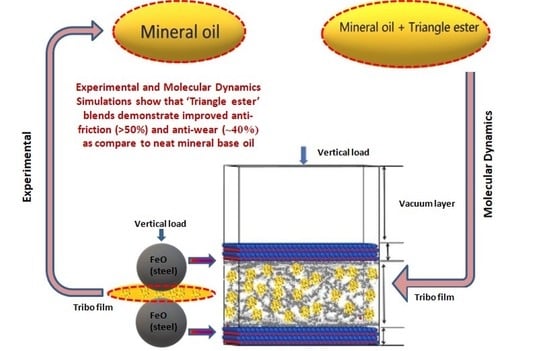
‘Triangle Ester’ Molecules as Blending Components in Mineral Oil: A Theoretical and Experimental Investigation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Details
2.2. Determination of Tribo-Performance Behavior
2.3. Molecular Dynamics Model and Calculation Details
3. Results and Discussion
3.1. Tribological Characteristics of the Base Oil
3.2. Tribological Characteristics of the Blends
3.3. Molecular Dynamics Analysis
3.3.1. Influence of Contact Stress
3.3.2. Molecular Dynamics Analysis at Different Stresses
3.3.3. Temperature Profile
3.4. Application in Commercial Engine Oil (CEO)
4. Conclusions
- (1)
- The optimized concentration of EE improved friction by 61.3% and 42.5% and wear by 12.75% and 41.1% in Gp I and Gp II base oils, respectively. In the case of TE blends, the friction reduced by 64.8% and 40.2%, while wear scar diameter was reduced by about 93.5% and 49.6% in Gp I and Gp II base oils.
- (2)
- The Gp I base oil, being more polar due to the presence of unsaturated olefins and sulfur content, engaged well in like interactions with EE that reflect in lower friction of EE as compared with TE in Gp I base oil. The TE, however, showed better antifriction behavior in Gp II base oil.
- (3)
- The blank MO, which is high in unsaturates (Gp I base oil), displayed higher friction due to weak bonding with the metal oxide substrate.
- (4)
- MD simulations showed that Van der Waals forces were the primary drivers for the interaction of the fluid with the substrate. These forces showed high values for TE and EE blends as compared with the neat base oil.
- (5)
- The nature of relative concentration distribution curves gave information about the Boltzmann distribution of the adsorbed and desorbed state of the entrained fluid within the walls. Bimodal curves suggest that the flux of molecules between adsorbed and desorbed states was high, resulting in higher friction and wear, as in the case of MO, while a single peak distribution of ester blends suggests stable SAMs.
- (6)
- Tribological properties under varying stresses indicated a weakening of the non-bonding forces, accompanied by elevated temperatures, near the walls due to slip heating which caused an increase in friction and wear. Optimized TE blends, with their higher thermal conductivity, can distribute the heat within the film and displayed better tribological behavior than EE under all conditions of stress. TE also has potential value as an additive for booster dosing of commercial engine oil for improved tribological performance.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sharma, N.; Bari, S.K.; Nagendramma, P.; Thakre, G.D.; Ray, A. 3 Tribological Investigations. Green Tribol. Emerg. Technol. Appl. 2021, 71. [Google Scholar]
- Suhane, A.; Sarviya, R.; Siddiqui, A.; Khaira, H. Optimization of wear performance of castor oil based lubricant using Taguchi technique. Mater Today Proc. 2017, 4, 2095–2104. [Google Scholar] [CrossRef]
- Nagendramma, P. Study of pentaerythritol tetraoleate ester as industrial gear oil. Lubr. Sci. 2011, 23, 355–362. [Google Scholar] [CrossRef]
- Aziz, N.A.M.; Robiah, Y.; Umer, R. Temperature effect on tribological properties of polyol ester-based environmentally adapted lubricant. Tribol. Int. 2016, 93, 43–49. [Google Scholar] [CrossRef]
- Erhan, S.Z.; Asadauskas, S. Lubricant base stocks from vegetable oils. Ind. Crop. Prod. 2000, 11, 277–282. [Google Scholar] [CrossRef]
- Farfán-Cabrera, L.I.; Gallardo-Hernández, E.A.; Vite-Torres, M.; Laguna-Camacho, J.R. Frictional behavior of a wet clutch using blends of automatic transmission fluid (ATF) and biolubricant (Jatropha oil) in a pin-on-disk tester. Tribol. Trans. 2015, 58, 941–946. [Google Scholar] [CrossRef]
- Fox, N.J.; Stachowiak, G.W. Vegetable oil-based lubricants: A review of oxidation. Tribol. Int. 2007, 40, 1035–1046. [Google Scholar] [CrossRef]
- Havet, L.; Blouet, J.; Valloire, F.R.; Brasseur, E.; Slomka, D. Tribological characteristics of some environmentally friendly lubricants. Wear 2011, 248, 140–146. [Google Scholar] [CrossRef]
- Habibullah, M.; Masjuki, H.H.; Kalam, M.A.; Gulzar, M.; Arslan, A.; Zahid, R. Tribological characteristics of Calophyllum inophyllum–based TMP (trimethylolpropane) ester as energy-saving and biodegradable lubricant. Tribol. Trans. 2015, 58, 1002–1011. [Google Scholar] [CrossRef]
- Karmakar, G.; Ghosh, P.; Sharma, B.K. Chemically modifying vegetable oils to prepare green lubricants. Lubricants 2017, 5, 44. [Google Scholar] [CrossRef] [Green Version]
- Shah, R.; Woydt, M.; Zhang, S. The economic and environmental significance of sustainable lubricants. Lubricants 2021, 9, 21. [Google Scholar] [CrossRef]
- Faes, J.; González, R.; Blanco, D.; Fernández-González, A.; Hernández-Battez, A.; Iglesias, P.; Viesca, J.L. Methyltrioctylammonium Octadecanoate as Lubricant Additive to Different Base Oils. Lubricants 2022, 10, 128. [Google Scholar] [CrossRef]
- Karmakar, G.; Dey, K.; Ghosh, P.; Sharma, B.K.; Erhan, S.Z. A short review on polymeric biomaterials as additives for lubricants. Polymers 2021, 13, 1333. [Google Scholar] [CrossRef] [PubMed]
- Cañellas, G.; Emeric, A.; Combarros, M.; Navarro, A.; Beltran, L.; Vilaseca, M.; Vives, J. Tribological Performance of Esters, Friction Modifier and Antiwear Additives for Electric Vehicle Applications. Lubricants 2023, 11, 109. [Google Scholar] [CrossRef]
- Liaocheng Ruijie Chemical Co., Ltd. Available online: http://www.made-in-china.com (accessed on 14 March 2023).
- Cook, B.A.; Brown, T.W. Ester Based Metal Working Lubricants. US Patent 4178260, 11 December 1979. [Google Scholar]
- Kawasaki, H. Biodegradable Lubricating Oil Composition. EP 1 707 617 A1, 2990107 (JP). 4 October 2006. [Google Scholar]
- Heikal, E.K.; Elmelawy, M.; Khalil, S.A.; Elbasuny, N. Manufacturing of environment friendly biolubricants from vegetable oils. Egypt. J. Pet. 2017, 26, 53–59. [Google Scholar] [CrossRef] [Green Version]
- Kamalakar, K.; Rajak, A.K.; Prasad, R.B.; Karuna, M.S. Rubber seed oil-based biolubricant base stocks: A potential source for hydraulic oils. Ind. Crop. Prod. 2013, 51, 249–257. [Google Scholar] [CrossRef]
- Maleque, M.A.; Masjuki, H.H.; Sapuan, S.M. Vegetable based biodegradable lubricating oil additives. Ind. Lubr. Tribol. 2003, 55, 137–143. [Google Scholar] [CrossRef]
- Panchal, T.M.; Patel, A.; Chauhan, D.; Thomas, M.; Patel, J.V. A methodological review on bio-lubricants from vegetable oil-based resources. Renew. Sustain. Energy Rev. 2017, 70, 65–70. [Google Scholar] [CrossRef]
- Mobarak, H.M.; Mohamad, E.N.; Masjuki, H.H.; Kalam, M.A.; Al Mahmud, K.A.; Habibullah, M.; Ashraful, A.M. The prospects of bio lubricants as alternatives in automotive applications. Renew. Sustain. Energy Rev. 2014, 33, 34–43. [Google Scholar] [CrossRef]
- Zulkifli, N.W.; Azman, S.S.; Kalam, M.A.; Masjuki, H.H.; Yunus, R.; Gulzar, M. Lubricity of bio-based lubricant derived from different chemically modified fatty acid methyl ester. Tribol. Int. 2016, 93, 555–562. [Google Scholar] [CrossRef]
- Jayadas, N.; Nair, K. Coconut oil as base oil for industrial lubricants-evaluation and modification of thermal, oxidative and low temperature properties. Tribol. Int. 2006, 39, 873–878. [Google Scholar] [CrossRef]
- Ting, C.C.; Chen, C.C. Viscosity and working efficiency analysis of soybean oil-based bio-lubricants. Measurement 2011, 44, 1337–1341. [Google Scholar] [CrossRef]
- Hamid, H.A.; Yunus, R.; Rashid, U.; Choong, T.S.; Ali, S.; Syam, A.M. Synthesis of high oleic palm oil-based trimethylolpropane esters in a vacuum operated pulsed loop reactor. Fuel 2016, 166, 560–566. [Google Scholar] [CrossRef]
- Kodali, D.R. High performance ester lubricants from natural oils. Ind. Lubr. Tribol. 2002, 54, 165–170. [Google Scholar] [CrossRef]
- Salih, N.; Jumat, S.; Emad. Y.; The physicochemical and tribological properties of oleic acid-based trimester biolubricants. Ind. Crop. Prod. 2011, 34, 1089–1096. [Google Scholar] [CrossRef]
- Sharma, N.; Thakre, G.D.; Ray, A. Hitherto unexplored three-membered heterocyclic rings favorably alter tribological properties of fatty acid linear esters. Tribol. Trans. 2021, 64, 996–1021. [Google Scholar] [CrossRef]
- Sharma, N.; Kumar, S.; Thakre, G.D.; Ray, A. Novel synthetic ‘Triangle Ester’ Lubricants: Useful Correlations for Wetting and Tribological Phenomena over Common Engineering Substrates. Tribol. Trans. 2022, 66, 1–18. [Google Scholar] [CrossRef]
- Liu, Y.; Li, Z.; Li, G.; Tang, L. Friction and wear behavior of Ni-based alloy coatings with different amount of WC–TiC ceramic particles. J. Mater. Sci. 2023, 58, 1116–1126. [Google Scholar] [CrossRef]
- Bucolo, M.; Buscarino, A.; Famoso, C.; Fortuna, L.; Gagliano, S. Imperfections in integrated devices allow the emergence of unexpected strange attractors in electronic circuits. IEEE Access 2021, 9, 29573–29583. [Google Scholar] [CrossRef]




















| Blend Composition in Hexadecane | Density (g/cm3) | Number of Molecules of Hexadecane for 1 Molecule of EE/TE | Cell Size (Å × Å × Å) |
|---|---|---|---|
| 1% EE | 0.858 | 250 | 48.0 × 48.0 × 48.0 |
| 2% EE | 0.859 | 120 | 37.7 × 37.7 × 37.7 |
| 3% EE | 0.862 | 80 | 33.0 × 33.0 × 33.0 |
| 4% EE | 0.869 | 60 | 30.0 × 30.0 × 30.0 |
| 5% EE | 0.870 | 50 | 28.3 × 28.3 × 28.3 |
| 1% TE | 0.870 | 250 | 47.8 × 47.8 × 47.8 |
| 2% TE | 0.873 | 120 | 37.5 × 37.5 × 37.5 |
| 3% TE | 0.878 | 80 | 32.8 × 32.8 × 32.8 |
| 4% TE | 0.879 | 60 | 29.9 × 29.9 × 29.9 |
| 5% TE | 0.881 | 50 | 28.2 × 28.2 × 28.2 |
| Blend | Kinematic Viscosity (cSt) | Viscosity Index (VI) | |
|---|---|---|---|
| 40 °C | 100 °C | ||
| 1% TE + Gp I | 32.84 | 5.72 | 115.05 |
| 2% TE + Gp I | 34.65 | 5.73 | 104.84 |
| 3% TE + Gp I | 39.69 | 6.11 | 97.95 |
| 4% TE + Gp I | 38.82 | 5.77 | 89.67 |
| 5% TE + Gp I | 37.79 | 5.98 | 80.67 |
| 1% TE + Gp II | 32.34 | 5.84 | 124.78 |
| 2% TE + Gp II | 34.48 | 5.23 | 117.08 |
| 3% TE + Gp II | 36.63 | 5.98 | 106.76 |
| 4% TE + Gp II | 31.14 | 4.92 | 106.12 |
| 5% TE + Gp II | 30.31 | 5.16 | 104.44 |
| Blend | Kinematic Viscosity (cSt) | Viscosity Index (VI) | |
|---|---|---|---|
| 40 °C | 100 °C | ||
| Gp I | 30.42 | 5.52 | 109.61 |
| 1% EE + Gp I | 34.39 | 5.88 | 105.79 |
| 2% EE + Gp I | 34.03 | 5.62 | 104.63 |
| 3% EE + Gp I | 34.09 | 5.62 | 102.25 |
| 4% EE + Gp I | 34.22 | 5.68 | 95.54 |
| 5% EE + Gp I | 31.62 | 5.66 | 111.23 |
| Gp II | 30.47 | 5.36 | 119.40 |
| 1% EE + Gp II | 30.61 | 5.27 | 114.14 |
| 2% EE + Gp II | 30.72 | 5.38 | 104.76 |
| 3% EE + Gp II | 30.88 | 5.21 | 102.87 |
| 4% EE + Gp II | 31.02 | 5.13 | 102.85 |
| 5% EE + Gp II | 30.30 | 5.37 | 119.61 |
| Change in C-O Bond Length (Å) | Change in C-S Bond Length (Å) | |
|---|---|---|
| Pure EE/TE | 1.43 to 1.47 | 1.81 to 1.83 |
| Blend of 4% EE/3%TE | 1.81 to 2.53 | 1.43 to 1.59 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, N.; Kumar, S.; Thakre, G.D.; Ray, A. ‘Triangle Ester’ Molecules as Blending Components in Mineral Oil: A Theoretical and Experimental Investigation. Lubricants 2023, 11, 144. https://doi.org/10.3390/lubricants11030144
Sharma N, Kumar S, Thakre GD, Ray A. ‘Triangle Ester’ Molecules as Blending Components in Mineral Oil: A Theoretical and Experimental Investigation. Lubricants. 2023; 11(3):144. https://doi.org/10.3390/lubricants11030144
Chicago/Turabian StyleSharma, Neha, Sunil Kumar, Gananath D. Thakre, and Anjan Ray. 2023. "‘Triangle Ester’ Molecules as Blending Components in Mineral Oil: A Theoretical and Experimental Investigation" Lubricants 11, no. 3: 144. https://doi.org/10.3390/lubricants11030144