and et al. 2022. "The Symbiotic Fungus Leucoagaricus gongylophorus (Möller) Singer (Agaricales, Agaricaceae) as a Target Organism to Control Leaf-Cutting Ants" Insects 13, no. 4: 359. https://doi.org/10.3390/insects13040359