Temperature-Dependent Demographic Characteristics and Control Potential of Aphelinus asychis Reared from Sitobion avenae as a Biological Control Agent for Myzus persicae on Chili Peppers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Insect Cultures
2.2. Life Table, Parasitism, and Host Feeding
3. Results
3.1. Life Table
3.2. Population Parameters
3.3. Host Feeding
3.4. Non-Effective Parasitism
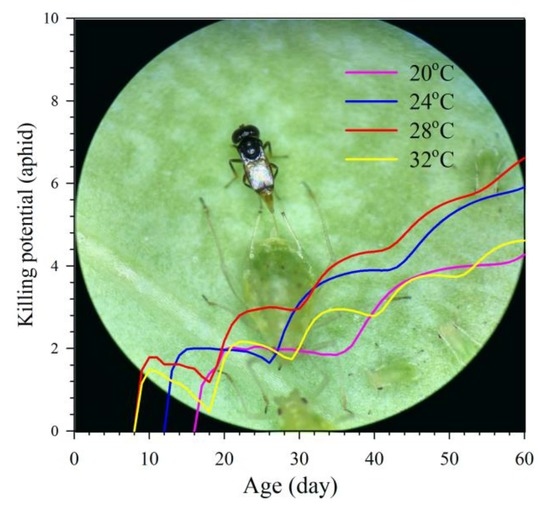
3.5. Aphid Killing Rate
3.6. Relationship between Population Fitness and Temperatures
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Q.B.; Zhang, Y.Y.; Zhuang, M.; Yang, L.M.; Liu, Y.M.; Lü, H.H.; Fang, Z.Y. EST–SSR fingerprinting of fifty cabbage representative varieties from China. Sci. Agric. Sin. 2014, 47, 111–121. [Google Scholar]
- Van Emden, H.F.; Van Eastop, V.F.; Hughes, R.D.M.J.; Way, M.J. The Ecology of Myzus persicae. Annu. Rev. Entomol. 2003, 14, 197–270. [Google Scholar] [CrossRef]
- Sun, H.; Song, Y. Establishment of a wheat banker plant system for the parasitoid Aphidius gifuensis against Myzus persicae in greenhouse chili pepper. Appl. Entomol. Zool. 2019, 54, 1–9. [Google Scholar] [CrossRef]
- Voudouris, C.C.; Kati, A.N.; Sadikoglou, E.; Williamson, M.; Skouras, P.; Dimotsiou, O.; Georgiou, S.; Fenton, B.; Skavdis, G.; Margaritopoulos, J.T. Insecticide resistance status of Myzus persicae in Greece: Long term surveys and new diagnostics for resistance mechanisms.: Resistance status of M. persicae in Greece. Pest Manag. Sci. 2015, 72, 671–683. [Google Scholar] [CrossRef] [PubMed]
- Needham, P.H.; Sawicki, R.M. Diagnosis of resistance to organophosphorus insecticides in Myzus persicae. Nature 1971, 230, 125–126. [Google Scholar] [CrossRef]
- Martinez–Torres, D.; Foster, S.P.; Field, L.M.; Devonshire, A.L.; Williamson, M.S. A sodium channel point mutation is associated with resistance to DDT and pyrethroid insecticides in the peach–potato aphid, Myzus persicae (Sulzer) (Hemiptera: Aphididae). Insect Biochem. Mol. 1999, 8, 339–346. [Google Scholar] [CrossRef]
- Ffrench–Constant, R.H.; Anthony, N.; Aronstein, K.; Rocheleau, T.; Stilwell, G. Cyclodiene insecticide resistance: From molecular to population genetics. Annu. Rev. Entomol. 2000, 45, 449–466. [Google Scholar] [CrossRef]
- Philippou, D.; Field, L.M.; Moores, G.D. Metabolic enzyme(s) confer imidacloprid resistance in a clone of Myzus persicae (Sulzer) (Hemiptera: Aphididae) from Greece. Pest Manag. Sci. 2009, 66, 390–395. [Google Scholar]
- Suwignyo, R.A.; Napoleon, A. Insecticides residue in the centre of paddy field in Musi Rawas, South Sumatera, Indonesia. E3s Web Conf. 2018, 68, 04014. [Google Scholar]
- Bucy, M.T.; Melathopoulos, A. Labels of insecticides to which Oregon honey bee (Apis mellifera L.) hives could be exposed do not align with federal recommendations in their communication of acute and residual toxicity to honey bees. Pest Manag. Sci. 2020, 76, 1664–1672. [Google Scholar] [CrossRef]
- Heller, S.; Joshi, N.K.; Chen, J.; Rajotte, E.G.; Mullin, C.; Biddinger, D.J. Pollinator exposure to systemic insecticides and fungicides applied in the previous fall and pre–bloom period in apple orchards. Environ. Pollut. 2020, 265, 114589. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.D. Biological control of arthropod pests using banker plant systems: Past progress and future directions. Biol. Control 2010, 52, 8–16. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chi, H.; Liu, T.X. Demography and parasitic effectiveness of Aphelinus asychis reared from Sitobion avenae as a biological control agent of Myzus persicae reared on chili pepper and cabbage. Biol. Control 2016, 92, 111–119. [Google Scholar] [CrossRef]
- Wang, S.Y.; Feng, Y.; Liang, N.N.; Tang, R.; Liu, Y.H.; Zhang, D.Y.; Liu, T.X. Starving Aphelinus asychis negatively affects host feeding and parasitism on Myzus persicae. J. Asia-Pac. Entomol. 2018, 21, 676–681. [Google Scholar] [CrossRef]
- Hayat, M. The genera of Aphelinidae (Hymenoptera) of the world. Syst. Entomol. 1983, 8, 63–102. [Google Scholar] [CrossRef]
- Japoshvili, G.; Karaca, I. A review of the species of Aphelinus Dalman, 1820 (Hymenoptera: Aphelinidae) from Georgia. J. Entomol. Res. Soc. 2009, 11, 41–52. [Google Scholar]
- Li, C.D.; Byeon, Y.W.; Choi, B.R. An aphelinid species, Aphelinus asychis Walker (Hymenoptera: Aphelinidae) new to Korea. J. Asia-Pac. Entomol. 2007, 10, 13–15. [Google Scholar] [CrossRef]
- Kumar, S.; Kashyap, S.; Soni, S. The foraging behaviour of Aphelinus asychis Walker (Hymenoptera: Aphelinidae) and Aphidius ervi (Haliday) (Hymenoptera: Braconidae) on Myzus persicae (Sulzer) (Hemiptera: Aphididae). Phytoparasitica 2019, 47, 1–10. [Google Scholar] [CrossRef]
- Gavkare, O.; Kumar, S.; Japoshvili, G. Effectiveness of native parasitoids of Myzus persicae in greenhouse environments in India. Phytoparasitica 2014, 42, 141–144. [Google Scholar] [CrossRef]
- Pan, M.Z.; Wang, L.; Zhang, C.Y.; Zhang, L.X.; Liu, T.X. The influence of feeding and host deprivation on egg load and reproduction of an aphid parasitoid, Aphidius gifuensis, (hymenoptera: Braconidae). Appl. Entomol. Zool. 2017, 52, 1–9. [Google Scholar] [CrossRef]
- Takada, H. Parasitoids (Hymenoptera: Braconidae, Aphidiinae, Aphelinidae) of four principal pest aphids (Homoptera: Aphididae) on greenhouse vegetable crops in Japan. Appl. Entomol. Zool. 2002, 37, 237–249. [Google Scholar] [CrossRef] [Green Version]
- Tatsumi, E.; Takada, H. Evaluation of Aphelinus asychis and Aphelinus albipodus (Hymenoptera: Aphelinidae) as biological control agents against three pest aphids. Appl. Entomol. Zool. 2005, 40, 379–385. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Y.; Osborne, L.S.; Chen, J.; Mckenzie, C.; Houben, K.; Irizarry, F. Evaluation of corn plant as potential banker plant for supporting predatory gall midge, Feltiella acarisuga (Diptera: Cecidomyiidae) against Tetranychus urticae (Acari: Tetranychidae) in greenhouse vegetable production. Crop Prot. 2011, 30, 1635–1642. [Google Scholar] [CrossRef]
- Xiao, Y.; Chen, J.; Cantliffe, D.; Mckenzie, C.; Houben, K.; Osborne, L.S. Establishment of papaya banker plant system for parasitoid, Encarsia sophia (Hymenoptera: Aphilidae) against Bemisia tabaci (Hemiptera: Aleyrodidae) in greenhouse tomato production. Biol. Control 2011, 58, 239–247. [Google Scholar] [CrossRef]
- Messelink, G.J.; Bennison, J.; Alomar, O.; Ingegno, B.L.; Tavella, L.; Shipp, L.; Palevsky, E.; Wäckers, F.L. Approaches to conserving natural enemy populations in greenhouse crops: Current methods and future prospects. BioControl 2014, 59, 1–17. [Google Scholar] [CrossRef]
- Pan, M.Z.; Liu, T.X. Suitability of three aphid species for Aphidius gifuensis (Hymenoptera: Braconidae): Parasitoid performance varies with hosts of origin. Biol. Control 2014, 69, 90–96. [Google Scholar] [CrossRef]
- Pan, M.Z.; Cao, H.H.; Liu, T.X. Effects of winter wheat cultivars on the life history traits and olfactory response of Aphidius gifuensis. Biocontrol 2014, 59, 539–546. [Google Scholar] [CrossRef]
- Inward, D.J.G.; Wainhouse, D.; Peace, A. The effect of temperature on the development and life cycle regulation of the pine weevil Hylobius abietis and the potential impacts of climate change. Agric. For. Entomol. 2012, 14, 348–357. [Google Scholar] [CrossRef]
- Romo, C.M.; Tylianakis, J.M. Elevated temperature and drought interact to reduce parasitoid effectiveness in suppressing hosts. PLoS ONE 2013, 8, e58136. [Google Scholar] [CrossRef] [Green Version]
- Piyaphongkul, J.; Pritchard, J.; Bale, J. Effects of acclimation on the thermal tolerance of the brown planthopper Nilaparvata lugens (Stål). Agric. For. Entomol. 2014, 16, 174–183. [Google Scholar] [CrossRef]
- Zhang, S.Z.; Cao, Z.; Zhang, F.; Liu, T.X. Exposing eggs to high temperatures affects the development, survival and reproduction of Harmonia axyridis. J. Therm. Biol. 2014, 39, 40–44. [Google Scholar] [CrossRef]
- Qin, Y.J.; Lan, S.; Zhao, Z.H.; Sun, H.Y.; Zhu, X.M.; Yang, P.Y.; Li, Z.H. Potential geographical distribution of the fall armyworm (Spodoptera frugiperda) in China. Plant Protect. 2019, 45, 43–47. [Google Scholar]
- Wu, X. Temperature effects on development and fecundity of Aphidius gifuensis ashmead. Zool. Res. 2000, 21, 192–198. [Google Scholar]
- Vet, L.; Lenteren, J.C.; Woets, J. The parasite-host relationship between Encarsia formosa (Hymenoptera: Aphelinidae) and Trialeurodes vaporariorum (Homoptera: Aleyrodidae). IX. A review of the biological control of the greenhouse whitefly with suggestions for future research. Entomol. Exp. Appl. 2010, 90, 26–51. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Guo, J.Y.; Chen, H.S.; Wan, F.H. Effects of temperature on survival, development, longevity, and fecundity of Ophraella communa (Coleoptera: Chrysomelidae), a potential biological control agent against Ambrosia artemisiifolia (Asterales: Asteraceae). Environ. Entomol. 2010, 39, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Khan, J.; Saljoki, A.U.; Rehman, A. Effect of temperature on biological attributes and predatory potential of Harmonia dimidiata (Fab.) (Coleoptera: Coccinellidae) fed on Rhopalosiphum padi aphid. J. Entomol. Zool. Stud. 2016, 4, 1016–1022. [Google Scholar]
- Tougeron, K.; Brodeur, J.; Le Lann, C.; Van Baaren, J. How climate change affects the seasonal ecology of insect parasitoids. Ecol. Entomol. 2020, 45, 167–181. [Google Scholar] [CrossRef] [Green Version]
- Hansen, L.S.; Jensen, K.M.V. Effect of temperature on parasitism and host–feeding of Trichogramma turkestanica (Hymenoptera: Trichogrammatidae) on Ephestia kuehniella (Lepidoptera: Pyralidae). J. Econ. Entomol. 2002, 95, 50–56. [Google Scholar] [CrossRef]
- Daane, K.M.; Malakar–Kuenen, R.D.; Walton, V.M. Temperature dependent development of Anagyrus pseudococci (Hymenoptera: Encyrtidae) as a parasitoid of the vine mealybug, Planococcus ficus (Homoptera: Pseudococcidae). Biol. Control 2004, 31, 123–132. [Google Scholar] [CrossRef]
- Chi, H.; Liu, H. Two new methods for the study of insect population ecology. Bull. Inst. Zool. Acad. Sin. 1985, 24, 225–240. [Google Scholar]
- Chi, H. Life-table analysis incorporating both sexes and variable development rates among individuals. Environ. Entomol. 1988, 17, 26–34. [Google Scholar] [CrossRef]
- Chi, H. TWOSEX–MSChart: Computer Program for Age Stage, Two-Sex Life Table Analysis. 2020. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 20 July 2020).
- Yu, J.Z.; Chi, H.; Chen, B.H. Comparison of the life tables and predation rates of Harmonia dimidiata (F.) (Coleoptera: Coccinellidae) fed on Aphis gossypii Glover (Hemiptera: Aphididae) at different temperatures. Biol. Control 2013, 64, 1–9. [Google Scholar] [CrossRef]
- Chi, H. CONSUME–MSChart: Computer Program for Consumption Rate Analysis Based on the Age Stage, Two-Sex Life Table. 2020. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 9 July 2020).
- Chi, H. TIMING–MSChart: Computer Program for Consumption Rate Analysis Based on the Age Stage, Two-Sex Life Table. 2020. Available online: http://140.120.197.173/Ecology/prod02.htm (accessed on 9 July 2020).
- Efron, B.; Tibshirani, R.J. An Introduction to the Bootstrap; Chapman and Hall: New York, NY, USA, 1993. [Google Scholar]
- Akköprü, E.; Atlıhan, R.; Okut, H.; Chi, H. Demographic assessment of plant cultivar resistance to insect pests: A case study of the dusky-veined walnut aphid (Hemiptera: Callaphididae) on five walnut cultivars. J. Econ. Entomol. 2015, 108, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Qiu, B.L.; De Barro, P.J.; Xu, C.X.; Ren, S.X. Effect of temperature on the life history of Encarsia bimaculata (Hymenoptera: Aphelinidae), a parasitoid of Bemisia tabaci (Hemiptera: Aleyrodidae). Eur. J. Entomol. 2006, 103, 787–792. [Google Scholar] [CrossRef]
- McClay, A.S.; Hughes, R.B. Temperature and host–plant effects on development and population growth of Mecinus janthinus (Coleoptera: Curculionidae), a biological control agent for invasive Linaria spp. Biol. Control 2007, 40, 405–410. [Google Scholar] [CrossRef]
- Liu, S.S. The influence of temperature on the population increase of Myzus persicae and Lipaphis erysimi. Acta Entomol. Sin. 1991, 34, 189–197. [Google Scholar]
- Flanders, S.E. Aphelinid biologies with implication for taxonomy. Ann. Entomol. Soc. Am. 1953, 46, 84–94. [Google Scholar] [CrossRef]
- Schirmer, S.; Sengonca, C.; Blaeser, P. Influence of abiotic factors on some biological and ecological characteristics of the aphid parasitoid Aphelinus asychis (Hymenoptera: Aphelinidae) parasitizing Aphis gossypii (Sternorrhyncha: Aphididae). Eur. J. Entomol. 2008, 105, 121–129. [Google Scholar] [CrossRef] [Green Version]
- Sengonca, C.; Schirmer, S.; Blaeser, P. Life table of the aphid parasitoid Aphelinus asychis (Walker) (Hymenoptera, Aphelinidae) parasitizing different age groups of Aphis gossypii Glover (Homoptera, Aphididae). J. Plant Dis. Protect. 2008, 115, 122–128. [Google Scholar] [CrossRef]
- Raney, H.G.; Coles, L.W.; Eikenbary, R.D.; Morrison, R.D.; Starks, K. Host preference, longevity, developmental period and sex ratio of Aphelinus asychis with three sorghum-fed species of aphids held at controlled temperatures. Ann. Entomol. Soc. Am. 1971, 64, 169–176. [Google Scholar] [CrossRef]
- Byeon, Y.W.; Tuda, M.; Takagi, M.; Kim, J.H.; Choi, M.Y. Life history parameters and temperature requirements for development of an aphid parasitoid Aphelinus asychis (Hymenoptera: Aphelinidae). Environ. Entomol. 2011, 40, 431–440. [Google Scholar] [CrossRef]
- Kang, E.J.; Byeon, Y.W.; Kim, J.H.; Choi, M.Y.; Choi, Y.S. The effect of temperatures on the biological characteristics of two aphid parasitoids Aphelinus asychis (Walker) and Aphelinus varipes (Forster) (Hymenoptera: Aphelinidae) on two aphid hosts. Korean J. Appl. Entomol. 2012, 51, 397–403. [Google Scholar] [CrossRef]
- Jackson, H.B.; Eikenbary, R.D. Bionomics of Aphelinus asychis (Hymenoptera: Eulophidae) an introduced parasite of sorghum greenbug (Homoptera: Aphididae). Ann. Entomol. Soc. Am. 1971, 64, 81–85. [Google Scholar] [CrossRef]
- Cate, R.H.; Eikenbary, R.D.; Morrison, R.D. Morrison. Preference for and effect of greenbug parasitism and feeding by Aphelinus asychis. Environ. Entomol. 1977, 6, 547–550. [Google Scholar] [CrossRef]
- Bernal, J.; Gonzalez, D. Temperature requirements of 4 parasites of the Russian wheat aphid Diuraphis noxia. Entomol. Exp. Appl. 1993, 69, 173–182. [Google Scholar] [CrossRef]
- Wilbert, H. Das Ausleseverfahren von Aphelinus semiflavus Howard und die Abwehrreaktionen seiner Wirte (Hymenoptera, Aphelinidae). Beitr. Entomol. 1964, 14, 159–219. [Google Scholar]
- Gerling, D.; Roitberg, B.D.; Mackauer, M. Instar–specific defense of the pea aphid, Acyrthosiphon pisum—Influence on oviposition success of the parasite Aphelinus asychis (Hymenoptera: Aphelinidae). J. Insect Behav. 1990, 3, 501–514. [Google Scholar] [CrossRef]
- Tang, Y.Q.; Yokomi, R.K. Biology of Aphelinus spiraecolae (Hymenoptera: Aphelinidae), a parasitoid of the Spirea aphid (Homoptera: Aphididae). Environ. Entomol. 1996, 25, 519–523. [Google Scholar] [CrossRef]
- Byeon, Y.W.; Tuda, M.; Takagi, M.; Kim, J.H.; Kim, Y.H. Non-reproductive host killing caused by Aphelinus asychis (Hymenoptera: Aphelinidae), a parasitoid of cotton aphid, Aphis gossypii (Homoptera: Aphididae). J. Fac. Agric. Kyushu Univ. 2009, 54, 369–372. [Google Scholar]
- Chi, H.; Mou, D.F.; Allahyari, H.; Yu, J.Z.; Huang, Y.B.; Yang, T.C.; Farhadi, R.; Gholizadeh, M. Finite predation rate: A novel parameter for the quantitative measurement of predation potential of predator at population level. Nat. Preced. 2011. [Google Scholar] [CrossRef]
- Noyes, J.S. Universal Chalcidoidea Databas. 2006. Available online: http://www.nhm.ac.uk/jdsml/research-curation/projects/chalcidoids/ (accessed on 5 June 2006).
- Nowierski, R.M.; Fitzgerald, B.C. Supercooling capacity of Eurasian and North American populations of parasitoids of the Russian wheat aphid, Diuraphis noxia. Biocontrol 2002, 47, 279–292. [Google Scholar] [CrossRef]
- Kalinkat, G.; Rall, B.C.; Björkman, C.; Niemelä, P. Effects of climate change on the interactions between insect pests and their natural enemies. Clim. Chang. Insect Pests 2015, 119–135. [Google Scholar]
- Angilletta, M.J. Thermal Adaptation: A Theoretical and Empirical Synthesis; Oxford University Press: Oxford, UK, 2009. [Google Scholar]
- Abram, P.K.; Boivin, G.; Moiroux, J.; Brodeur, J. Behavioural effects of temperature on ectothermic animals: Unifying thermal physiology and behavioural plasticity. Biol. Rev. 2016, 92, 1859–1876. [Google Scholar] [CrossRef] [PubMed]
- Sánchez–Guillén, R.A.; Córdoba–Aguilar, A.; Hansson, B.; Ott, J.; Wellenreuther, M. Evolutionary consequences of climate-induced range shifts in insects. Biol. Rev. 2016, 91, 1050–1064. [Google Scholar] [CrossRef] [PubMed]
- Córdoba-Aguilar, A.; González-Tokman, D.; González-Santoyo, I. Insect Behavior: From Mechanisms to Ecological and Evolutionary Consequences; Oxford University Press: Oxford, UK, 2018. [Google Scholar]







| Parameter | Definition | Formula |
|---|---|---|
| sxj | Age-stage-specific survival rate | |
| lx | Age-specific survival rate | |
| mx | Age-specific fecundity | |
| r | Intrinsic rate of increase | |
| R0 | Net reproductive rate | |
| T | Mean generation time | |
| kx | Age-specific host feeding rate | |
| qx | Age-specific net host feeding rate | |
| C0 | Net host feeding rate | |
| ψ | Stable host feeding rate | |
| ω | Finite host feeding rate | |
| gx | Age-specific non-effective parasitism rate | |
| hx | Age-specific net non-effective parasitism rate | |
| N0 | Net non-effective parasitism rate | |
| γ | Stable non-effective parasitism rate | |
| ε | Finite non-effective parasitism rate | |
| μx | Age-specific aphid killing rate | |
| wx | Age-specific net aphid killing rate | |
| Z0 | Net aphid killing rate | |
| ϑ | Stable aphid killing rate | |
| θ | Finite aphid killing rate | |
| Qp | Transformation rate | |
| p(t) | Population growth | t: the simulation time. m: number of life stages. nxj: number of individuals of age x and stage j. |
| v(t) | Killing potential |
| Parameters | 20 °C | 24 °C | 28 °C | 32 °C | ||||
|---|---|---|---|---|---|---|---|---|
| n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | n | Mean ± SE | |
| Emergence rate of the parent cohort (%) | 43 | 86% | 40 | 80% | 40 | 80% | 26 | 52% |
| Female proportion of the parent cohort (%) | 43 | 74.4 ± 6.7% a | 40 | 62.5 ± 7.7% ab | 40 | 52.5 ± 7.9% bc | 26 | 65.4 ± 9.3% ab |
| Female preadult duration (d) | 32 | 20.1 ± 0.3 a | 25 | 14.1 ± 0.2 b | 21 | 9.5 ± 0.1 c | 17 | 9.7 ± 0.2 c |
| Female longevity (d) | 32 | 38.0 ± 2.1 a | 25 | 26.6 ± 0.8 b | 21 | 16.0 ± 0.7 c | 17 | 14.0 ± 0.6 d |
| Male preadult duration (d) | 11 | 20.4 ± 0.7 a | 15 | 13.1 ± 0.2 b | 19 | 9.6 ± 0.1 c | 9 | 9.4 ± 0.2 c |
| Male longevity (d) | 11 | 34.6 ± 0.9 a | 15 | 23.4 ± 0.9 b | 19 | 13.0 ± 0.3 c | 9 | 11.7± 0.5 d |
| Reproduction period (d) | 32 | 16.0 ± 2.0 a | 25 | 11.5±0.8 b | 21 | 5.9±0.6 c | 17 | 4.2±0.6 d |
| Fecundity (progeny adults/female) | 32 | 238.6 ± 31.0 a | 25 | 150.3 ± 11.8 b | 21 | 50.1 ± 5.9 c | 17 | 11.7 ± 2.1 d |
| Parameters | 20 °C | 24 °C | 28 °C | 32 °C |
|---|---|---|---|---|
| r (d−1) | 0.1848 ± 0.0051 a | 0.2360 ± 0.0082 b | 0.2441± 0.0140 b | 0.1676 ± 0.0186 a |
| λ (d−1) | 1.2030 ± 0.0061 a | 1.2662 ± 0.0103 b | 1.2765 ± 0.0179 b | 1.1825 ± 0.0219 a |
| R0 (progeny adults) | 177.5 ± 27.6 a | 94.0 ± 13.6 b | 26.3 ± 5.0 c | 7.6 ± 1.7 d |
| T (d) | 28.0 ± 0.5 a | 19.2 ± 0.2 b | 13.4 ± 0.3 c | 12.1 ± 0.2 d |
| C0 (aphids) | 26.6 ± 4.0 a | 18.2 ± 2.7 b | 6.9 ± 1.4 c | 3.0 ± 0.6 d |
| N0 (aphids) | 18.7 ± 3.0 a | 11.9 ± 1.7 a | 5.5 ± 1.3 b | 3.3 ± 0.8 b |
| Z0 (aphids) | 222.8 ± 34.4 a | 124.0 ± 17.4 b | 38.6 ± 7.3 c | 14.0 ± 2.9 d |
| ϑ | 0.2610 ± 0.0145 a | 0.3550 ± 0.0235 b | 0.4241 ± 0.0412 b | 0.3647 ± 0.0371 b |
| θ | 0.3140 ± 0.0190 a | 0.4495 ± 0.0333 b | 0.5414 ± 0.0595 b | 0.4312 ± 0.0511 b |
| Qp | 1.2552 ± 0.0124 a | 1.3201 ± 0.0166 b | 1.4691 ± 0.0429 c | 1.8333 ± 0.0680 d |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.Y.; Wang, B.L.; Yan, G.L.; Liu, Y.H.; Zhang, D.Y.; Liu, T.X. Temperature-Dependent Demographic Characteristics and Control Potential of Aphelinus asychis Reared from Sitobion avenae as a Biological Control Agent for Myzus persicae on Chili Peppers. Insects 2020, 11, 475. https://doi.org/10.3390/insects11080475
Wang SY, Wang BL, Yan GL, Liu YH, Zhang DY, Liu TX. Temperature-Dependent Demographic Characteristics and Control Potential of Aphelinus asychis Reared from Sitobion avenae as a Biological Control Agent for Myzus persicae on Chili Peppers. Insects. 2020; 11(8):475. https://doi.org/10.3390/insects11080475
Chicago/Turabian StyleWang, Sheng Yin, Bo Li Wang, Gai Lan Yan, Yan Hong Liu, Da Yu Zhang, and Tong Xian Liu. 2020. "Temperature-Dependent Demographic Characteristics and Control Potential of Aphelinus asychis Reared from Sitobion avenae as a Biological Control Agent for Myzus persicae on Chili Peppers" Insects 11, no. 8: 475. https://doi.org/10.3390/insects11080475