SARS-CoV-2 Neutralizing Antibodies in Mexican Population: A Five Vaccine Comparison
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
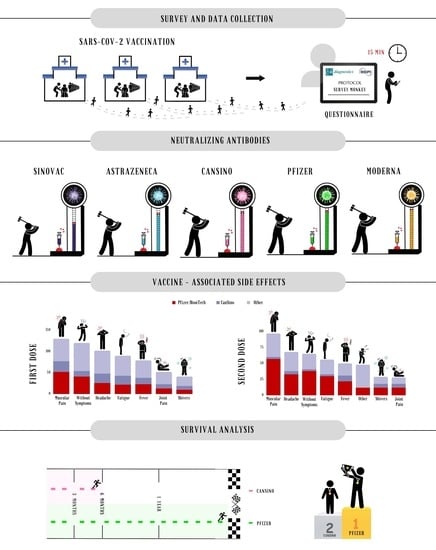
2.2. Survey and Data Collection
2.3. Neutralizing Antibodies Detection
2.4. Statistical Analysis
3. Results
3.1. Description of Study Groups
3.2. Quantification of Neutralizing Antibodies
3.3. Survival Analysis
3.4. Vaccine-Associated Side Effects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, L.D.; Lian, C.; Yeap, L.S.; Meng, F.L. The development of neutralizing antibodies against SARS-CoV-2 and their common features. J. Mol. Cell. Biol. 2020, 12, 980–986. [Google Scholar] [CrossRef]
- WHO. Coronavirus Disease (COVID-19) Pandemic. Available online: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed on 12 December 2022).
- WHO. WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/data (accessed on 13 December 2022).
- WHO. COVID-19 Vaccine Tracker and Landscape. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 11 November 2022).
- Fiolet, T.; Kherabi, Y.; MacDonald, C.J.; Ghosn, J.; Peiffer-Smadja, N. Comparing COVID-19 vaccines for their characteristics, efficacy and effectiveness against SARS-CoV-2 and variants of concern: A narrative review. Clin. Microbiol. Infect. 2022, 28, 202–221. [Google Scholar] [CrossRef]
- Alter, G.; Yu, J.; Liu, J.; Chandrashekar, A.; Borducchi, E.N.; Tostanoski, L.H.; McMahan, K.; Jacob-Dolan, C.; Martinez, D.R.; Chang, A.; et al. Immunogenicity of Ad26.COV2.S vaccine against SARS-CoV-2 variants in humans. Nature 2021, 596, 268–272. [Google Scholar] [CrossRef]
- Guzman-Martinez, O.; Guardado, K.; de Guevara, E.L.; Navarro, S.; Hernandez, C.; Zenteno-Cuevas, R.; Montero, H. IgG Antibodies Generation and Side Effects Caused by Ad5-nCoV Vaccine (CanSino Biologics) and BNT162b2 Vaccine (Pfizer/BioNTech) among Mexican Population. Vaccines 2021, 9, 999. [Google Scholar] [CrossRef]
- Grupo Técnico Asesor de Vacunación Covid. Priorización inicial y consecutiva para la vacunación contra SARS-CoV-2 en la población mexicana. Recomendaciones preliminares. Salud. Publica Mex 2020, 63, 288–309. [Google Scholar] [CrossRef]
- Mongua-Rodriguez, N.; Rodriguez-Alvarez, M.; De-la-Rosa-Zamboni, D.; Jimenez-Corona, M.E.; Castaneda-Cediel, M.L.; Miranda-Novales, G.; Cruz-Pacheco, G.; Ferreira-Guerrero, E.; Ferreyra-Reyes, L.; Delgado-Sanchez, G.; et al. Knowledge, attitudes, perceptions, and COVID-19 hesitancy in a large public university in Mexico city during the early vaccination rollout. BMC Public Health 2022, 22, 1853. [Google Scholar] [CrossRef]
- Gobierno de Mexico/Comisión Federal Para la Protección Contra Riesgos Sanitarios. Vacunas COVID-19 Autorizadas. Available online: https://www.gob.mx/cofepris/acciones-y-programas/vacunas-covid-19-autorizadas (accessed on 13 December 2022).
- Gobierno de Mexico. Vacunas Contra COVID-19. Available online: https://www.gob.mx/salud/prensa/315-aplicadas-en-mexico-209-6-millones-de-vacunas-contra-covid-19 (accessed on 13 December 2022).
- He, X.; Su, J.; Ma, Y.; Zhang, W.; Tang, S. A comprehensive analysis of the efficacy and effectiveness of COVID-19 vaccines. Front. Immunol. 2022, 13, 945930. [Google Scholar] [CrossRef]
- Feikin, D.R.; Higdon, M.M.; Abu-Raddad, L.J.; Andrews, N.; Araos, R.; Goldberg, Y.; Groome, M.J.; Huppert, A.; O’Brien, K.L.; Smith, P.G.; et al. Duration of effectiveness of vaccines against SARS-CoV-2 infection and COVID-19 disease: Results of a systematic review and meta-regression. Lancet 2022, 399, 924–944. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020, 383, 2439–2450. [Google Scholar] [CrossRef]
- Taylor, S.C.; Hurst, B.; Martiszus, I.; Hausman, M.S.; Sarwat, S.; Schapiro, J.M.; Rowell, S.; Lituev, A. Semi-quantitative, high throughput analysis of SARS-CoV-2 neutralizing antibodies: Measuring the level and duration of immune response antibodies post infection/vaccination. Vaccine 2021, 39, 5688–5698. [Google Scholar] [CrossRef]
- Hernandez-Bello, J.; Morales-Nunez, J.J.; Machado-Sulbaran, A.C.; Diaz-Perez, S.A.; Torres-Hernandez, P.C.; Balcazar-Felix, P.; Gutierrez-Brito, J.A.; Lomeli-Nieto, J.A.; Munoz-Valle, J.F. Neutralizing Antibodies against SARS-CoV-2, Anti-Ad5 Antibodies, and Reactogenicity in Response to Ad5-nCoV (CanSino Biologics) Vaccine in Individuals with and without Prior SARS-CoV-2. Vaccines 2021, 9, 1047. [Google Scholar] [CrossRef] [PubMed]
- Nam, M.; Seo, J.D.; Moon, H.W.; Kim, H.; Hur, M.; Yun, Y.M. Evaluation of Humoral Immune Response after SARS-CoV-2 Vaccination Using Two Binding Antibody Assays and a Neutralizing Antibody Assay. Microbiol. Spectr. 2021, 9, e0120221. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mao, Q.; Wu, X.; He, Q.; Bian, L.; Bai, Y.; Wang, Z.; Wang, Q.; Zhang, J.; Liang, Z.; et al. Considerations for the Feasibility of Neutralizing Antibodies as a Surrogate Endpoint for COVID-19 Vaccines. Front. Immunol. 2022, 13, 814365. [Google Scholar] [CrossRef] [PubMed]
- WHO. Mexico Situation. Available online: https://covid19.who.int/region/amro/country/mx (accessed on 12 December 2022).
- Momentive.ai. Survey Monkey. Get Answers with Surveys. Available online: https://es.surveymonkey.com/welcome/sem/?program=7013A000000mweBQAQ&utm_bu=CR&utm_campaign=71700000059189691&utm_adgroup=58700005408386346&utm_content=43700049190940759&utm_medium=cpc&utm_source=adwords&utm_term=p49190940759&utm_kxconfid=s4bvpi0ju&language=non-english&gclid=Cj0KCQiA4uCcBhDdARIsAH5jyUkOyOpZmHYKlHaF5TMzijeAzKWqvc77OohLVsNTqjx1ulghGOhoHbQaAgorEALw_wcB&gclsrc=aw.ds (accessed on 10 November 2022).
- Al-Amer, R.; Maneze, D.; Everett, B.; Montayre, J.; Villarosa, A.R.; Dwekat, E.; Salamonson, Y. COVID-19 vaccination intention in the first year of the pandemic: A systematic review. J. Clin. Nurs. 2022, 31, 62–86. [Google Scholar] [CrossRef] [PubMed]
- Taborda, A.R.; Murillo, D.A.; Moreno, C.L.; Taborda, P.A.R.; Fuquen, M.; Diaz, P.A.; Londono, D. Analysis of budgetary impact of COVID-19 vaccination in Latin AmericaAnalise do impacto orcamentario da vacinacao contra a COVID-19 na America Latina. Rev. Panam. Salud. Publica 2022, 46, e5. [Google Scholar] [CrossRef] [PubMed]
- Au, W.Y.; Cheung, P.P. Effectiveness of heterologous and homologous covid-19 vaccine regimens: Living systematic review with network meta-analysis. BMJ 2022, 377, e069989. [Google Scholar] [CrossRef]
- Chiu, N.C.; Chi, H.; Tu, Y.K.; Huang, Y.N.; Tai, Y.L.; Weng, S.L.; Chang, L.; Huang, D.T.; Huang, F.Y.; Lin, C.Y. To mix or not to mix? A rapid systematic review of heterologous prime-boost covid-19 vaccination. Expert Rev. Vaccines 2021, 20, 1211–1220. [Google Scholar] [CrossRef]
- Voysey, M.; Costa Clemens, S.A.; Madhi, S.A.; Weckx, L.Y.; Folegatti, P.M.; Aley, P.K.; Angus, B.; Baillie, V.L.; Barnabas, S.L.; Bhorat, Q.E.; et al. Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: A pooled analysis of four randomised trials. Lancet 2021, 397, 881–891. [Google Scholar] [CrossRef]
- Pormohammad, A.; Zarei, M.; Ghorbani, S.; Mohammadi, M.; Razizadeh, M.H.; Turner, D.L.; Turner, R.J. Efficacy and Safety of COVID-19 Vaccines: A Systematic Review and Meta-Analysis of Randomized Clinical Trials. Vaccines 2021, 9, 467. [Google Scholar] [CrossRef]
- Thomas, S.J.; Moreira, E.D., Jr.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Polack, F.P.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine through 6 Months. N. Engl. J. Med. 2021, 385, 1761–1773. [Google Scholar] [CrossRef]
- Sahin, U.; Muik, A.; Vogler, I.; Derhovanessian, E.; Kranz, L.M.; Vormehr, M.; Quandt, J.; Bidmon, N.; Ulges, A.; Baum, A.; et al. BNT162b2 vaccine induces neutralizing antibodies and poly-specific T cells in humans. Nature 2021, 595, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Favresse, J.; Gillot, C.; Di Chiaro, L.; Eucher, C.; Elsen, M.; Van Eeckhoudt, S.; David, C.; Morimont, L.; Dogne, J.M.; Douxfils, J. Neutralizing Antibodies in COVID-19 Patients and Vaccine Recipients after Two Doses of BNT162b2. Viruses 2021, 13, 1364. [Google Scholar] [CrossRef] [PubMed]
- Halperin, S.A.; Ye, L.; MacKinnon-Cameron, D.; Smith, B.; Cahn, P.E.; Ruiz-Palacios, G.M.; Ikram, A.; Lanas, F.; Lourdes Guerrero, M.; Munoz Navarro, S.R.; et al. Final efficacy analysis, interim safety analysis, and immunogenicity of a single dose of recombinant novel coronavirus vaccine (adenovirus type 5 vector) in adults 18 years and older: An international, multicentre, randomised, double-blinded, placebo-controlled phase 3 trial. Lancet 2022, 399, 237–248. [Google Scholar] [CrossRef]
- Levin, E.G.; Lustig, Y.; Cohen, C.; Fluss, R.; Indenbaum, V.; Amit, S.; Doolman, R.; Asraf, K.; Mendelson, E.; Ziv, A.; et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N. Engl. J. Med. 2021, 385, e84. [Google Scholar] [CrossRef] [PubMed]
- Klein, S.L.; Flanagan, K.L. Sex differences in immune responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Faizo, A.A.; Qashqari, F.S.; El-Kafrawy, S.A.; Barasheed, O.; Almashjary, M.N.; Alfelali, M.; Bawazir, A.A.; Albarakati, B.M.; Khayyat, S.A.; Hassan, A.M.; et al. A potential association between obesity and reduced effectiveness of COVID-19 vaccine-induced neutralizing humoral immunity. J. Med. Virol. 2022, 95, e28130. [Google Scholar] [CrossRef]
- World Obesity. Global Obesity Observatory/Mexico. Available online: https://data.worldobesity.org/country/mexico-139 (accessed on 13 December 2022).
- Krutikov, M.; Palmer, T.; Tut, G.; Fuller, C.; Azmi, B.; Giddings, R.; Shrotri, M.; Kaur, N.; Sylla, P.; Lancaster, T.; et al. Prevalence and duration of detectable SARS-CoV-2 nucleocapsid antibodies in staff and residents of long-term care facilities over the first year of the pandemic (VIVALDI study): Prospective cohort study in England. Lancet Healthy Longev. 2022, 3, e13–e21. [Google Scholar] [CrossRef]
- Krammer, F.; Srivastava, K.; Alshammary, H.; Amoako, A.A.; Awawda, M.H.; Beach, K.F.; Bermudez-Gonzalez, M.C.; Bielak, D.A.; Carreno, J.M.; Chernet, R.L.; et al. Antibody Responses in Seropositive Persons after a Single Dose of SARS-CoV-2 mRNA Vaccine. N. Engl. J. Med. 2021, 384, 1372–1374. [Google Scholar] [CrossRef] [PubMed]
- Ontanon, J.; Blas, J.; de Cabo, C.; Santos, C.; Ruiz-Escribano, E.; Garcia, A.; Marin, L.; Saez, L.; Beato, J.L.; Rada, R.; et al. Influence of past infection with SARS-CoV-2 on the response to the BNT162b2 mRNA vaccine in health care workers: Kinetics and durability of the humoral immune response. EBioMedicine 2021, 73, 103656. [Google Scholar] [CrossRef]
- Polack, F.P.; Thomas, S.J.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Perez, J.L.; Perez Marc, G.; Moreira, E.D.; Zerbini, C.; et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020, 383, 2603–2615. [Google Scholar] [CrossRef]
- Frenck, R.W., Jr.; Klein, N.P.; Kitchin, N.; Gurtman, A.; Absalon, J.; Lockhart, S.; Perez, J.L.; Walter, E.B.; Senders, S.; Bailey, R.; et al. Safety, Immunogenicity, and Efficacy of the BNT162b2 Covid-19 Vaccine in Adolescents. N. Engl. J. Med. 2021, 385, 239–250. [Google Scholar] [CrossRef] [PubMed]
- Kaur, R.J.; Dutta, S.; Bhardwaj, P.; Charan, J.; Dhingra, S.; Mitra, P.; Singh, K.; Yadav, D.; Sharma, P.; Misra, S. Adverse Events Reported From COVID-19 Vaccine Trials: A Systematic Review. Indian J. Clin. Biochem. 2021, 36, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Ghasemiyeh, P.; Mohammadi-Samani, S.; Firouzabadi, N.; Dehshahri, A.; Vazin, A. A focused review on technologies, mechanisms, safety, and efficacy of available COVID-19 vaccines. Int. Immunopharmacol. 2021, 100, 108162. [Google Scholar] [CrossRef] [PubMed]
- Galipeau, Y.; Greig, M.; Liu, G.; Driedger, M.; Langlois, M.A. Humoral Responses and Serological Assays in SARS-CoV-2 Infections. Front. Immunol. 2020, 11, 610688. [Google Scholar] [CrossRef]
- Meyer, B.; Reimerink, J.; Torriani, G.; Brouwer, F.; Godeke, G.J.; Yerly, S.; Hoogerwerf, M.; Vuilleumier, N.; Kaiser, L.; Eckerle, I.; et al. Validation and clinical evaluation of a SARS-CoV-2 surrogate virus neutralisation test (sVNT). Emerg. Microbes. Infect. 2020, 9, 2394–2403. [Google Scholar] [CrossRef]



| Characteristics | n/Median |
|---|---|
| Gender, n (%) | |
| Female | 165 (68%) |
| Male | 77 (32%) |
| Age, median (IQR) | 32 (25–42) |
| Occupation, n (%) | |
| Nurse/Admission | 90 (37%) |
| Medical doctor | 52 (21%) |
| Other | 100 (42%) |
| BMI, median (IQR) | 26.5 (23.6–29.9.16) |
| Overweight | 83 (34%) |
| Obesity | 59 (24%) |
| Physical activity, n (%) | 127 (52%) |
| Income, n (%) | |
| USD 200–550 | 112 (46%) |
| >USD 550 | 94 (39%) |
| Nociceptive habits, n (%) | |
| Alcohol | 160 (66%) |
| Tobacco | 74 (30%) |
| Comorbidities, n (%) | |
| Hypertension | 22 (9%) |
| Diabetes mellitus | 5 (2%) |
| Other | 45 (19%) |
| COVID-19 cases, n (%) | |
| Positive before vaccine | 75 (31%) |
| Positive after vaccine | 33 (14%) |
| Characteristics | n/median |
| Gender, n (%) | |
| Female | 165 (68%) |
| Male | 77 (32%) |
| Age, median (IQR) | 32 (25–42) |
| Occupation, n (%) | |
| Nurse/Admission | 90 (37%) |
| Medical doctor | 52 (21%) |
| BMI, median (IQR) | 26.5 (23.6–29.9.16) |
| Overweight | 83 (34%) |
| Obesity | 59 (24%) |
| Physical activity, n (%) | 127 (52%) |
| Income, n (%) | |
| USD 200–550 | 112 (46%) |
| >USD 550 | 94 (39%) |
| Nociceptive habits, n (%) | |
| Alcohol | 160 (66%) |
| Tobacco | 74 (30%) |
| Comorbidities, n (%) | |
| Hypertension | 22 (9%) |
| Diabetes mellitus | 5 (2%) |
| Other | 45 (19%) |
| COVID-19 background, n (%) | |
| Positive before vaccine | 75 (31%) |
| Positive after vaccine | 33 (14%) |
| Vaccines | n (%) |
|---|---|
| Pfizer/BioNTech | 140 (58%) |
| CanSino | 49 (19%) |
| AstraZeneca | 17 (7%) |
| Sinovac | 21 (8%) |
| Moderna | 15 (6%) |
| Pfizer/BioNTech | CanSino | AstraZeneca | Sinovac | Moderna | ||
|---|---|---|---|---|---|---|
| n | 143 | 49 | 17 | 21 | 17 | |
| IQR | ||||||
| 25 | 94.24 | 73.21 | 78.19 | 62.39 | 97.23 | |
| median | 97.23 | 97.23 | 97.18 | 74.05 | 97.61 | |
| 75 | 97.70 | 97.48 | 97.92 | 97.87 | 97.94 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alcorta-Nuñez, F.; Pérez-Ibave, D.C.; Burciaga-Flores, C.H.; Garza, M.Á.; González-Escamilla, M.; Rodríguez-Niño, P.; González-Guerrero, J.F.; Alcorta-Garza, A.; Vidal-Gutiérrez, O.; Ramírez-Correa, G.A.; et al. SARS-CoV-2 Neutralizing Antibodies in Mexican Population: A Five Vaccine Comparison. Diagnostics 2023, 13, 1194. https://doi.org/10.3390/diagnostics13061194
Alcorta-Nuñez F, Pérez-Ibave DC, Burciaga-Flores CH, Garza MÁ, González-Escamilla M, Rodríguez-Niño P, González-Guerrero JF, Alcorta-Garza A, Vidal-Gutiérrez O, Ramírez-Correa GA, et al. SARS-CoV-2 Neutralizing Antibodies in Mexican Population: A Five Vaccine Comparison. Diagnostics. 2023; 13(6):1194. https://doi.org/10.3390/diagnostics13061194
Chicago/Turabian StyleAlcorta-Nuñez, Fernando, Diana Cristina Pérez-Ibave, Carlos Horacio Burciaga-Flores, Miguel Ángel Garza, Moisés González-Escamilla, Patricia Rodríguez-Niño, Juan Francisco González-Guerrero, Adelina Alcorta-Garza, Oscar Vidal-Gutiérrez, Genaro A. Ramírez-Correa, and et al. 2023. "SARS-CoV-2 Neutralizing Antibodies in Mexican Population: A Five Vaccine Comparison" Diagnostics 13, no. 6: 1194. https://doi.org/10.3390/diagnostics13061194