A New Approach in Prebiotic Chemistry Studies: Proline Sorption Triggered by Mineral Surfaces Analysed Using XPS
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Interaction Process/Adsorption Experiments
2.3. Characterization by XPS, IR, and XRD Analysis
3. Results and Discussion
3.1. L-Proline Adsorption on Mineral Surfaces
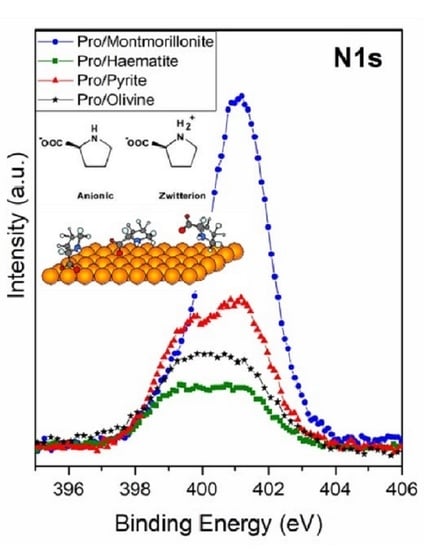
3.1.1. XPS Studies
3.1.2. FT-IR Studies
3.1.3. XRD Studies
3.2. The Role of the Mineral Surface in the Competitive Adsorption of Zwitterionic and Anionic Species
3.2.1. Montmorillonite
3.2.2. Iron Disulphide
3.2.3. Haematite
3.2.4. Basaltic Olivine
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Walton, C.R.; Shorttle, O.; Jenner, F.E.; Williams, H.M.; Golden, J.; Morrison, S.M.; Downs, R.T.; Zerkle, A.; Hazen, R.M.; Pasek, M. Phosphorus mineral evolution and prebiotic chemistry: From minerals to microbes. Earth-Sci. Rev. 2021, 221, 103806. [Google Scholar] [CrossRef]
- Martin, S.; Alexander, S.; Corey, C. A Perspective on the Role of Minerals in Prebiotic Synthesis. AMBIO J. Hum. Environ. 2004, 33, 539–551. [Google Scholar] [CrossRef]
- Duval, M.; Deboos, V.; Hallonet, A.; Sagorin, G.; Denicourt-Nowicki, A.; Roucoux, A. Selective palladium nanoparticles-catalyzed hydrogenolysis of industrially targeted epoxides in water. J. Catal. 2021, 396, 261–268. [Google Scholar] [CrossRef]
- Belmonte, L.; Mansy, S.S. Metal Catalysts and the Origin of Life. Elements 2016, 12, 413–418. [Google Scholar] [CrossRef]
- Bartnikas, T.B.; Gitlin, J.D. How to make a metalloprotein. Nat. Struct. Biol. 2001, 8, 733–734. [Google Scholar] [CrossRef]
- Aithal, A.; Dagar, S.; Rajamani, S. Metals in Prebiotic Catalysis: A Possible Evolutionary Pathway for the Emergence of Metalloproteins. ACS Omega 2023, 8, 5197–5208. [Google Scholar] [CrossRef]
- Frenkel-Pinter, M.; Sargon, A.B.; Glass, J.B.; Hud, N.V.; Williams, L.D. Transition metals enhance prebiotic depsipeptide oligomerization reactions involving histidine. RSC Adv. 2021, 11, 3534–3538. [Google Scholar] [CrossRef]
- Muchowska, K.B.; Varma, S.J.; Chevallot-Beroux, E.; Lethuillier-Karl, L.; Li, G.; Moran, J. Metals promote sequences of the reverse Krebs cycle. Nat. Ecol. Evol. 2017, 1, 1716–1721. [Google Scholar] [CrossRef] [Green Version]
- Bray, M.S.; Lenz, T.K.; Haynes, J.W.; Bowman, J.C.; Petrov, A.S.; Reddi, A.R.; Hud, N.V.; Williams, L.D.; Glass, J.B. Multiple prebiotic metals mediate translation. Proc. Natl. Acad. Sci. USA 2018, 115, 12164–12169. [Google Scholar] [CrossRef] [Green Version]
- Shock, E.L.; Boyd, E.S. Principles of Geobiochemistry. Elements 2015, 11, 395–401. [Google Scholar] [CrossRef]
- Russell, M.J.; Martin, W. The rocky roots of the acetyl-CoA pathway. Trends Biochem. Sci. 2004, 29, 358–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, S.; Zuchan, K.; Baymann, F.; Schoepp-Cothenet, B.; Branscomb, E.; Russell, M.; Nitschke, W. Minerals and the Emergence of Life. In Metals in Life Sciences; Kroneck, P., Sosa Torres, M.E., Eds.; De Gruyter: Berlin, Germany, 2021; pp. 135–157. [Google Scholar]
- Zaia, D.A.M. Adsorption of amino acids and nucleic acid bases onto minerals: A few suggestions for prebiotic chemistry experiments. Int. J. Astrobiol. 2012, 11, 229–234. [Google Scholar] [CrossRef]
- Rimola, A.; Sodupe, M.; Ugliengo, P. Role of Mineral Surfaces in Prebiotic Chemical Evolution. In Silico Quantum Mechanical Studies. Life 2019, 9, 10. [Google Scholar] [CrossRef] [Green Version]
- Bernal, J.D. The Physical Basis of Life. J. D. Bernal. London: Routledge and Kegan Paul, 1951. 80 pp. 6s.; The Structure and Mechanical Properties of Metals. Bruce Chalmers. New York: Wiley, 1951. 132 pp. $3.50.; Selective Toxicity with Special Reference to Chemotherapy. Adrien Albert. New York: Wiley; London: Methuen, 1951. 228 pp. $1.75. Science 1952, 115, 50. [Google Scholar] [CrossRef]
- Liu, R.; Orgel, L.E. Polymerization on the Rocks: β-amino Acids and Arginine. Orig. Life Evol. Biosph. 1998, 28, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Orgel, L.E. Polymerization on the Rocks: Theoretical Introduction. Orig. Life Evol. Biosph. 1998, 28, 227–234. [Google Scholar] [CrossRef] [PubMed]
- Marti, E.M.; Quash, A.; Methivier, C.; Dubot, P.; Pradier, C.M. Interaction of S-histidine, an amino acid, with copper and gold surfaces, a comparison based on RAIRS analyses. Colloids Surf. A Physicochem. Eng. Asp. 2004, 249, 85–89. [Google Scholar] [CrossRef]
- Mateo-Martí, E.; Rogero, C.; Gonzalez, C.; Sobrado, J.M.; de Andrés, P.L.; Martin-Gago, J.A. Interplay between Fast Diffusion and Molecular Interaction in the Formation of Self-Assembled Nanostructures of S-Cysteine on Au(111). Langmuir 2010, 26, 4113–4118. [Google Scholar] [CrossRef] [PubMed]
- Kayushina, R.; Vainshtein, B. Kristallografiya 10 (1965) 333. Sov. Phys. Crystallogr. 1966, 10, 698. [Google Scholar]
- Reva, I.D.; Stepanian, S.G.; Plokhotnichenko, A.M.; Radchenko, E.D.; Sheina, G.G.; Blagoi, Y.P. Infrared matrix isolation studies of amino acids. Molecular structure of proline. J. Mol. Struct. 1994, 318, 1–13. [Google Scholar] [CrossRef]
- Sapse, A.M.; Mallah-Levy, L.; Daniels, S.B.; Erickson, B.W. The.gamma. turn: Ab initio calculations on proline and N-acetylproline amide. J. Am. Chem. Soc. 1987, 109, 3526–3529. [Google Scholar] [CrossRef]
- Williamson, M.P. The structure and function of proline-rich regions in proteins. Biochem. J. 1994, 297, 249–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kay, B.K.; Williamson, M.P.; Sudol, M. The importance of being proline: The interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000, 14, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Fox, S.W.; Windsor, C.R. Synthesis of Amino Acids by the Heating of Formaldehyde and Ammonia. Science 1970, 170, 984–986. [Google Scholar] [CrossRef] [PubMed]
- Janda, M.; Morvova, M.; Machala, Z.; Morva, I. Study of plasma induced chemistry by DC discharges in CO2/N2/H2O mixtures above a water surface. Orig. Life Evol. Biosph. 2008, 38, 23–35. [Google Scholar] [CrossRef]
- Marshall, W.L. Hydrothermal synthesis of amino acids. Geochim. Et Cosmochim. Acta 1994, 58, 2099–2106. [Google Scholar] [CrossRef]
- Plankensteiner, K.; Reiner, H.; Rode, B.M. Amino acids on the rampant primordial Earth: Electric discharges and the hot salty ocean. Mol. Divers. 2006, 10, 3–7. [Google Scholar] [CrossRef]
- Ring, D.; Wolman, Y.; Friedmann, N.; Miller, S.L. Prebiotic Synthesis of Hydrophobic and Protein Amino Acids. Proc. Natl. Acad. Sci. USA 1972, 69, 765–768. [Google Scholar] [CrossRef] [Green Version]
- Ruiz-Bermejo, M.; Menor-Salván, C.; Osuna-Esteban, S.; Veintemillas-Verdaguer, S. The effects of ferrous and other ions on the abiotic formation of biomolecules using aqueous aerosols and spark discharges. Orig. Life Evol. Biosph. 2007, 37, 507–521. [Google Scholar] [CrossRef]
- Pizzarello, S.; Schrader, D.L.; Monroe, A.A.; Lauretta, D.S. Large enantiomeric excesses in primitive meteorites and the diverse effects of water in cosmochemical evolution. Proc. Natl. Acad. Sci. USA 2012, 109, 11949–11954. [Google Scholar] [CrossRef] [Green Version]
- Shimoyama, A.; Ogasawara, R. Dipeptides and diketopiperazines in the Yamato-791198 and Murchison carbonaceous chondrites. Orig. Life Evol. Biosph. 2002, 32, 165–179. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Martinez, S.; Escamilla-Roa, E.; Zorzano, M.-P.; Mateo-Marti, E. Defects on a pyrite(100) surface produce chemical evolution of glycine under inert conditions: Experimental and theoretical approaches. Phys. Chem. Chem. Phys. 2019, 21, 24535–24542. [Google Scholar] [CrossRef] [Green Version]
- Galvez-Martinez, S.; Escamilla-Roa, E.; Zorzano, M.-P.; Mateo-Marti, E. Ar+ ion bombardment dictates glycine adsorption on pyrite (100) surface: X-ray photoemission spectroscopy and DFT approach. Appl. Surf. Sci. 2020, 530, 147182. [Google Scholar] [CrossRef]
- Pérez-Fernández, C.; Ruiz-Bermejo, M.; Gálvez-Martínez, S.; Mateo-Martí, E. An XPS study of HCN-derived films on pyrite surfaces: A prebiotic chemistry standpoint towards the development of protective coatings. RSC Adv. 2021, 11, 20109–20117. [Google Scholar] [CrossRef] [PubMed]
- Berndt, M.E.; Allen, D.E.; Seyfried, W.E., Jr. Reduction of CO2 during serpentinization of olivine at 300 °C and 500 bar. Geology 1996, 24, 351–354. [Google Scholar] [CrossRef]
- Russell, M.J.; Hall, A.J.; Martin, W. Serpentinization as a source of energy at the origin of life. Geobiology 2010, 8, 355–371. [Google Scholar] [CrossRef] [PubMed]
- de Souza, C.M.D.; Carneiro, C.E.A.; Baú, J.P.T.; da Costa, A.C.S.; Ivashita, F.F.; Paesano, A.; di Mauro, E.; de Santana, H.; Holm, N.G.; Neubeck, A.; et al. Interaction of forsterite-91 with distilled water and artificial seawater: A prebiotic chemistry experiment. Int. J. Astrobiol. 2013, 12, 135–143. [Google Scholar] [CrossRef]
- Deer, W.L.H.; Howie, R.A.; Zussman, J. Disilicates and Ring Silicates (Rock-Forming Minerals); Geological Society of London: London, UK, 1986. [Google Scholar]
- Murray, H.H. Chapter 2 Structure and Composition of the Clay Minerals and their Physical and Chemical Properties. In Developments in Clay Science; Murray, H.H., Ed.; Elsevier: Amsterdam, The Netherlands; Oxford, UK, 2006; Volume 2, pp. 7–31. [Google Scholar]
- Mateo-Martí, E.; Briones, C.; Rogero, C.; Gomez-Navarro, C.; Methivier, C.; Pradier, C.M.; Martín-Gago, J.A. Nucleic acid interactions with pyrite surfaces. Chem. Phys. 2008, 352, 11–18. [Google Scholar] [CrossRef]
- Berner, R.A. Sedimentary pyrite formation: An update. Geochim. Cosmochim. Acta 1984, 48, 605–615. [Google Scholar] [CrossRef]
- Keith, M.; Häckel, F.; Haase, K.M.; Schwarz-Schampera, U.; Klemd, R. Trace element systematics of pyrite from submarine hydrothermal vents. Ore Geol. Rev. 2016, 72, 728–745. [Google Scholar] [CrossRef]
- Luther, G.W.; Kostka, J.E.; Church, T.M.; Sulzberger, B.; Stumm, W. Seasonal iron cycling in the salt-marsh sedimentary environment: The importance of ligand complexes with Fe(II) and Fe(III) in the dissolution of Fe(III) minerals and pyrite, respectively. Mar. Chem. 1992, 40, 81–103. [Google Scholar] [CrossRef]
- Wächtershäuser, G. On the chemistry and evolution of the pioneer organism. Chem. Biodivers. 2007, 4, 584–602. [Google Scholar] [CrossRef]
- Sanchez-Arenillas, M.; Mateo-Marti, E. Spectroscopic study of cystine adsorption on pyrite surface: From vacuum to solution conditions. Chem. Phys. 2015, 458, 92–98. [Google Scholar] [CrossRef]
- Sanchez-Arenillas, M.; Mateo-Marti, E. Pyrite surface environment drives molecular adsorption: Cystine on pyrite(100) investigated by X-ray photoemission spectroscopy and low energy electron diffraction. Phys. Chem. Chem. Phys. 2016, 18, 27219–27225. [Google Scholar] [CrossRef] [PubMed]
- Xian, H.; Zhu, J.; Tan, W.; Tang, H.; Liu, P.; Zhu, R.; Liang, X.; Wei, J.; He, H.; Teng, H.H. The mechanism of defect induced hydroxylation on pyrite surfaces and implications for hydroxyl radical generation in prebiotic chemistry. Geochim. Cosmochim. Acta 2019, 244, 163–172. [Google Scholar] [CrossRef]
- Mateo-Marti, E.; Galvez-Martinez, S.; Gil-Lozano, C.; Zorzano, M.-P. Pyrite-induced uv-photocatalytic abiotic nitrogen fixation: Implications for early atmospheres and Life. Sci. Rep. 2019, 9, 15311. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colín-García, M.; Heredia, A.; Cordero, G.; Camprubí, A.; Negrón-Mendoza, A.; Ortega-Gutiérrez, F.; Beraldi, H.; Ramos-Bernal, S. Hydrothermal vents and prebiotic chemistry a review. Boletín De La Soc. Geológica Mex. 2016, 68, 599–620. [Google Scholar] [CrossRef]
- Attard, G.; Barnes, C. Surfaces; Oxford University Press: Oxford, UK, 1998. [Google Scholar]
- Hüfner, S. Photoelectron Spectroscopy; Springer: Berlin/Heidelberg, Germany, 1995; Volume 82, p. 27. [Google Scholar]
- Moulder, J.F.; Stickle, W.F.; Sobol, W.M.; Bomben, K.D. Handbook of X-Ray Photoelectron Spectroscopy. Perkin-Elmer Corp. 1992, 40, 221. [Google Scholar]
- Siegbahn, K. ESCA atomic, molecular and solid state structure studies by means of electron spectroscopy. In Nova acta Regiae Societatis Scientiarum Upsaliensis; Almqvist & Wiksell: Uppsala, Sweden, 1967. [Google Scholar]
- Siegbahn, K.M. Electron Spectroscopy for Atoms, Molecules and Condensed Matter. Rev. Mod. Phys. 1982, 54, 709. [Google Scholar] [CrossRef]
- Commission on New Minerals, Nomenclature and Classification. Available online: http://cnmnc.units.it/ (accessed on 20 March 2023).
- Mineralogy Database. Available online: http://webmineral.com/ (accessed on 20 March 2023).
- Warr, L.N. Recommended abbreviations for the names of clay minerals and associated phases. Clay Miner. 2020, 55, 261–264. [Google Scholar] [CrossRef]
- Jozefaciuk, G.; Bowanko, G. Effect of Acid and Alkali Treatments on Surface Areas and Adsorption Energies of Selected Minerals. Clays Clay Miner. 2002, 50, 771–783. [Google Scholar] [CrossRef]
- Yotsuji, K.; Tachi, Y.; Sakuma, H.; Kawamura, K. Effect of interlayer cations on montmorillonite swelling: Comparison between molecular dynamic simulations and experiments. Appl. Clay Sci. 2021, 204, 106034. [Google Scholar] [CrossRef]
- Wu, R.; McMahon, T.B. Stabilization of the Zwitterionic Structure of Proline by an Alkylammonium Ion in the Gas Phase. Angew. Chem. Int. Ed. 2007, 46, 3668–3671. [Google Scholar] [CrossRef]
- Mateo Marti, E.; Barlow, S.M.; Haq, S.; Raval, R. Bonding and assembly of the chiral amino acid S-proline on Cu(110): The influence of structural rigidity. Surf. Sci. 2002, 501, 191–202. [Google Scholar] [CrossRef]
- Clark, D.; Peeling, J.; Colling, L. An experimental and theoretical investigation of the core level spectra of a series of amino acids, dipeptides and polypeptides. Biochim. Et Biophys. Acta (BBA)-Protein Struct. 1976, 453, 533–545. [Google Scholar] [CrossRef]
- Uvdal, K.; Bodö, P.; Ihs, A.; Liedberg, B.; Salaneck, W.R. X-ray photoelectron and infrared spectroscopy of glycine adsorbed upon copper. J. Colloid Interface Sci. 1990, 140, 207–216. [Google Scholar] [CrossRef]
- Uvdal, K.; Bodö, P.; Liedberg, B. l-cysteine adsorbed on gold and copper: An X-ray photoelectron spectroscopy study. J. Colloid Interface Sci. 1992, 149, 162–173. [Google Scholar] [CrossRef]
- Gao, F.; Wang, Y.; Burkholder, L.; Tysoe, W.T. Chemistry of l-proline on Pd(111): Temperature-programmed desorption and X-ray photoelectron spectroscopic study. Surf. Sci. 2007, 601, 3579–3588. [Google Scholar] [CrossRef]
- Scheglov, A.; Helmke, A.; Loewenthal, L.; Ohms, G.; Vioel, W. XPS and ATR-FTIR study on chemical modifications of cold atmospheric plasma (CAP) operated in air on the amino acids L-proline and trans-4-Hydroxy-l-proline. Plasma Process. Polym. 2018, 15, 1800078. [Google Scholar] [CrossRef] [Green Version]
- Madejová, J. FTIR techniques in clay mineral studies. Vib. Spectrosc. 2003, 31, 1–10. [Google Scholar] [CrossRef]
- Mary, Y.S.; Ushakumari, L.; Harikumar, B.; Varghese, H.T.; Panicker, C.Y. FT-IR, FT-raman and SERS spectra of L-proline. J. Iran. Chem. Soc. 2009, 6, 138–144. [Google Scholar] [CrossRef]
- Patel, H.A.; Somani, R.S.; Bajaj, H.C.; Jasra, R.V. Nanoclays for polymer nanocomposites, paints, inks, greases and cosmetics formulations, drug delivery vehicle and waste water treatment. Bull. Mater. Sci. 2006, 29, 133–145. [Google Scholar] [CrossRef]
- Paques-Ledent, M.T.; Tarte, P. Vibrational studies of olivine-type compounds—I. The i.r. and Raman spectra of the isotopic species of Mg2SiO4. Spectrochim. Acta Part A Mol. Spectrosc. 1973, 29, 1007–1016. [Google Scholar] [CrossRef]
- Manfred, K.; Alexandr, A.; Thomas, O.; Cathcart, J.M.; Boris, M. The influence of wetting and drying cycles on mid-infrared attenuated total-reflection spectra of quartz: Understanding spectroscopy of disturbed soil. In Proceedings of the Detection and Remediation Technologies for Mines and Minelike Targets IX, Orlando, FL, USA, 12–16 April 2004; pp. 629–637. [Google Scholar]
- Tadic, M.; Panjan, M.; Tadic, B.V.; Lazovic, J.; Damnjanovic, V.; Kopani, M.; Kopanja, L. Magnetic properties of hematite (−FeO) nanoparticles synthesized by sol-gel synthesis method: The influence of particle size and particle size distribution. J. Electr. Eng. 2019, 70, 71–76. [Google Scholar] [CrossRef] [Green Version]
- Yu, C.; Ke, Y.; Deng, Q.; Lu, S.; Ji, J.; Hu, X.; Zhao, Y. Synthesis and Characterization of Polystyrene-Montmorillonite Nanocomposite Particles Using an Anionic-Surfactant-Modified Clay and Their Friction Performance. Appl. Sci. 2018, 8, 964. [Google Scholar] [CrossRef] [Green Version]
- Yuniati, M.D.; Hirajima, T.; Miki, H.; Sasaki, K. Silicate Covering Layer on Pyrite Surface in the Presence of Silicon–Catechol Complex for Acid Mine Drainage Prevention. Mater. Trans. 2015, 56, 1733–1741. [Google Scholar] [CrossRef] [Green Version]
- Au, P.-I.; Leong, Y.-K. Surface Chemistry and Rheology of Slurries of Kaolinite and Montmorillonite from Different Sources. KONA Powder Part. J. 2016, 33, 17–32. [Google Scholar] [CrossRef] [Green Version]
- Gil-Lozano, C.; Fairén, A.G.; Muñoz-Iglesias, V.; Fernández-Sampedro, M.; Prieto-Ballesteros, O.; Gago-Duport, L.; Losa-Adams, E.; Carrizo, D.; Bishop, J.L.; Fornaro, T.; et al. Constraining the preservation of organic compounds in Mars analog nontronites after exposure to acid and alkaline fluids. Sci. Rep. 2020, 10, 15097. [Google Scholar] [CrossRef] [PubMed]
- Elmi, C.; Guggenheim, S.; Gieré, R. Surface Crystal Chemistry of Phyllosilicates Using X-Ray Photoelectron Spectroscopy: A Review. Clays Clay Miner. 2016, 64, 537–551. [Google Scholar] [CrossRef] [Green Version]
- Rath, R.K.; Subramanian, S.; Pradeep, T. Surface Chemical Studies on Pyrite in the Presence of Polysaccharide-Based Flotation Depressants. J. Colloid Interface Sci. 2000, 229, 82–91. [Google Scholar] [CrossRef] [Green Version]
- Balomenou, G.; Stathi, P.; Enotiadis, A.; Gournis, D.; Deligiannakis, Y. Physicochemical study of amino-functionalized organosilicon cubes intercalated in montmorillonite clay: H-binding and metal uptake. J. Colloid Interface Sci. 2008, 325, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Mustafa, S.; Tasleem, S.; Naeem, A.; Safdar, M. Solvent effect on the electrophoretic mobility and adsorption of Cu on iron oxide. Colloids Surf. A Physicochem. Eng. Asp. 2008, 330, 8–13. [Google Scholar] [CrossRef]
- Oelkers, E.H.; Golubev, S.V.; Chairat, C.; Pokrovsky, O.S.; Schott, J. The surface chemistry of multi-oxide silicates. Geochim. Et Cosmochim. Acta 2009, 73, 4617–4634. [Google Scholar] [CrossRef]









| Mineral | Chemical Formula | Mineral Group | Environment |
|---|---|---|---|
| Montmorillonite 1 | (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2 ·nH2O | Phyllosilicate | Sedimentary, hydrothermal, diagenetic |
| Olivine 2 | (Mg,Fe)2SiO4 | Silicate | Basic and ultra-basic igneous rocks, Group species name |
| Pyrite 1 | FeS2 | Sulphide | Sedimentary, magmatic, metamorphic, and hydrothermal deposits |
| Haematite 1 | Fe2O3 | Oxide | Magmatic, hydrothermal, metamorphic, and sedimentary |
| System of Study | Anion | Zwitterion | Mineral Composition |
|---|---|---|---|
| Pro/Mnt | 19% | 81% | Mg, Ca, Na silicate |
| Pro/Iron disulphide | 65% | 35% | Iron disulphide |
| Pro/Ol | 57% | 43% | Fe Mg silicate |
| Pro/Hem | 50% | 50% | Iron oxide |
| Wavenumber (cm−1) | Vibration Mode | Refs. | |
|---|---|---|---|
| Mnt | Pro/Mnt | ||
| 3625 | 3614 | ν(OH) | [68,69] |
| - | 3069 | νas(CH2) | [69] |
| - | 2981 | νs(CH2) | [69] |
| 1633 | - | ν(H-O-H) | [68] |
| - | 1611 | ν(COO−) | [69] |
| - | 1559 | δ(NH2+) | [69] |
| - | 1407 | ν(COO−) | [69] |
| - | 1372 | ν(C-H) | [69] |
| 1114 | 1115 | ν(Si-O) out of plane | [70] |
| 1000 | 1007 | ν(Si-O) in plane | [70] |
| 797 | 796 | Platy form of tridymite | [70] |
| Hem | Pro/Hem | ||
| - | 1611 | ν(COO−) | [69] |
| - | 1549 | δ(NH2+) | [69] |
| - | 1405 | ν(COO−) | [69] |
| - | 1376 | ν(C-H) | [69] |
| 1089 | 1082 | ν(SiO4) 1 | [71,72] |
| 1047 | 1030 | ν(Si-O-Si) 1 | [73] |
| 908 | 903 | ν(Si-OH) 1 | [73] |
| 799 | 796 | ν(Si-O-Si) 1 | [73] |
| 518 | 519 | δ(Fe-O) | [73] |
| 441 | 441 | δ(Fe-O) | [73] |
| Ol | Pro/Ol | ||
| - | 3068 | νas(CH2) | [69] |
| - | 2975 | νs(CH2) | [69] |
| - | 1612 | ν(COO−) | [69] |
| - | 1559 | δ(NH2+) | [69] |
| - | 1449 | ν(COO−) | [69] |
| - | 1379 | ν(C-H) | [69] |
| 1086 | 1087 | ν(Si-O) | [70] |
| 959 | 959 | ν(SiO4) tetrahedron structure | [71,72] |
| 929 | 930 | ν(SiO4) tetrahedron structure | [71,72] |
| 740 | 743 | ν(SiO4) tetrahedron structure | [71,72] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cueto-Díaz, E.J.; Gálvez-Martínez, S.; Colin-García, M.; Mateo-Martí, E. A New Approach in Prebiotic Chemistry Studies: Proline Sorption Triggered by Mineral Surfaces Analysed Using XPS. Life 2023, 13, 908. https://doi.org/10.3390/life13040908
Cueto-Díaz EJ, Gálvez-Martínez S, Colin-García M, Mateo-Martí E. A New Approach in Prebiotic Chemistry Studies: Proline Sorption Triggered by Mineral Surfaces Analysed Using XPS. Life. 2023; 13(4):908. https://doi.org/10.3390/life13040908
Chicago/Turabian StyleCueto-Díaz, Eduardo J., Santos Gálvez-Martínez, María Colin-García, and Eva Mateo-Martí. 2023. "A New Approach in Prebiotic Chemistry Studies: Proline Sorption Triggered by Mineral Surfaces Analysed Using XPS" Life 13, no. 4: 908. https://doi.org/10.3390/life13040908