Current State of DNA Barcoding of Sciaroidea (Diptera)—Highlighting the Need to Build the Reference Library
Abstract
:Simple Summary
Abstract
1. Introduction
2. Results and Discussion
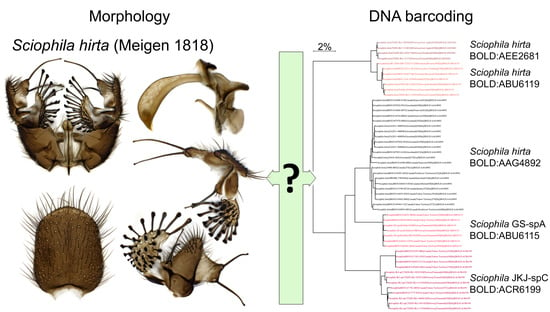
2.1. The Hype around Blind Barcode Scanning
2.2. Integrative Sciaroidea Taxonomy and Ecology
2.3. The Nordic Initiative to Build a Reference Library of Sciaroidea
Integrative Disclosures and Discoveries
2.4. Secondary DNA Barcoding Outside of BOLD
2.5. Alternatives to BOLD
2.6. There Is Hope for You Yet, Taxonomy
3. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ševčík, J.; Kaspřák, D.; Mantič, M.; Fitzgerald, S.; Ševčíková, T.; Tóthová, A.; Jaschhof, M. Molecular phylogeny of the megadiverse insect infraorder Bibionomorpha sensu lato (Diptera). PeerJ 2016, 4, e2563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Courtney, G.; Pape, T.; Skevington, J.; Sinclair, B. Biodiversity of Diptera: Science and Society. In Insect Biodiversity: Science and Society, Volume I, 2nd ed.; Foottit, R.G., Adler, P.H., Eds.; John Wiley & Sons Ltd.: HoBoken, NJ, USA, 2017; Volume 1, pp. 229–278. [Google Scholar]
- Hebert, P.D.N.; Ratnasingham, S.; Zakharov, S.E.; Telfer, A.C.; Levesque-Beaudin, V.; Milton, M.A.; Pedersen, S.; Jannetta, P.; deWaard, J.R. Counting animal species with DNA barcodes: Canadian insects. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ronquist, F.; Forshage, M.; Häggqvist, S.; Karlsson, D.; Hovmöller, R.; Bergsten, J.; Holston, K.; Britton, T.; Abenius, J.; Andersson, B.; et al. Completing Linnaeus’s inventory of the Swedish insect fauna: Only 5000 species left? PLoS ONE 2020, 15, e0228561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dziedzicki, H. Revue des espèces europeennes du genre Phronia Winnertz, avec la description des deux genres nouveaux: Macrobrachius et Megophthalmidia. Trudy Russk. Ent. Obshch. 1889, 23, 404–532. [Google Scholar]
- Dziedzicki, H. Zur Monographie der Gattung Rymosia Winn. Horae Soc. Ent. Ross. 1910, 77, 89–104. [Google Scholar]
- Edwards, F.W. Notes on British Mycetophilidae. Trans. R. Entomol. Soc. Lond. 1913, 1913, 334–382. [Google Scholar]
- Dziedzicki, H. Atlas des organes genitaux des types de Winnertz et des genres de la collection de Mycetophiles. Publ. Soc. Scient. Varsovie. 1915, 3, 1–16, pls. I–XXI. [Google Scholar]
- Hutson, A.M.; Ackland, D.M.; Kidd, L.N. Mycetophilidae (Bolitophilinae, Ditomyiinae, Diadocidiinae, Keroplatinae, Sciophilinae and Manotinae), Diptera Nematocera. Handb. Identif. Br. Insects 1980, 9, 1–111. [Google Scholar]
- Zaitzev, A.I. Грибные кoмары рoда Sciophila Meig. (Diptera, Mycetophilidae) Гoларктики; Holarctic fungus gnats of the genus Sciophila Meig; NAUK: Moscow, Russia, 1982; pp. 1–75. (In Russian) [Google Scholar]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. Lond. B 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Michel, J.B.; Shen, Y.K.; Aiden, A.P.; Veres, A.; Gray, M.K.; The Google Books Team; Pickett, J.P.; Hoiberg, D.; Clancy, D.; Norvig, P.; et al. Quantitative analysis of culture using millions of digitized books. Science 2011, 331, 176–182. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://books.google.com/ngrams/graph?content=DNA+barcoding,morphological+description,integrative+taxonomy,taxonomic+revision&year_start=1920&year_end=2019&corpus=26&smoothing=4 (accessed on 14 October 2021).
- Hajibabaei, M.; Singer, G.A.C.; Hebert, P.D.N.; Hickey, D.A. DNA barcoding:how it complements taxonomy, molecular phylogenetics and population genetics. Trends Genet. 2007, 23, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Jinbo, U.; Kato, T.; Ito, M. Current progress in DNA barcoding and future implications for entomology. Entomol. Sci. 2011, 14, 107–124. [Google Scholar] [CrossRef]
- Pentinsaari, M.; Blagoev, G.A.; Hogg, I.D.; Levesque-Beaudin, V.; Perez, K.; Sobel, C.N.; Vandenbrink, B.; Borisenko, A. A DNA Barcoding Survey of an Arctic Arthropod Community: Implications for Future Monitoring. Insects 2020, 11, 46. [Google Scholar] [CrossRef] [Green Version]
- Weigand, H.; Beermann, A.J.; Čiampor, F.; Costa, F.O.; Csabai, Z.; Duarte, S.; Geiger, M.F.; Grabowski, M.; Rimet, F.; Rulik, B.; et al. DNA barcode reference libraries for the monitoring of aquatic biota in Europe: Gap-analysis and recommendations for future work. Sci. Total Environ. 2019, 678, 499–524. [Google Scholar] [CrossRef] [PubMed]
- Ratnasingham, S.; Hebert, P.D.N. A DNA-based registry for all animal species: The Barcode Index Number (BIN) system. PLoS ONE 2013, 8, e66213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Souza, M.L.; van der Bank, M.; Shongwe, Z.; Rattray, R.D.; Stewart, R.; van Rooyen, J.; Govender, D.; Hebert, P.D.N. Biodiversity baselines: Tracking insects in Kruger National Park with DNA barcodes. Biol. Conserv. 2021, 25, 109034. [Google Scholar] [CrossRef]
- Svenningsen, C.S.; Frøslev, T.; Guldberg, B.J.; Pedersen, L.B.; Larsen, J.C.; Ejrnæs, R.; Fløjgaard, C.; Hansen, A.J.; Heilmann-Clausen, J.; Dunn Robert, R.; et al. 2021. Detecting flying insects using car nets and DNA metabarcoding. Biol. Lett. 2021, 17, 20200833. [Google Scholar] [CrossRef]
- Telfer, A.; Young, M.; Quinn, J.; Perez, K.; Sobel, C.; Sones, J.; Levesque-Beaudin, V.; Derbyshire, R.; Fernandez-Triana, J.; Rougerie, R.; et al. Biodiversity inventories in high gear: DNA barcoding facilitates a rapid biotic survey of a temperate nature reserve. Biodivers. Data J. 2015, 3, e6313. [Google Scholar] [CrossRef]
- Jin, S.; Kim, K.Y.; Kim, M.S.; Park, C. An assessment of the taxonomic reliability of DNA barcode sequences in publicly available databases. Algae 2020, 35, 293–301. [Google Scholar] [CrossRef]
- Hartop, E.A.; Srivathsan, A.; Ronquist, F.; Meier, R. Large-scale Integrative Taxonomy (LIT): Resolving the data conundrum for dark taxa. bioRxiv 2021. Available online: https://www.biorxiv.org/content/10.1101/2021.04.13.439467v2 (accessed on 14 October 2021).
- Kjærandsen, J.; Søli, G.E.E. Updated checklist of Norwegian Mycetophilidae (Diptera) with 92% DNA barcode reference coverage. Nor. J. Entomol. 2020, 67, 201–234. [Google Scholar]
- Page, R.D.M. DNA barcoding and taxonomy: Dark Taxa and Dark Texts. Philos. Trans. R. Soc. B Biol. Sci. 2016, 371, 20150334. [Google Scholar] [CrossRef] [Green Version]
- Roslin, T.; Somervuo, P.; Pentinsaari, M.; Hebert, P.D.N.; Agda, J.; Ahlroth, P.; Anttonen, P.; Aspi, J.; Blagoev, G.; Blanco, S.; et al. A molecular-based identification resource for the arthropods of Finland. Mol. Ecol. Resour. 2022, 22, 803–822. [Google Scholar] [CrossRef]
- Elven, H.; Søli, G. Kunnskapsstatus for artsmangfoldet i Norge 2015. In Utredning for Artsdatabanken 1/2016; Artsdatabanken: Trondheim, Norway, 2016; Available online: https://www.artsdatabanken.no/Files/16197/Kunnskapsstatus_for_artsmangfoldet_i_Norge_2015_(PDF) (accessed on 14 October 2021). (In Norwegian)
- Janzen, D.H.; Hallwachs, W.; Blandin, P.; Burns, J.M.; Cadiou, J.M.; Chacon, I.; Dapkey, T.; Deans, A.R.; Epstein, M.E.; Espinoza, B.; et al. Integration of DNA barcoding into an ongoing inventory of complex tropical biodiversity. Mol. Ecol. Resour. 2009, 9 (Suppl. S1), 1–26. [Google Scholar] [CrossRef] [Green Version]
- Morinière, J.; Balke, M.; Doczkal, D.; Doczkal, D.; Geiger, M.F.; Hardulak, L.A.; Haszprunar, G.; Hausmann, A.; Hendrich, L.; Regalado, L.; et al. A DNA barcode library for 5,200 German flies and midges (Insecta: Diptera) and its implications for metabarcoding-based biomonitoring. Mol. Ecol. Resour. 2019, 19, 900–928. [Google Scholar] [CrossRef] [Green Version]
- Kotrba, M. The DNA barcoding project on German Diptera: An appreciative and critical analysis with four suggestions for improving the development and reliability of DNA-based identification. Eur. J. Entomol. 2020, 117, 315–327. [Google Scholar] [CrossRef]
- Chimeno, C.; Hausmann, A.; Schmidt, S.; Raupach, M.J.; Doczkal, D.; Baranov, V.; Hübner, J.; Höcherl, A.; Albrecht, R.; Jaschhof, M.; et al. Peering into the Darkness: DNA Barcoding Reveals Surprisingly High Diversity of Unknown Species of Diptera (Insecta) in Germany. Insects 2022, 13, 82. [Google Scholar] [CrossRef]
- Engel, M.S.; Ceríaco, L.M.P.; Daniel, G.M.; Dellapé, P.M.; Löbl, I.; Marinov, M.; Reis, R.E.; Young, M.T.; Dubois, A.; Agarwal, I.; et al. The taxonomic impediment: A shortage of taxonomists, not the lack of technical approaches. Zool. J. Linn. Soc. 2021, 193, 381–387. [Google Scholar] [CrossRef]
- Kjærandsen, J.; Jaschhof, M. New records and first DNA barcodes of the family Canthyloscelidae (Diptera) in Fennoscandia. Nor. J. Entomol. 2019, 66, 81–93. [Google Scholar]
- Sutou, M.; Kato, T.; Ito, M. Description of the final larval stage and the pupa of Ctenosciara japonica (Diptera: Sciaridae) and their DNA barcodes. Studia Dipterol. 2007, 14, 17–22. [Google Scholar]
- Kurina, O.; Õunap, E.; Ramel, G. Baeopterogyna mihalyii Matile (Diptera, Mycetophilidae): Association of sexes using morphological and molecular approaches with the first description of females. ZooKeys 2011, 114, 15–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurina, O.; Õunap, E.; Põldmaa, K. Two new Neuratelia Rondani (Diptera, Mycetophilidae) species from Western Palaearctic: A case of limited congruence between morphology and DNA sequence data. ZooKeys 2015, 496, 105–129. [Google Scholar] [CrossRef] [Green Version]
- Jürgenstein, S.; Kurina, O.; Põldmaa, K. The Mycetophila ruficollis Meigen (Diptera, Mycetophilidae) group in Europe: Elucidating species delimitation with COI and ITS2 sequence data. ZooKeys 2015, 508, 15–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hippa, H.; Kaspřák, D.; Kahar, S.R.H.; Ševčík., J. Two new Oriental species of Paramanota Tuomikoski (Diptera: Mycetophilidae), with DNA sequence data. Raffles Bull. Zool. 2016, 64, 360–367. [Google Scholar]
- Ševčík, J.; Kaspřák, D.; Rulik, B. A new species of Docosia Winnertz from Central Europe, with DNA barcoding based on four gene markers (Diptera, Mycetophilidae). ZooKeys 2016, 549, 127–143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaspřák, D.; Borkent, C.J.; Wahab, R.A. Leptomorphus sevciki sp. nov., a remarkable new wasp-mimicking fungus gnat from Brunei (Diptera: Mycetophilidae). Acta Entomol. Musei Natl. Pragae 2017, 57, 195–203. [Google Scholar] [CrossRef] [Green Version]
- Salmela, J.; Kolcsár, L. New and poorly known Palaearctic fungus gnats (Diptera, Sciaroidea). Biodivers. Data J. 2017, 5, e11760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnussen, T.; Kjærandsen, J.; Johnsen, A.; Søli, G.E.E. Six new species of Afrotropical Allodia (Diptera: Mycetophilidae): DNA barcodes indicate recent diversification with a single origin. Zootaxa 2018, 4407, 301–320. [Google Scholar] [CrossRef] [Green Version]
- Ševčík, J.; Burdíková, N.; Kaspřák, D.; Kurina, O. Five new Palaearctic species of Docosia (Diptera: Mycetophilidae), with updated molecular phylogeny of the genus. Eur. J. Taxon. 2020, 717, 3–26. [Google Scholar] [CrossRef]
- Kjærandsen, J.; Polevoi, A.; Salmela, J. Coelosynapha, a new genus of the subfamily Gnoristinae (Diptera: Mycetophilidae) with a circumpolar, Holarctic distribution. Biodivers. Data J. 2020, 8, e54834. [Google Scholar] [CrossRef] [PubMed]
- Lindemann, J.P.; Søli, G.; Kjærandsen, J. Revision of the Exechia parva group (Diptera: Mycetophilidae). Biodivers. Data J. 2021, 9, e67134. [Google Scholar] [CrossRef] [PubMed]
- Kurina, O.; Kirik, H. Every Single Specimen Counts: A New Docosia Winnertz (Diptera: Mycetophilidae) Species Described from a Singleton. Insects 2021, 12, 1069. [Google Scholar] [CrossRef] [PubMed]
- Sutou, M.; Maruyama, M.; Komatsu, T.; Kanao, T. Discovery of a remarkable new species of black fungus gnat (Diptera, Sciaridae) from termite nests in Malaysia. J. Nat. Hist. 2012, 46, 969–978. [Google Scholar] [CrossRef]
- Shin, S.; Jung, S.; Heller, K.; Menzel, F.; Hong, T.K.; Shin, J.S.; Lee, S.H.; Lee, H.; Lee, S. DNA barcoding of Bradysia (Diptera: Sciaridae) for detection of the immature stages on agricultural crops. J. Appl. Entomol. 2015, 139, 638–645. [Google Scholar] [CrossRef]
- Heller, K.; Köhler, A.; Menzel, F.; Olsen, K.M.; Gammelmo, Ø. Two formerly unrecognized species of Sciaridae (Diptera) revealed by DNA barcoding. Nor. J. Entomol. 2016, 63, 96–115. [Google Scholar]
- Heller, K.; Rulik, B. Ctenosciara alexanderkoenigi sp. n. (Diptera: Sciaridae), an exotic invader in Germany? Biodivers. Data J. 2016, 4, e6460. [Google Scholar] [CrossRef]
- Ye, L.; Leng, R.; Huang, J.; Qu, C.; Wu, H. Review of three black fungus gnat species (Diptera: Sciaridae) from greenhouses in China: Three greenhouse sciarids from China. J. Asia-Pac. Entomol. 2017, 20, 179–184. [Google Scholar] [CrossRef]
- Eiseman, C.S.; Heller, K.; Rulik, B. A New Leaf-Mining Dark-Winged Fungus Gnat (Diptera: Sciaridae), with Notes on Other Insect Associates of Marsh Marigold (Ranunculaceae: Caltha palustris L.). Proc. Entomol. Soc. Wash. 2016, 118, 519–532. [Google Scholar] [CrossRef]
- Vilkamaa, P.; Rudzinski, H.-G.; Burdíková, N.; Ševčík, J. Phylogenetic position of Aerumnosa Mohrig (Diptera, Sciaridae) as revealed by multigene analysis, with the description of four new Oriental species. Zootaxa 2018, 4399, 248–260. [Google Scholar] [CrossRef]
- Vilkamaa, P.; Halenius, P.; Ševčík, J. Review of Pseudoaerumnosa Rudzinski (Diptera, Sciaridae), with the description of twenty-four new species. Zootaxa 2019, 4656, 248–260. [Google Scholar] [CrossRef]
- Yang, X.; Shi, K.; Heller, K.; Menzel, F.; Huang, J.; Wu, H. Morphology and DNA barcodes of two species of Bradysia Winnertz from China (Diptera, Sciaridae), with the description of Bradysia minorlobus Yang, Shi amp; Huang sp. n. Zootaxa 2019, 4612, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Lee, H.; Menzel, F.; Lee, S. Taxonomic study on the Phytosciara genus group (Diptera: Sciaridae) in Korea, including the description of a new species. J. Asia-Pac. Entomol. 2020, 23, 358–363. [Google Scholar] [CrossRef]
- Menzel, F.; Salmela, J.; Vilkamaa, P. New species and new records of black fungus gnats (Diptera: Sciaridae) from the Viidumäe Nature Reserve, Estonia. Eur. J. Taxon. 2020, 720, 62–76. [Google Scholar] [CrossRef]
- Chen, Z.; Yang, X.; Menzel, F.; Wu, H.; Huang, J. Three Oriental species of Pseudoaerumnosa Rudzinski (Diptera, Sciaridae) from China. Zootaxa 2021, 4969, 551–562. [Google Scholar] [CrossRef] [PubMed]
- Jaschhof, M. A review of the Aprionus flavidus group, with description of two new species close to Aprionus betulae Jaschhof (Diptera: Cecidomyiidae, Micromyinae). Studia Dipterol. 2014, 21, 221–229. [Google Scholar]
- Dorchin, N.; Joy, J.B.; Wise, M.J.; Abrahamson, W.G.; Hilke, L.K.; Wise, M.J.; Abrahamson, W.G. Taxonomy and phylogeny of the Asphondylia species (Diptera: Cecidomyiidae) of North American goldenrods: Challenging morphology, complex host associations, and cryptic speciation. Zool. J. Linn. Soc. 2015, 174, 265–304. [Google Scholar] [CrossRef] [Green Version]
- Duque-Gamboa, D.; Castillo-Cárdenas, M.F.; Hernández, L.M.; Guzmán, Y.C.; Manzano, M.R.; Toro-Perea, N. Mitochondrial DNA suggests cryptic speciation in Prodiplosis longifila Gagné (Diptera: Cecidomyiidae) associated with geographic distance and host specialization. Bull. Entomol. Res. 2018, 108, 739–749. [Google Scholar] [CrossRef] [PubMed]
- Jaschhof, M.; Jaschhof, C. Reevaluation of species richness in Winnertzia (Diptera, Cecidomyiidae, Winnertziinae), with descriptions of 37 new species from Sweden, Peru and Australia. Zootaxa 2020, 4829, 1–72. [Google Scholar] [CrossRef]
- Bernardo, U.; Nugnes, F.; Gargiulo, S.; Nicoletti, R.; Becchimanzi, A.; Stinca, A.; Viggiani, G. An Integrative Study on Asphondylia spp. (Diptera: Cecidomyiidae), Causing Flower Galls on Lamiaceae, with Description, Phenology, and Associated Fungi of Two New Species. Insects 2021, 12, 958. [Google Scholar] [CrossRef]
- Kurina, O.; Mantič, M.; Ševčík, J. A remarkable new genus of Keroplatidae (Insecta, Diptera) from the Afrotropical region, with DNA sequence data. Afr. Invertebr. 2017, 58, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Mantič, M.; Ševčík, J. Macrocera rohaceki sp. nov. and other interesting records of Keroplatidae (Diptera) from southern and central Europe, with DNA sequence data. Acta Entomol. Musei Natl. Pragae 2017, 57, 751–764. [Google Scholar] [CrossRef] [Green Version]
- Kjærandsen, J. Defying the northern limit: New records and DNA barcodes of Symmerus Walker, 1848 (Diptera, Ditomyiidae) from Northern Norway. Nor. J. Entomol. 2020, 67, 44–51. [Google Scholar]
- Hippa, H.; Ševčík, J. Notes on Nepaletricha (Diptera: Sciaroidea incertae sedis), with description of three new species from India and Vietnam. Acta Entomol. Musei Natl. Pragae 2014, 54, 729–739. [Google Scholar]
- Kjærandsen, J.; Hagenlund, L.K. New records and first DNA barcodes of Sciarosoma nigriclava (Strobl, 1898) (Diptera, Sciaroidea incertae sedis) from Norway. Nor. J. Entomol. 2019, 66, 94–98. [Google Scholar]
- Okuyama, Y.; Okamoto, T.; Kjærandsen, J.; Kato, M. Bryophytes facilitate outcrossing of Mitella by functioning as larval food for pollinating fungus gnats. Ecology 2018, 99, 1890–1893. [Google Scholar] [CrossRef] [Green Version]
- Bowser, M.; Bowser, M.; Bowser, E.; Bowser, A.; Bowser, E.; Melvin, T. DNA barcoding Alaskan willow rosette gall makers (Diptera: Cecidomyiidae: Rabdophaga). Newsl. Alsk. Entomol. Soc. 2018, 11, 8–14. [Google Scholar] [CrossRef]
- Meyer, C.P.; Paulay, G. DNA barcoding: Error rates based on comprehensive sampling. PLoS Biol. 2005, 3, e422. [Google Scholar] [CrossRef] [Green Version]
- Owens, B. Most insect studies lack crucial species information. Survey results suggest that a lot of entomology research could be impossible to replicate. Nat. News 2018. [CrossRef]
- Monckton, S.K.; Johal, S.; Packer, L. Inadequate treatment of taxonomic information prevents replicability of most zoological research. Can. J. Zool. 2020, 98, 633–642. [Google Scholar] [CrossRef]
- MacLeod, N.; Benfield, M.; Culverhouse, P. Time to automate identification. Nature 2010, 467, 154–155. [Google Scholar] [CrossRef]
- Culverhouse, P.F.; Macleod, N.; Williams, R.; Benfield, M.C.; Lopes, R.M.; Picheral, M. An empirical assessment of the consistency of taxonomic identifications. Mar. Biol. Res. 2014, 10, 73–84. [Google Scholar] [CrossRef]
- Chandler, P.J. The Holarctic species of the Mycetophila fungorum (De Geer) group (Diptera, Mycetophilidae). Br. J. Entomol. Nat. Hist. 1993, 6, 5–11. [Google Scholar]
- Wu, H. The Chinese species of the Mycetophila fungorum group (Diptera: Mycetophilidae). Zool. Meded. 1997, 71, 171–175. [Google Scholar]
- Rheindt, F.E.; Christidis, L.; Norman, J.A. Genetic introgression, incomplete lineage sorting and faulty taxonomy create multiple cases of polyphyly in a montane clade of tyrant-flycatchers (Elaenia, Tyrannidae). Zool. Scr. 2009, 38, 143–153. [Google Scholar] [CrossRef]
- Magnussen, T.; Johnsen, A.; Kjærandsen, J.; Struck, T.H.; Søli, G.E.E. Molecular phylogeny of Allodia (Diptera: Mycetophilidae) constructed using genome skimming. Syst. Entomol. 2022, 47, 267–281. [Google Scholar] [CrossRef]
- Caspers, N. Mycetophiliden aus Lunz, Niederösterreich (Diptera, Nematocera, Mycetophilidae). Entomofauna 1984, 5, 173–205. [Google Scholar]
- Zaitzev, A.I. Fungus gnats of the sericoma, griseicolle and ruficorne species groups of the genus Brevicornu Marshall (Diptera, Mycetophilidae) of Holarctic fauna. Entomol. Obozr. 1988, 67, 391–404. [Google Scholar]
- Ševčík, J.; Kjærandsen, J.; Marshall, S. Revision of Speolepta (Diptera: Mycetophilidae), with descriptions of new Nearctic and Oriental species. Can. Entomol. 2012, 144, 93–107. [Google Scholar] [CrossRef]
- Dörge, D.; Zaenker, S.; Klussmann-Kolb, A.; Weigand, A. Traversing worlds—Dispersal potential and ecological classification of Speolepta leptogaster (Winnertz, 1863) (Diptera, Mycetophilidae). Subterr. Biol. 2014, 13, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Ahrens, D.; Ahyong, S.T.; Ballerio, A.; Barclay, M.V.L.; Eberle, J.; Espeland, M.; Huber, B.A.; Mengual, X.; Pacheco, T.L.; Peters, R.S.; et al. Is it time to describe new species without diagnoses?—A comment on Sharkey et al. Zootaxa 2021, 5027, 151–159. [Google Scholar] [CrossRef]
- Rindal, E.; Søli, G.E.E.; Kjærandsen, J.; Bachmann, L. Molecular phylogeny of the fungus gnat tribe Exechiini (Mycetophilidae, Diptera). Zool. Scr. 2007, 36, 327–335. [Google Scholar] [CrossRef]
- Rindal, E.; Søli, G.E.E.; Bachmann, L. Molecular phylogeny of the fungus gnat family Mycetophilidae (Diptera, Mycetophiliformia). Syst. Entomol. 2009, 34, 524–532. [Google Scholar] [CrossRef]
- Rindal, E.; Søli, G.E.E.; Bachmann, L. On the systematics of the fungus gnat subfamily Mycetophilinae (Diptera): A combined morphological and molecular approach. J. Zool. Syst. Evol. Res. 2009, 47, 227–233. [Google Scholar] [CrossRef]
- Martinsson, S.; Kjærandsen, J.; Sundberg, P. Towards a molecular phylogeny of the fungus gnat genus Boletina (Diptera: Mycetophilidae). Zool. Scr. 2011, 40, 272–281. [Google Scholar] [CrossRef]
- Ševčík, J.; Kaspřák, D.; Tóthová, A. Molecular phylogeny of fungus gnats (Diptera: Mycetophilidae) revisited: Position of Manotinae, Metanepsiini, and other enigmatic taxa as inferred from multigene analysis. Syst. Entomol. 2013, 38, 654–660. [Google Scholar] [CrossRef]
- Ševčík, J.; Kaspřák, D.; Mantič, M.; Ševčíková, T.; Tóthová, A. Molecular phylogeny of the fungus gnat family Diadocidiidae and its position within the infraorder Bibionomorpha (Diptera). Zool. Scr. 2014, 43, 370–378. [Google Scholar] [CrossRef]
- Sikora, T.; Jaschhof, M.; Mantič, M.; Kaspřák, D.; Ševčík, J. Considerable congruence, enlightening conflict: Molecular analysis largely supports morphology-based hypotheses on Cecidomyiidae (Diptera) phylogeny. Zool. J. Linn. Soc. 2019, 185, 98–110. [Google Scholar] [CrossRef]
- Kaspřák, D.; Kerr, P.; Sýkora, V.; Tóthová, A.; Ševčík, J. Molecular phylogeny of the fungus gnat subfamilies Gnoristinae and Mycomyinae, and their position within Mycetophilidae (Diptera). Syst. Entomol. 2019, 44, 128–138. [Google Scholar] [CrossRef]
- Burdíková, N.; Kjærandsen, J.; Lindemann, J.P.; Kaspřák, D.; Tóthová, A.; Ševčík, J. Molecular phylogeny of the Paleogene fungus gnat tribe Exechiini (Diptera: Mycetophilidae) revisited: Monophyly of genera established and rapid radiation confirmed. J. Zool. Syst. Evol. Res. 2019, 57, 806–821. [Google Scholar] [CrossRef]
- Dorchin, N.; Harris, K.M.; Stireman, J.O. Phylogeny of the gall midges (Diptera, Cecidomyiidae, Cecidomyiinae): Systematics, evolution of feeding modes and diversification rates. Mol. Phylogenet. Evol. 2019, 140, 106602. [Google Scholar] [CrossRef]
- Mantič, M.; Sikora, T.; Burdíková, N.; Blagoderov, V.; Kjærandsen, J.; Kurina, O.; Ševčík, J. Hidden in Plain Sight: Comprehensive Molecular Phylogeny of Keroplatidae and Lygistorrhinidae (Diptera) Reveals Parallel Evolution and Leads to a Revised Family Classification. Insects 2020, 11, 348. [Google Scholar] [CrossRef] [PubMed]
- Gibbs, J. DNA barcoding a nightmare taxon: Assessing barcode index numbers and barcode gaps for sweat bees. Genome 2018, 61, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Puillandre, N.; Lambert, A.; Brouillet, S.; Achaz, G. ABGD, Automatic Barcode Gap Discovery for primary species delimitation. Mol. Ecol. 2012, 21, 1864–1877. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Kapli, P.; Pavlidis, P.; Stamatakis, A. A general species delimitation method with applications to phylogenetic placements. Bioinformatics 2013, 29, 2869–2876. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivathsan, A.; Hartop, E.; Puniamoorthy, J.; Lee, W.T.; Kutty, S.N.; Kurina, O.; Meier, R. Rapid, large-scale species discovery in hyperdiverse taxa using 1D MinION sequencing. BMC Biol. 2019, 17, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kutty, S.N.; Wang, W.; Ang, Y.; Tai, Y.C.; Ho, J.K.I.; Meier, R. Next-Generation Sequencing Identification Tools for Nee Soon freshwater swamp forest, Singapore. Gard. Bull. Singap. 2018, 70 (Suppl. S1), 155–174. [Google Scholar] [CrossRef]
- Cotterill, F.P.; Foissner, W. A pervasive denigration of natural history misconstrues how biodiversity inventories and taxonomy underpin scientific knowledge. Biodivers. Conserv. 2010, 19, 291–303. [Google Scholar] [CrossRef] [Green Version]
- Sharkey, M.J.; Janzen, D.H.; Hallwachs, W.; Chapman, E.G.; Smith, M.A.; Dapkey, T.; Brown, A.; Ratnasingham, S.; Naik, S.; Manjunath, R.; et al. Minimalist revision and description of 403 new species in 11 subfamilies of Costa Rican braconid parasitoid wasps, including host records for 219 species. ZooKeys 2021, 1013, 1–665. [Google Scholar] [CrossRef]
- Meier, R.; Blaimer, B.B.; Buenaventura, E.; Hartop, E.; von Rintelen, T.; Srivathsan, A.; Yeo, D. A re-analysis of the data in Sharkey et al.’s (2021) minimalist revision reveals that BINs do not deserve names, but BOLD Systems needs a stronger commitment to open science. Cladistics 2021, 1–12. [Google Scholar] [CrossRef]
- Sternkopf, V.; Liebers-Helbig, D.; Ritz, M.S.; Zhang, J.; Helbig, A.J.; de Knijff, P. Introgressive hybridization and the evolutionary history of the herring gull complex revealed by mitochondrial and nuclear DNA. BMC Evol. Biol. 2010, 10, 348. [Google Scholar] [CrossRef] [Green Version]
- Huemer, P.; Karsholt, O.; Aarvik, L.; Berggren, K.; Bidzilya, O.; Junnilainen, J.; Landry, J.-F.; Mutanen, M.; Nupponen, K.; Segerer, A.; et al. DNA barcode library for European Gelechiidae (Lepidoptera) suggests greatly underestimated species diversity. ZooKeys 2020, 921, 141–157. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Vaamonde, C.; Kirichenko, N.; Cama, A.; Doorenweerd, C.; Godfray, H.C.J.; Guiguet, A.; Gomboc, S.; Huemer, P.; Landry, J.-F.; Laštůvka, A.; et al. Evaluating DNA Barcoding for Species Identification and Discovery in European Gracillariid Moths. Front. Ecol. Evol. 2021, 9, 66. [Google Scholar] [CrossRef]








| Taxon | BOLD, World Totals (14 October 2021) | |||||||
|---|---|---|---|---|---|---|---|---|
| Specimens | % BOLD | BINs | % BOLD | ID Species | % ID Species | SPM/BINs | BINs/SPP | |
| Sciaroidea | 1,224,877 | 9.2% | 56,648 | 9.5% | 2843 | 5.0% | 22 | 20 |
| Cecidomyiidae | 661,414 | 5.0% | 43,763 | 7.3% | 594 | 1.4% | 15 | 74 |
| Sciaridae | 479,666 | 3.6% | 9077 | 1.5% | 755 | 8.3% | 53 | 12 |
| Mycetophilidae | 73,622 | 0.6% | 3267 | 0.5% | 1301 | 40% | 23 | 3 |
| Keroplatidae | 8500 | 0.1% | 435 | 0.07% | 134 | 31% | 20 | 3 |
| Bolitophilidae | 515 | 0.004% | 37 | 0.006% | 35 | 95% | 14 | 1 |
| Ditomyiidae | 823 | 0.006% | 40 | 0.007% | 6 | 15% | 21 | 7 |
| Diadocidiidae | 329 | 0.002% | 26 | 0.004% | 15 | 58% | 13 | 2 |
| Incertae sedis | 8 | 0.0001% | 3 | 0.001% | 3 | 100% | 3 | 1 |
| Taxon | Nordic barcoding, including private data | |||||||
| Specimens | % BOLD | BINs | % BOLD | ID species | % BOLD | SPM/BINs | BINs/SPP | |
| Sciaroidea | 14,908 | 1.22% | 2191 | 3.9% | 1557 | 55% | 7 | 1.4 |
| Cecidomyiidae | 2560 | 0.39% | 614 | 1.4% | 81 | 14% | 4 | 8 |
| Sciaridae | 4972 | 1.04% | 601 | 6.6% | 414 | 55% | 8 | 1.5 |
| Mycetophilidae | 6685 | 9.08% | 877 | 27% | 964 | 74% | 8 | 0.9 |
| Keroplatidae | 391 | 4.60% | 54 | 12% | 55 | 41% | 7 | 1.0 |
| Bolitophilidae | 217 | 42% | 36 | 97% | 34 | 97% | 6 | 1.1 |
| Ditomyiidae | 13 | 1.58% | 2 | 5.0% | 2 | 33% | 7 | 1.0 |
| Diadocidiidae | 66 | 20% | 6 | 23% | 6 | 40% | 11 | 1.0 |
| Incertae sedis | 4 | 50% | 1 | 33% | 1 | 33% | 4 | 1.0 |
| Taxon | Nordic species estimates as proportion of Nordic BINs | |||||||
| DS-FINPRO ref. library | BINs/DS-FINPRO | Norwegian published species | BINs/Norwegian species | Swedish species estimate | BINs/Swedish estimate | Nordic species estimate | BINs/Nordic estimate | |
| Sciaroidea | 440 | 4.98 | 1289 | 1.70 | 2720 | 0.81 | 3727 | 0.59 |
| Cecidomyiidae | 16 | 38.38 | 245 | 2.51 | 1250 | 0.49 | 1800 | 0.34 |
| Sciaridae | 81 | 7.42 | 143 | 4.20 | 470 | 1.28 | 750 | 0.80 |
| Mycetophilidae | 305 | 2.88 | 835 | 1.05 | 890 | 0.99 | 1050 | 0.84 |
| Keroplatidae | 33 | 1.64 | 38 | 1.42 | 60 | 0.90 | 75 | 0.72 |
| Bolitophilidae | 2 | 18.00 | 21 | 1.71 | 38 | 0.95 | 40 | 0.90 |
| Ditomyiidae | 0 | - | 2 | 1.00 | 3 | 0.67 | 3 | 0.67 |
| Diadocidiidae | 3 | 2.00 | 4 | 1.50 | 8 | 0.75 | 8 | 0.75 |
| Incertae sedis | 0 | - | 1 | 1.00 | 1 | 1.00 | 1 | 1.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kjærandsen, J. Current State of DNA Barcoding of Sciaroidea (Diptera)—Highlighting the Need to Build the Reference Library. Insects 2022, 13, 147. https://doi.org/10.3390/insects13020147
Kjærandsen J. Current State of DNA Barcoding of Sciaroidea (Diptera)—Highlighting the Need to Build the Reference Library. Insects. 2022; 13(2):147. https://doi.org/10.3390/insects13020147
Chicago/Turabian StyleKjærandsen, Jostein. 2022. "Current State of DNA Barcoding of Sciaroidea (Diptera)—Highlighting the Need to Build the Reference Library" Insects 13, no. 2: 147. https://doi.org/10.3390/insects13020147